Abstract
The influence of inhibitors of P-glycoprotein (verapamil [VE], cyclosporine [CY], and GF120918 [GF]) on the cell handling of macrolides (erythromycin [ERY], clarithromycin [CLR], roxithromycin [ROX], azithromycin [AZM], and telithromycin [TEL]) was examined in J774 murine macrophages. The net influx rates of AZM and TEL were increased from 2- to 3.5-fold in the presence of these inhibitors, but their efflux was slowed only marginally. At 3 h, the inhibitors increased the levels of AZM, ERY, and TEL accumulation approximately three- to fourfold (the effect of VE, however, was lower) but did not influence CLR accumulation (the inhibitors had an intermediate behavior on ROX accumulation). The effect was concentration dependent (half-maximal increases in the level of accumulation of AZM were obtained with GF, CY, and VE at 0.5, 5, and 10 μM, respectively). ATP depletion also caused an approximately threefold increase in the level of accumulation of AZM. Two inhibitors of MRP (probenecid [2.5 mM] and gemfibrozil [0.25 mM]) had no effect. Monensin (a proton ionophore) completely suppressed the accumulation of AZM in control cells as well as in cells incubated in the presence of VE, demonstrating that transmembrane proton gradients are the driving force causing the accumulation of AZM in both cases. Yet, VE did not alter the pH of the lysosomes (approximately 5) or of the cytosol (approximately 7.1). P-glycoprotein was detected by immunostaining at the cell surface as well as in intracellular vacuoles (endosomes and lysosomes). The data suggest that the influx of AZM, ERY, TEL, and ROX is adversely influenced by the activity of P-glycoprotein in J774 macrophages, resulting in suboptimal drug accumulation.
Active drug transporters have been described in both procaryotic and eucaryotic cells. Originally described as conferring resistance to anticancer agents in cancer cells, antibiotics in bacteria, or antifungal agents in fungi, these proteins appear today to be part of a very general mechanism that cells have developed to protect themselves from invasion by diffusible, foreign molecules (for a review, see reference 37). In this context, the occurrence of antibiotic transporters in eucaryotic cells has become a common observation (7, 33). More specifically, P-glycoprotein (also referred to as MDR1) and MRP, which are expressed in most cell types and which transport a large variety of drugs, have received much attention. These two types of transporters belong to the superfamily of ATP binding cassette transporters and use ATP hydrolysis as an energy source (28). They play a key role in drug disposition by modulating drug transport through epithelia and other biological barriers to an extent that was completely unsuspected only a few years ago (1).
Focusing on macrolides, erythromycin has been shown to be transported by P-glycoprotein in Caco-2 intestinal cells (29, 34). In parallel, erythromycin and azithromycin are capable of inhibiting the transport of various substrates of the P-glycoprotein in epithelial cells in vitro as well as in vivo (9, 12, 13, 23, 30, 31, 39). Yet, little is known about the role of efflux transporters in the handling of macrolides by macrophages, in which these drugs are known to accumulate in large amounts (2, 3, 20, 24).
In the present study, we have examined directly in macrophages the potential influence of P-glycoprotein and MRP on the accumulation and efflux of five macrolides of clinical interest. We used both broad-spectrum, nonspecific inhibitors of P-glycoprotein (verapamil and cyclosporine) and MRP (probenecid and gemfibrozil) and the specific P-glycoprotein modulator GF120918 (11, 15). We selected the murine J774 murine macrophage line since much is already known about the dispositions of macrolides in these cells (2, 3, 36).
MATERIALS AND METHODS
Cells.
We used J774 murine macrophages, which were cultivated as described previously (25). Cell viability was assessed by measurement of lactate dehydrogenase release (19).
Determination of cellular antibiotic accumulation.
