Abstract
We assessed the pharmacokinetics and interaction of ABT-773 in 12 volunteers receiving ABT-773 alone or concomitantly with ranitidine or sucralfate. Data for 150 mg of ABT-773 were as follows: the maximum concentration of the drug in plasma (Cmax) was 318 ng/ml, its half-life was 5.66 h, and its area under the plasma concentration-time curve from 0 h to ∞ (AUC0-∞) was 1,662 ng · h/ml. Coadministration of ranitidine, reduced the Cmax (−25.7%) and AUC0-∞ (−15.8%) significantly. Sucralfate had no impact on the bioavailability of ABT-773.
ABT-773 is a new ketolide with a high protein binding ratio. It has a potent antibacterial spectrum against penicillin- and macrolide-resistant gram-positive bacteria. Ketolides act by stimulating the dissociation of peptidyl-tRNA from the ribosome during the translocation process (3).
Ranitidine is a H2 receptor antagonist which inhibits gastric acid production and is widely available without prescription. ABT-773 exhibits great stability in an acidic environment like the stomach and shows better solubility at low pH than at high pH. Therefore, concomitant administration of ABT-773 with ranitidine might lead to a lower rate and reduced extent of absorption of ABT-773.
Sucralfate is a nonsystemic antiulcer drug that mainly protects the stomach and duodenum by coating the mucosa. Coadministration of sucralfate reduces the bioavailability of several drugs, including tetracyclines (2) and quinolones, by forming aluminum chelate complexes (1). ABT-773 absorption may be influenced in the same way.
Volunteers.
Twelve healthy Caucasian volunteers (six males and six females; mean age, 32 ± 5.7 years; mean body weight, 79.0 ± 11.2 kg; mean creatinine clearance, 121.5 ± 25.5 ml/min/1.73 m2) participated in the study. After approval by the local ethics committee according to German law, informed written consent was obtained from all subjects.
Study design.
According to the three-way-crossover design, each volunteer received the following drug combinations in a random order: ABT-773 alone (treatment A), ABT-773 and ranitidine (treatment B), and ABT-773 and sucralfate (treatment C). All drugs were administered orally. The study consisted of three study periods separated from each other by a washout period of at least 14 days. ABT-773 was given after an overnight fast as a single oral dose of 150 mg. In treatments B and C, 150 mg of ranitidine or 2,000 mg of sucralfate was administered 1 h before ABT-773.
Sample collection and processing.
Plasma samples (6 ml) were taken from a peripheral vein before drug administration and then at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h after dosing.
Urine was collected during the following periods: 0 to 3, 3 to 6, 6 to 12, and 12 to 24 h after dosing. The volume of urine was measured after each collection interval, and a 5-ml aliquot of well-mixed urine was saved.
Microbiological assay.
The concentrations of ABT-773 and its active metabolite(s) in urine were measured by a validated agar diffusion method described previously by Reeves and Bywater (4). The coefficients of variation determined on three different days for concentrations of 0.5 and 2 μg/ml were 6.1 and 4.8%, respectively. The lower limit of detection for the assay was 0.048 μg/ml.
LC-MS.
The liquid chromatography-mass spectrometry (LC-MS) method consists of solid-phase extraction followed by introduction into an LC-MS system for separation and detection. The lower limit of quantitation for the assay was approximately 2.0 ng/ml, with 0.5 ml of human plasma being used for both analytes.
Pharmacokinetic calculations.
Drug concentrations in plasma were analyzed by standard noncompartmental methods using the software program WinNonlin, professional edition, version 2.0. The primary pharmacokinetic parameters to determine the effects of ranitidine and sucralfate on the bioavailability of ABT-773 were the maximum observed concentration of the drug in plasma (Cmax) and the area under the plasma concentration-time curve from time zero extrapolated to infinity (AUC0-∞). Secondary parameters included the AUC from time zero to the time of the last sample collection, i.e., 24 h (AUC0-last), the elimination half-life (t1/2), the time to Cmax (Tmax), and mean residence time (MRT). Values for primary and secondary parameters were compared by analysis of variance by using the factors sequence, subject nested within the sequence, period, and treatment. A 90% confidence interval for the ratio of the mean of values for test treatment B or C to the mean of values for reference treatment A was calculated by parametric methods by using Schuirmann's procedure that uses two one-sided t tests (5). A statistically significant difference was accepted when P was <0.05.
Safety and tolerance.
The volunteers' overall tolerance to ABT-773 was good. No severe adverse events occurred. Seven out of the 12 volunteers reported having a mild headache, which was possibly related to the study drug. There were no significant changes in blood pressure, temperature, or heart rate or in hematological and biochemistry parameters.