Studies of cellular antibiotic accumulation were performed by the general procedure described in previous publications (3, 25). Antibiotic assays were performed with cell lysates by the diffusion disk method (17) with antibiotic medium 2 (Difco, Becton Dickinson & Co., Sparks, Md.) seeded with Micrococcus luteus ATCC 9341. The pH of the medium was adjusted to 9.5 for all drugs except azithromycin (for which the pHs were adjusted to 9.5 for samples with drug concentrations <0.5 mg/liter and 8.0 for higher drug concentrations). The lowest limits of detection and the typical ranges of drug concentrations measured were 0.2 and 0.9 to 4 mg/liter, respectively, for erythromycin; 0.2 and 0.25 to 0.4 mg/liter, respectively, for roxithromycin; 0.08 and 0.25 to 0.9 mg/liter, respectively, for azithromycin; 0.2 and 0.4 to 0.8 mg/liter, respectively, for clarithromycin; and 0.08 and 0.3 to 1.3 mg/liter, respectively, for telithromycin. Linearity was obtained up to a concentration of 2 mg/liter for all drugs (the concentration at which linearity was obtained for azithromycin at pH 8 was 32 mg/liter), with R2 being ≥0.96 (n = 18 for each drug). Inter- and intraday coefficients of variation for azithromycin were 5.3 and 2.2%, respectively. All assays were performed on plates 22.5 by 22.5 cm, with standards of the corresponding drug incubated on the same plate as the samples (typically, six standards covering the observed range of concentrations of samples were used, and these were tested in triplicate [similar inhibition zones were observed for standards prepared in water or cell lysates]). The cell drug content (cellular concentration) was systematically expressed by reference to the protein content, and the apparent cellular concentration-to-extracellular concentration ratio was determined by using a conversion factor of 5 μl of cell volume per mg of cell protein (2, 3).
Determination of cytosolic and lysosomal pHs.
The pHs of intracellular compartments were measured with the specific fluorescent probes 2-(4-pyridyl)-5-{[4-(2-dimethylaminoethylamino-carbamoyl)methoxy]phenyl}oxazole dextran(lysosensor yellow/blue-labeled dextran [LYBD] for lysosomes (5) and 2′,7′-bis-(2-carboxyethyl)-5-(and -6)-carboxyfluorescein [BCECF] (26) for the cytosol. Cells were incubated overnight with 2 mg of LYBD per ml or for 1 h at 37°C with 2 μM BCECF-AM (acetoxymethyl ester). The fluorescence of LYBD was recorded at 515 nm upon successive excitation at 340 and 405 nm, and that of BCECF was also recorded at 515 nm upon successive excitation at 440 and 490 nm (the ratio of the readings allows calculation of the local pH [5, 26]).
Confocal microscopy.
Cells were incubated overnight with rhodamine B-labeled dextran (molecular weight, 10,000; 2.5 mg/ml) to vitally stain endosomes and lysosomes, washed, and then used for immunolabeling of P-glycoprotein with rabbit polyclonal anti-P-glycoprotein antibodies (12.5 mg/liter) and Alexa Fluor 488-labeled anti-rabbit antibodies (5 mg/liter) by a previously described method (36). Observations were made with MRC1024 confocal scanning equipment (Bio-Rad, Richmond, Calif.) mounted on an Axiovert confocal microscope (the excitation wavelength was 495 nm and the emission wavelength was 519 nm for green signals; the excitation wavelength was 578 nm and the emission wavelength was 603 nm for red signals; Carl Zeiss, Oberkochen, Germany).
Reagents.
Erythromycin was obtained as Erythrocine (erythromycin lactobionate), which is the registered commercial product for intravenous administration in Belgium and which was supplied by Abbott s.a., Ottignies-Louvain-la-Neuve, Belgium. All other antibiotics were obtained as microbiological standards from their corresponding manufacturers (azithromycin [dihydrate salt; potency, 94.4%] was from Pfizer Inc., Groton, Conn.; telithromycin [potency, 99.3%] and roxithromycin [potency, 99.7%] were from Aventis Pharma, Romainville, France; and clarithromycin [potency, 98.4%] was from Abbott Laboratories Ltd., Queenborough, England). Verapamil, cyclosporine, and 2-deoxyglucose were products from Fluka Chemie, Buchs, Switzerland; GF120918 was kindly donated by GlaxoWellcome Research and Development, Laboratoire GlaxoWellcome, Les Ulis, France. Probenecid and gemfibrozil were supplied by Sigma-Aldrich Chemie, Steinheim, Germany; monensin was from Sigma Chemical Co., St. Louis, Mo.; cell culture media and serum were from Gibco Biocult (Paisley, Scotland); rabbit polyclonal anti-P-glycoprotein (Ab-1) was from Oncogene, Boston, Mass.; Alexa Fluor 488 anti-rabbit immunoglobulin G, LYBD, and BCECF-AM were from Molecular Probes, Eugene, Oreg.; and all other reagents were from E. Merck AG (Darmstadt, Germany).
Statistical analyses.
Curve-fitting analyses were done with GraphPad Prism software (version 2.01; GraphPad Prism Software, San Diego, Calif.), and group comparisons (Student's t test, one-way analysis of variance) were done with Instat Prism software (version 3.01; GraphPad Prism Software).
RESULTS
Influence of P-glycoprotein inhibitors on the kinetics of macrolide accumulation and efflux.
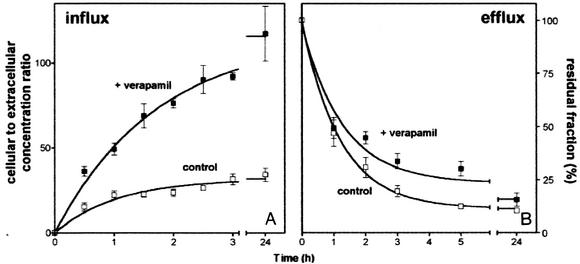
Figure 1A shows that azithromycin (5 mg/liter) is gradually accumulated by J774 murine macrophages, with the levels reaching a plateau after approximately 3 h (apparent cellular concentration-to-extracellular concentration ratio, approximately 30-fold). Similar kinetics were observed in the presence of verapamil (20 μM, added simultaneously with azithromycin); however, systematically higher levels of accumulation (2- to 3.5-fold) were obtained at all time points (yielding an apparent cellular concentration-to-extracellular concentration ratio at equilibrium of approximately 100-fold). Similar observations were made in the presence of 20 μM cyclosporine and 2 μM GF120918. Unless stated otherwise, subsequent experiments were therefore performed with cells incubated for 3 h with the macrolides under study (5 mg/liter) and with or without the P-glycoprotein inhibitors. To examine azithromycin efflux, cells loaded with this antibiotic (extracellular concentration, 20 mg/liter) in the presence of inhibitors for 3 h were reincubated in antibiotic-free medium in the continuing presence of the same inhibitors. The same protocol was used for the controls, except that the inhibitor was absent. Figure 1B shows that efflux was only modestly impaired by verapamil, with a maximal effect at 5 h (approximately 30% retention, versus 12% retention for the controls). Yet, no significant influence of verapamil could be demonstrated over the short-term (1-h) or long-term (24-h) treatment periods. Similar results were obtained with the two other inhibitors (20 μM cyclosporine and 2 μM GF120918). All these experiments were then repeated with telithromycin, with similar results.
FIG. 1.
(A) Kinetics of uptake of azithromycin (extracellular concentration, 5 mg/liter) in J774 murine macrophages incubated for up to 24 h in the absence (open squares) or in the presence (closed squares) of 20 μM verapamil. (B) Efflux of azithromycin (extracellular concentration, 20 mg/liter) from J774 murine macrophages incubated for 3 h with azithromycin in the presence or absence of verapamil and then reincubated in an azithromycin-free medium (chase). Open squares, controls; closed squares, cells incubated with 20 μM verapamil during uptake and efflux (half-lives for efflux, 50 min for the control and 53 min for verapamil, as calculated using one-phase exponential decay regression [Graph Pod Prism software]). Results are expressed as the percentage of the drug remaining associated with the cells at the end of the loading period. All data are the means ± standard deviations of three experiments.
Dose effects of P-glycoprotein and MRP inhibitors on azithromycin accumulation and comparison between macrolides.