Pharmacokinetics. (i) Plasma.
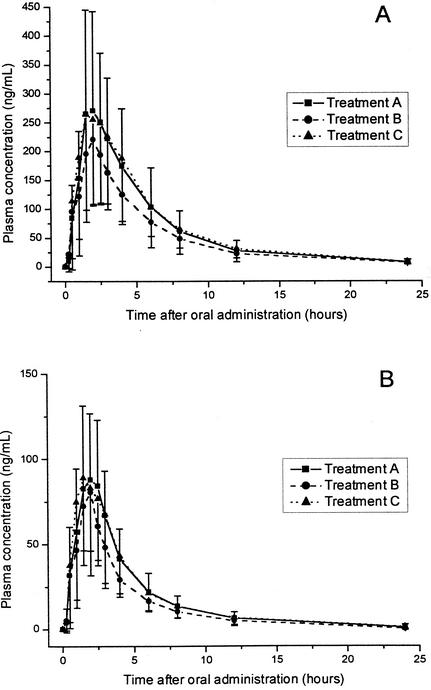
After oral administration of ABT-773 alone and 1 h after ranitidine and sucralfate intake, mean concentrations of ABT-773 and M-1 in plasma were as shown in Fig. 1. Table 1 summarizes the pharmacokinetic parameters for ABT-773 and M-1 obtained after the administration of ABT-773 alone and after administration with sucralfate or ranitidine. Prior administration of ranitidine reduced the AUC0-∞ by 15.8% and the Cmax by 25.7% (P = 0.02). Similar results were observed for the metabolite. Table 2 presents the results of the statistical comparisons between the reference treatment A and the test treatments B and C.
FIG. 1.
Arithmetic means of measured concentrations of ABT-773 (A) and its metabolite M-1 (B) in plasma after the administration of 150 mg of ABT-773 alone (treatment A) and with ranitidine (treatment B) or sucralfate (treatment C) in 12 healthy volunteers. The direction of the error bars (standard deviation) is upwards for treatment A and downwards for treatments B and C.
TABLE 1.
Pharmacokinetic parameters of ABT-773 and M-1 as determined by LC-MS after the administration of ABT-773 alone and with concomitant administration of ranitidine or sucralfate
| Analyte | Treatmenta | Mean ± SD
|
||||
|---|---|---|---|---|---|---|
| Cmax (ng/ml) | Tmax (h) | AUC0-last (ng · h/ml) | AUC0-∞ (ng · h/ml) | t1/2 (h) | ||
| ABT-773 | A | 318 ± 161 | 1.79 ± 0.50 | 1596 ± 876 | 1662 ± 907 | 5.66 ± 0.77 |
| B | 236 ± 105 | 2.04 ± 0.50 | 1242 ± 424 | 1310 ± 432 | 6.25 ± 1.08 | |
| C | 319 ± 180 | 1.92 ± 0.63 | 1680 ± 1030 | 1748 ± 1059 | 5.76 ± 0.81 | |
| M-1 | A | 112 ± 43.1 | 1.79 ± 0.54 | 401 ± 179 | 429 ± 186 | 4.12 ± 1.61 |
| B | 84.4 ± 46.6 | 1.88 ± 0.31 | 301 ± 124 | 328 ± 129 | 3.85 ± 1.30 | |
| C | 118 ± 55.4 | 1.92 ± 0.63 | 417 ± 208 | 444 ± 214 | 4.41 ± 1.72 | |
Treatment A, ABT-773 alone; treatment B, ABT-773 plus ranitidine; treatment C, ABT-773 plus sucralfate.
TABLE 2.
Statistical analysis of pharmacokinetic parameters after administration of ABT-773 alone and after coadministration with ranitidine or sucralfatea
| Variable | Test treatment | Reference treatment | Point estimate (%)
|
90% confidence interval limit
|
||||
|---|---|---|---|---|---|---|---|---|
| Lower limit
|
Upper limit
|
|||||||
| ABT-773 | M-1 | ABT-773 | M-1 | ABT-773 | M-1 | |||
| Cmaxb | B | A | 74.3 | 72.6 | 61.0 | 59.3 | 90.5d | 88.9 |
| C | A | 97.1 | 101.2 | 79.7 | 82.6 | 118.4 | 123.9 | |
| AUC0-lastb | B | A | 82.8 | 76.7 | 68.3 | 61.8 | 100.4 | 95.3 |
| C | A | 101.8 | 101.1 | 84.0 | 81.4 | 123.5 | 125.6 | |
| AUC0-∞b | B | A | 84.2 | 78.3 | 69.8 | 63.9 | 101.7 | 95.9 |
| C | A | 102.0 | 100.9 | 84.5 | 82.3 | 123.2 | 123.5 | |
| t1/2b | B | A | 109.8 | 95.3 | 100.4 | 83.4 | 120.0 | 108.9 |
| C | A | 101.7 | 106.2 | 93.0 | 92.9 | 111.2 | 121.3 | |
| Tmaxc | B | A | 114.0 | 104.7 | 92.4 | 84.9 | 135.5 | 124.4 |
| C | A | 107.0 | 107.0 | 85.4 | 87.2 | 128.5 | 126.7 | |
| MRTc | B | A | 111.6 | 98.1 | 104.7 | 88.9 | 118.4 | 107.3 |
| C | A | 101.7 | 101.6 | 94.8 | 92.3 | 108.5 | 110.8 | |
Treatment A, ABT-773 alone; treatment B, ABT-773 with ranitidine; treatment C, ABT-773 with sucralfate.