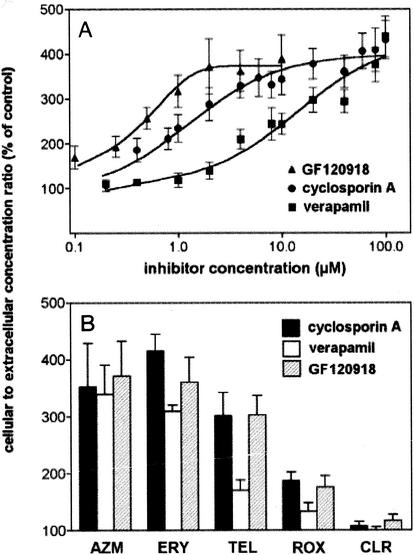
Figure 2A shows the results of experiments of the dose effects of verapamil, cyclosporine, and GF120918 on the accumulation of azithromycin at equilibrium. All three inhibitors displayed similar maximal effects (approximately fourfold), but with marked differences in potencies. In parallel experiments, we also examined the influences of two known inhibitors of the MRP efflux pump, namely, probenecid (2.5 mM) and gemfibrozil (0.25 mM), but did not observe any effect (data not shown; the expression of the MRP1 efflux pump has been demonstrated in J774 macrophages by Western blot analysis [J. M. Michot, F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens, 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 662, 2000]). In the next series of experiments, we examined the influence of verapamil, cyclosporine, and GF120918 at a fixed concentration on the level of accumulation of different macrolides. All antibiotics except erythromycin were used at 5 mg/liter; for the detection of erythromycin, it had to be present at 50 mg/liter due to its lower level of accumulation (3). As observed in Fig. 2B, erythromycin and telithromycin (with respect to cyclosporine and GF120918) behaved essentially like azithromycin (the effect of verapamil on telithromycin was, however, lower than those of the other inhibitors tested). In sharp contrast, the accumulation of clarithromycin was essentially unaffected. Roxithromycin showed an intermediate behavior.
FIG. 2.
(A) Azithromycin accumulation (extracellular concentration, 5 mg/liter) in J774 murine macrophages incubated for 3 h in the presence of increasing concentrations of P-glycoprotein inhibitors (closed squares, verapamil; closed circles, cyclosporine (cyclosporin A); closed triangles, GF120918). (B) Influence of P-glycoprotein inhibitors on cellular accumulation of macrolides in J774 murine macrophages after 3 h of incubation (closed bars, cyclosporine [20 μM]; open bars, verapamil [20 μM]; hatched bars, GF120918 [2 μM]). The extracellular concentration was 5 mg/liter for all macrolides except erythromycin, which was 50 mg/liter. Results are expressed as the percentage of the accumulation observed in the absence of inhibitor (the cellular concentration-to-extracellular concentration ratios for the controls were 20.7 ± 3.7 for azithromycin, 2.7 ± 0.1 for erythromycin, 16.3 ± 1.4 for telithromycin, 19.3 ± 1.7 for roxithromycin, and 46.6 ± 2.1 for clarithromycin; the values for azithromycin, erythromycin, and roxithromycin are consistent with those observed previously [2, 3] with the same cells but by use of radiolabeled drug). The effects of all inhibitors on antibiotic accumulation were significantly different (P < 0.05) for clarithromycin (CLR) compared to azithromycin (AZM), erythromycin (ERY), or telithromycin (TEL) and for roxithromycin (ROX) compared to azithromycin or erythromycin. The effect of verapamil on antibiotic accumulation was significantly different (P < 0.05) from the effects of the other inhibitors for erythromycin, telithromycin, and roxithromycin.
Influences of ATP depletion and cell exposure to monensin on azithromycin accumulation.
Because P-glycoprotein activity is ATP dependent, we examined the influence of ATP depletion on azithromycin accumulation in cells (obtained by preincubation of cells with 2-deoxyglucose and NaN3 and performance of uptake studies in the presence of these inhibitors; these conditions caused the cell ATP content to decrease to about 10% of its original value). As shown in Table 1, ATP depletion caused a marked increase in the level of azithromycin accumulation, which was as important as that seen with 20 μM verapamil. In parallel, cells were exposed to monensin, a natural carboxylic ionophore known to dissipate transmembrane proton gradients in eucaryotic cells (35). As shown in Table 1, monensin also almost completely suppressed the accumulation of azithromycin in control cells as well as cells incubated with verapamil.
TABLE 1.
Influence of an ATP-depleting treatment and monensin on cellular accumulation of azithromycin in control J774 macrophages and in J774 macrophages exposed to verapamila
| Treatment | %
Cellular accumulationb
|
|
|---|---|---|
| Control cells | Cells exposed to verapamil | |
| Control | 100.0 ± 7.8 | 287.6 ± 20.4 |
| ATP depletionc | 254.4 ± 30.7 | 310.7 ± 33.9 |
| Monensin | 5.9 ± 1.0 | 3.4 ± 0.6 |
ATP depleting treatment was for 90 min, monensin (20 μM) treatment was for 3 h, azithromycin was used at a concentration of 5 mg/liter, and verapamil was used at a concentration of 20 μM. By statistical analysis (one-way analysis of variance), P was <0.001 for control cells versus cells exposed to verapamil for the control treatment, the control treatment versus ATP depletion for control cells, and the control treatment versus monensin depletion for control cells.
Percentage of the cellular accumulation observed in cells without any treatment (azithromycin alone). Data are the means ± standard deviations of three experiments.
ATP depletion was obtained by 1 h of preincubation in the presence of 60 mM 2-deoxyglucose and 5 mM NaN3; these conditions were maintained during the incubation with azithromycin.
Influence of verapamil on lysosomal and cytoplasmic pHs.
We examined whether verapamil could increase the levels of macrolide accumulation by perturbing the pH gradient between the extracellular milieu and the lysosomes. This was investigated by using specific fluorescent tracers, the excitation spectra of which vary with a change in the pH (lysosensor yellow/blue-labeled dextran for lysosomes and BCECF for the cytosol; see Materials and Methods). No significant change from the control values was observed (pHs, 7.06 ± 0.93 [n = 17] and 4.95 ± 0.13 [n = 35] for the cytosol and lysosomes in the controls, respectively).
Localization of immunoreactive P-glycoprotein.
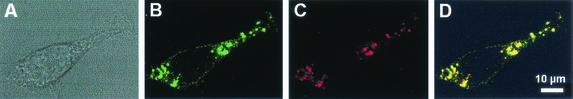
As shown in Fig. 3, anti-P-glycoprotein antibodies gave a faint pericellular labeling (plasma membrane), together with marked staining of discrete cytoplasmic granules of various sizes and a perinuclear distribution. Rhodamine-labeled dextran almost exclusively stained cytoplasmic granules. When merged images were examined, a large proportion of these granules turned yellow, demonstrating a partial common subcellular localization of rhodamine-labeled dextran and anti-P-glycoprotein antibodies.
FIG. 3.
Confocal fluorescence microscopy. (A) Phase-contrast microscopy; (B) green channel, with immunological detection of P-glycoprotein with rabbit polyclonal anti-P-glycoprotein antibodies (12.5 mg/liter) and Alexa Fluor 488-labeled secondary antibodies (5 mg/liter); (C) red channel, with vital staining of lysosomes and endosomes with rhodamine-B labeled dextran (2.5 mg/ml; the cells were incubated overnight with the tracer prior to their fixation and subsequent labeling with the anti-P-glycoprotein antibodies); (D) merged images.
DISCUSSION
The data presented in the paper disclose that verapamil, cyclosporine, and GF120918 markedly increase the cellular accumulation of the macrolide antibiotics azithromycin, erythromycin, and telithromycin in a model of J774 murine macrophages. The accumulation of roxithromycin is less affected, and the accumulation of clarithromycin showed no significant change. Verapamil and cyclosporine are known to inhibit the mammalian P-glycoprotein and to affect several other cell functions (15). In contrast, GF120918 is a much more specific inhibitor of P-glycoprotein and is not known, so far, to have other significant pharmacological properties (for reviews, see references 15 and 11). We have also shown that J774 macrophages express an immunoreactive P-glycoprotein, with a distribution consistent with what has been described in other cells (i.e., a partial localization at the cell surface, with a large quantity belonging to intracellular pools [the role of which is to maintain a constant amount of the active protein at the cell surface] [16]). All together, these data strongly suggest that modulation of P-glycoprotein is responsible for the observed effects on the accumulation of azithromycin, erythromycin, and telithromycin.