Analysis was based on ln-transformed data and parametric testing.
Analysis was based on untransformed data and parametric testing.
P < 0.05.
(ii) Urine.
The mean values (± standard deviations) for recovery from urine collected over 0 to 24 h postdose were 13.7% ± 4.7% for ABT-773 alone, 10.2% ± 4.1% for ABT-773 with ranitidine, and 11.1% ± 3.3% for ABT-773 with sucralfate. The Wilcoxon signed rank test revealed significantly decreased recovery from urine when ABT-773 was administered together with ranitidine compared to recovery after administration of ABT-773 alone (P < 0.05).
Concerning the relative bioavailability of ketolides, no studies have been published yet. This study revealed that coadministration of ranitidine 1 h prior to ABT-773 decreased the ABT-773 Cmax by 25.7% and the AUC0-∞ by 15.8% and prolonged the t1/2 and Tmax values slightly (approximately 10% for the t1/2 and 14% for the Tmax). Similar results were observed for the ABT-773 metabolite M-1.
In contrast, the coadministration of sucralfate had no significant impact on the bioavailability of ABT-773.
The data suggest that the decreased bioavailability of ABT-773 (represented by Cmax and AUC) following ranitidine administration is a consequence of a reduced rate and extent of absorption but that the effect on the elimination process (represented by the elimination t1/2) is small. This might be due to the decreased solubility of ABT-773 at higher gastric pHs.
The interaction of ABT-773 with ranitidine might be of clinical importance since the AUC/MIC ratio is the important pharmacokinetic-pharmacodynamic parameter for the in vivo efficacy of ABT-773 (D. R. Andes and W. A. Craig, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2139, 2000).
The statistically significantly reduced recovery of ABT-773 from urine after ranitidine coadministration is consistent with the reduced absorption of ABT-773. Since the urinary recovery of ABT-773, including that of its active metabolite(s), in general is low (13.7% ± 4.7%), these differences are not clinically important. Renal excretion is not the main pathway of elimination of ABT-773.
Conclusions.
The results of this study demonstrate that coadministration of ranitidine, but not of sucralfate, reduces the bioavailability of ABT-773. The Cmax is reduced by 25.7%, and the AUC0-∞ is decreased by 15.8%. The effect of ranitidine might be due to the decreased solubility of ABT-773 at higher gastric pH levels.
Acknowledgments
The expert technical assistance of K. Borner, M. Rieser, K. Hamel, M. Rau, and G. Schreiber is gratefully acknowledged.
This investigation was supported by a grant from Abbott Laboratories.
REFERENCES
- 1.Garrelts, J. C., P. J. Godley, J. D. Peterie, E. H. Gerlach, and C. C. Yakshe. 1990. Sucralfate significantly reduces ciprofloxacin concentrations in serum. Antimicrob. Agents Chemother. 34:931-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gugler, R., and H. Allgayer. 1990. Effects of antacids on the clinical pharmacokinetics of drugs. An update. Clin. Pharmacokinet. 18:210-219. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Serieys, C. 2000. Ketolides and oxazolidinones. Mechanisms of action and antibacterial spectrum. Presse Med. 29:2061-2064. (In French.) [PubMed]
- 4.Reeves, D. S., and M. J. Bywater. 1976. Assay of antimicrobial agents, p. 21-78. In J. de Louvois (ed.), Selected topics in clinical bacteriology. Bailliere & Tindall, London, United Kingdom.
- 5.Steinijans, V. W., and E. Diletti. 1983. Statistical analysis of bioavailability studies: parametric and nonparametric confidence intervals. Eur. J. Clin. Pharmacol. 24:127-136. [DOI] [PubMed] [Google Scholar]