Macrolide accumulation in cells is dependent upon transmembrane pH gradients, which cause these drugs to be preferentially sequestered in lysosomes and related acidic vacuoles (2, 3; see also our data with monensin). A first hypothesis could therefore be that P-glycoprotein inhibitors increase the pH gradient between lysosomes and the extracellular milieu and/or the cytosol, either directly or by modulating P-glycoprotein basal activity (pH gradients between cell compartments are indeed reduced upon overexpression of efflux transporters [16]). This hypothesis can be ruled out since we did not observe significant changes in lysosomal and cytosolic pHs after exposure to verapamil. Moreover, the pH variations induced by inhibitors should have affected the accumulation of all macrolides. A second and more likely hypothesis, therefore, is that P-glycoprotein defeats the cellular accumulation of certain macrolides because of its drug efflux capabilities. This hypothesis is consistent with our observation that ATP depletion is as effective as the addition of a P-glycoprotein inhibitor (P-glycoprotein is dependent upon ATP for activity) (for reviews, see references 27 and 28). It also explains why macrolides with only minor chemical differences (viz., clarithromycin versus erythromycin) may have markedly different sensitivities to inhibitors. Substrate recognition by P-glycoprotein is indeed known to be highly variable even between drugs that are very closely related chemically, and defined pharmacophores are still far from being clearly recognized, apart from their general properties related to their lipophilicities and sizes (32). It is intriguing, however, that P-glycoprotein inhibitors do not markedly influence macrolide efflux. Biophysical studies actually suggest that P-glycoprotein binds to its substrates from within the membrane and not from the cytosol, acting as a flippase (10) or a “vacuum cleaner” (27). In this type of model, a lower level of cell accumulation results more from a decreased influx than from an increased net efflux (6, 8), as substrates need not have access to the cytosol to be extruded (18). Experimental testing of this model for macrolides could be done by using sublines of J774 macrophages overexpressing the P-glycoprotein or appropriate reconstituted membrane models.
The present experiments have been limited to J774 macrophages, which were used as a model, and generalization of our conclusions to other phagocytic cells therefore remains unwarranted. In particular, the presence of a functional P-glycoprotein and its potential role in macrolide handling in polymorphonuclear leukocytes are controversial (4, 14, 21, 38). It is interesting, however, that human KB and G-185 cells overexpressing P-glycoprotein show decreased levels of macrolide accumulation and correspondingly reduced levels of antimicrobial activity against intracellular forms of Listeria monocytogenes (22). The influences of efflux proteins on cellular handling of macrolides and their effects on the intracellular activities of these antibiotics may therefore need to be studied systematically.
Acknowledgments
We thank B. Tombal (Unité de Physiologie Générale des Muscles, Université Catholique de Louvain, Brussels, Belgium) for help in pH determination studies. F. Renoird-Andries and M. C. Cambier provided skillful technical assistance. We thank the antibiotic manufacturers and GlaxoWellcome R&D for the kind gifts of their corresponding products.
C.S. was chercheur postdoctoral of the Belgian Fonds de la Recherche Scientifique Médicale (fellowship 3.4.549.00). J.-M.M. is supported by the Fonds Spécial de Recherches of the Université Catholique de Louvain. H.C. is aspirant, F.V.B. Chercheur Qualifié; and M.-P.M.-L. is Maître de Recherches of the Belgian Fonds National de la Recherche Scientifique. This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grants 3.4549.00 and 3.4542.02).
REFERENCES
- 1.Ayrton, A., and P. Morgan. 2001. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 31:469-497. [DOI] [PubMed] [Google Scholar]
- 2.Carlier, M. B., I. Garcia-Luque, J. P. Montenez, P. M. Tulkens, and J. Piret. 1994. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue React. 16:211-220. [PubMed] [Google Scholar]
- 3.Carlier, M. B., A. Zenebergh, and P. M. Tulkens. 1987. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J. Antimicrob. Chemother. 20(Suppl. B):47-56. [DOI] [PubMed] [Google Scholar]
- 4.Damiani, D., M. Michieli, A. Michelutti, A. Geromin, D. Raspadori, R. Fanin, C. Savignano, M. Giacca, S. Pileri, and F. Mallardi. 1993. Expression of multidrug resistance gene (MDR-1) in human normal leukocytes. Haematologica 78:12-17. [PubMed] [Google Scholar]
- 5.Diwu, Z., C. S. Chen, C. Zhang, D. H. Klaubert, and R. P. Haugland. 1999. A novel acidotropic pH indicator and its potential application in labeling acidic organelles of live cells. Chem. Biol. 6:411-418. [DOI] [PubMed] [Google Scholar]
- 6.Eytan, G. D., R. Regev, G. Oren, and Y. G. Assaraf. 1996. The role of passive transbilayer drug movement in multidrug resistance and its modulation. J. Biol. Chem. 271:12897-12902. [DOI] [PubMed] [Google Scholar]
- 7.Fanos, V., and L. Cataldi. 2001. Renal transport of antibiotics and nephrotoxicity: a review. J. Chemother. 13:461-472. [DOI] [PubMed] [Google Scholar]
- 8.Gaj, C. L., I. Anyanwutaku, Y. H. Chang, and Y. C. Cheng. 1998. Decreased drug accumulation without increased drug efflux in a novel MRP-overexpressing multidrug-resistant cell line. Biochem. Pharmacol. 55:1199-1211. [DOI] [PubMed] [Google Scholar]
- 9.Goldschmidt, N., T. Azaz-Livshits, Gotsman, R. Nir-Paz, A. Ben Yehuda, and M. Muszkat. 2001. Compound cardiac toxicity of oral erythromycin and verapamil. Ann. Pharmacother. 35:1396-1399. [DOI] [PubMed] [Google Scholar]
- 10.Higgins, C. F., and M. M. Gottesman. 1992. Is the multidrug transporter a flippase? Trends Biochem. Sci. 17:18-21. [DOI] [PubMed] [Google Scholar]
- 11.Hyafil, F., C. Vergely, V. P. Du, and T. Grand-Perret. 1993. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 53:4595-4602. [PubMed] [Google Scholar]
- 12.Kim, R. B., C. Wandel, B. Leake, M. Cvetkovic, M. F. Fromm, P. J. Dempsey, M. M. Roden, F. Belas, A. K. Chaudhary, D. M. Roden, A. J. Wood, and G. R. Wilkinson. 1999. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm. Res. 16:408-414. [DOI] [PubMed] [Google Scholar]
- 13.Kiso, S., S. H. Cai, K. Kitaichi, N. Furui, K. Takagi, K. Takagi, T. Nabeshima, and T. Hasegawa. 2000. Inhibitory effect of erythromycin on P-glycoprotein-mediated biliary excretion of doxorubicin in rats. Anticancer Res. 20:2827-2834. [PubMed] [Google Scholar]
- 14.Klimecki, W. T., B. W. Futscher, T. M. Grogan, and W. S. Dalton. 1994. P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood 83:2451-2458. [PubMed] [Google Scholar]
- 15.Krishna, R., and L. D. Mayer. 2000. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 11:265-283. [DOI] [PubMed] [Google Scholar]
- 16.Larsen, A. K., A. E. Escargueil, and A. Skladanowski. 2000. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 85:217-229. [DOI] [PubMed] [Google Scholar]
- 17.Mandell, G. L., and E. Coleman. 2001. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 45:1794-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marbeuf-Gueye, C., D. Ettori, W. Priebe, H. Kozlowski, and A. Garnier-Suillerot. 1999. Correlation between the kinetics of anthracycline uptake and the resistance factor in cancer cells expressing the multidrug resistance protein or the P-glycoprotein. Biochim. Biophys. Acta 1450:374-384. [DOI] [PubMed] [Google Scholar]
- 19.Montenez, J. P., F. Van Bambeke, J. Piret, R. Brasseur, P. M. Tulkens, and M. P. Mingeot-Leclercq. 1999. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol. Appl. Pharmacol. 156:129-140. [DOI] [PubMed] [Google Scholar]
- 20.Mor, N., J. Vanderkolk, and L. Heifets. 1994. Accumulation of clarithromycin in macrophages infected with Mycobacterium avium. Pharmacotherapy 14:100-104. [DOI] [PubMed] [Google Scholar]
- 21.Mtairag, E. M., H. Abdelghaffar, C. Douhet, and M. T. Labro. 1995. Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob. Agents Chemother. 39:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichterlein, T., M. Kretschmar, A. Schadt, A. Meyer, A. Wildfeuer, H. Laufen, and H. Hof. 1998. Reduced intracellular activity of antibiotics against Listeria monocytogenes in multidrug resistant cells. Int. J. Antimicrob. Agents 10:119-125. [DOI] [PubMed] [Google Scholar]
- 23.Page, R. L., J. M. Ruscin, D. Fish, and M. Lapointe. 2001. Possible interaction between intravenous azithromycin and oral cyclosporine. Pharmacotherapy 21:1436-1443. [DOI] [PubMed] [Google Scholar]
- 24.Pascual, A., S. Ballesta, I. Garcia, and E. J. Perea. 2001. Uptake and intracellular activity of ketolide HMR 3647 in human phagocytic and non-phagocytic cells. Clin. Microbiol. Infect. 7:65-69. [DOI] [PubMed] [Google Scholar]
- 25.Renard, C., H. J. Vanderhaeghe, P. J. Claes, A. Zenebergh, and P. M. Tulkens. 1987. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob. Agents Chemother. 31:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rink, T. J., R. Y. Tsien, and T. Pozzan. 1982. Cytoplasmic pH and free Mg2+ in lymphocytes. J. Cell Biol. 95:189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg, M. F., R. Callaghan, R. C. Ford, and C. F. Higgins. 1997. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J. Biol. Chem. 272:10685-10694. [DOI] [PubMed] [Google Scholar]
- 28.Saier, M. H., Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito, H., Y. Fukasawa, Y. Otsubo, K. Yamada, H. Sezaki, and S. Yamashita. 2000. Carrier-mediated transport of macrolide antimicrobial agents across Caco-2 cell monolayers. Pharm. Res. 17:761-765. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz, U. I., T. Gramatte, J. Krappweis, R. Oertel, and W. Kirch. 2000. P-glycoprotein inhibitor erythromycin increases oral bioavailability of talinolol in humans. Int. J. Clin. Pharmacol. Ther. 38:161-167. [DOI] [PubMed] [Google Scholar]
- 31.Siedlik, P. H., S. C. Olson, B. B. Yang, and R. H. Stern. 1999. Erythromycin coadministration increases plasma atorvastatin concentrations. J. Clin. Pharmacol. 39:501-504. [PubMed] [Google Scholar]
- 32.Stouch, T. R., and O. Gudmundsson. 2002. Progress in understanding the structure-activity relationships of P-glycoprotein. Adv. Drug Deliv. Rev. 54:315-328. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama, Y., H. Kusuhara, and H. Suzuki. 1999. Kinetic and biochemical analysis of carrier-mediated efflux of drugs through the blood-brain and blood-cerebrospinal fluid barriers: importance in the drug delivery to the brain. J. Control Release 62:179-186. [DOI] [PubMed] [Google Scholar]
- 34.Takano, M., R. Hasegawa, T. Fukuda, R. Yumoto, J. Nagai, and T. Murakami. 1998. Interaction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cells. Eur. J. Pharmacol. 358:289-294. [DOI] [PubMed] [Google Scholar]
- 35.Tartakoff, A. M. 1983. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell 32:1026-1028. [DOI] [PubMed] [Google Scholar]
- 36.Tyteca, D., P. Van Der Smissen, M. Mettlen, F. Van Bambeke, P. M. Tulkens, M. P. Mingeot-Leclercq, and P. J. Courtoy. 2002. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp. Cell Res. 281:86-100. [DOI] [PubMed] [Google Scholar]
- 37.Van Bambeke, F., E. Balzi, and P. M. Tulkens. 2000. Antibiotic efflux pumps. Biochem. Pharmacol. 60:457-470. [DOI] [PubMed] [Google Scholar]
- 38.Vazifeh, D., A. Preira, A. Bryskier, and M. T. Labro. 1998. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 42:1944-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L., K. Kitaichi, C. S. Hui, K. Takagi, K. Takagi, M. Sakai, K. Yokogawa, K. I. Miyamoto, and T. Hasegawa. 2000. Reversal of anticancer drug resistance by macrolide antibiotics in vitro and in vivo. Clin. Exp. Pharmacol. Physiol. 27:587-593. [DOI] [PubMed] [Google Scholar]