Abstract
Despite the marketing of a series of new antibiotics for antibiotic-resistant gram-positive bacteria, no new agents for multiple-antibiotic-resistant gram-negative infections will be available for quite some time. Clinicians will need to find more effective ways to utilize available agents. Colistin is an older but novel antibiotic that fell into disfavor with clinicians some time ago yet still retains a very favorable antibacterial spectrum, especially for Pseudomonas and Acinetobacter spp. Time-kill curves for two strains of multiantibiotic-resistant Pseudomonas aeruginosa were generated after exposure to colistin alone or in combination with ceftazidime or ciprofloxacin in an in vitro pharmacodynamic model. MICs of colistin, ceftazidime, ciprofloxacin, piperacillin-tazobactam, imipenem, and tobramycin were 0.125, ≥32, >4, >128/4, 16, and >16 mg/liter, respectively. Colistin showed rapid, apparently concentration-dependent bactericidal activity at concentrations between 3 and 200 mg/liter. We were unable to detect increased colistin activity at concentrations above 18 mg/liter due to extremely rapid killing. The combination of colistin and ceftazidime was synergistic (defined as at least a 2-log10 drop in CFU per milliliter from the count obtained with the more active agent) at 24 h. Adding ciprofloxacin to colistin did not enhance antibiotic activity. These data suggest that the antibacterial effect of colistin combined with ceftazidime can be maximized at a peak concentration of ≤18 mg/liter.
The polypeptide antibiotics of the polymyxin class, including polymyxin E (colistin), were first made available for clinical use in the late 1950s and early 1960s. These agents are rapidly bactericidal against many gram-negative bacteria (13). Colistin is available clinically with sulfomethylated amino groups, thought to minimize pain at injection sites as well as having other side effects (2). Studies have found the sulfomethylated derivates to have one-half to one-eighth the activity of the parent compound (8, 13), though the activity of the derivatives may change with time secondary to in vivo hydrolysis and it is not clear which moiety possesses the majority of clinical activity (1, 2). The MIC of the sulfomethyl colistin at which 90% of isolates are inhibited for 94 Pseudomonas aeruginosa isolates was found to be 4 mg/liter, with a range of 0.5 to 32 mg/liter (4).
Perhaps as a result of concerns about toxicity, the parenteral use of colistin has been rather limited. Colistin is thought to be nephro- and neurotoxic, though clinical trials involving this agent have not been in universal agreement on the rate of toxicity. (3, 12). The toxicity of colistin was thought to be dose dependent (9), though recent data from a pharmacokinetic study of colistin in 31 cystic fibrosis patients found no relationship between any adverse effects and concentration in plasma (15).
Resistance to beta-lactams, quinolones, and aminoglycosides in P. aeruginosa continues to increase. As no novel agents have been introduced to combat these multiple-antibiotic-resistant organisms, and as it seems unlikely that any new agents will be introduced soon, clinicians may become forced to rediscover older agents such as colistin. In fact, there is already a resurgence of interest in the use of this novel agent (13).
In this study we used an in vitro pharmacodynamic model to evaluate the killing of two strains of multidrug-resistant P. aeruginosa by colistin, both alone and in combination with ceftazidime or ciprofloxacin. P. aeruginosa was exposed to colistin alone over a 2-order-of-magnitude concentration range to determine if higher doses consistently produced a larger effect. Combination experiments, where typical human pharmacokinetics of colistin were simulated, were designed to determine if fewer daily doses of colistin could be given when either ciprofloxacin or ceftazidime was also added. Such data could be useful in predicting what dose and interval of colistin is likely to optimize activity and in forming a basis for combination therapy.
MATERIALS AND METHODS
Fifteen concentration-time-kill curve experiments with colistin given alone or in combination with either ceftazidime or ciprofloxacin against two strains of P. aeruginosa were performed. One experiment was done with ceftazidime given alone. Because previous in vivo studies that included pharmacokinetic measurements after exposure to sulfomethyl colistin measured concentrations of colistin in serum, and to avoid the possibility of equilibrium between the sulfomethyl derivative and the parent compound, this study used the parent compound exclusively. Intravenous 150-mg doses of sulfomethyl colistin produce peak concentrations in serum of approximately 18 mg/liter (colistin base equivalents); intramuscular administration of the same dose produces peak concentrations of approximately 6 mg/liter (colistin base equivalents) (10). The half-life (t1/2) of colistin is approximately 3 h (10, 15), and the degree of protein binding, while unknown, is likely low (11).
In vitro model.
Concentration-time-kill curve studies were conducted by using a previously described in vitro model (17). Growth control experiments were conducted for each of the two P. aeruginosa isolates. In the experiments that included antibiotic administration, an inoculum of P. aeruginosa was instilled into a chemostat followed by a bolus injection at time zero of colistin alone (nine experiments) or colistin in combination with ceftazidime (four experiments) or ciprofloxacin (two experiments) to produce the desired initial antibiotic combination for each experiment. Each experiment was run in duplicate for 24 (12 experiments) or 48 (4 experiments) h. Antibiotic-free cation-adjusted Mueller-Hinton broth (CAMHB; Ca2+, 50 mg/liter; Mg2+, 25 mg/liter) was pumped via a peristaltic pump into a chemostat at a predetermined rate. Simultaneously, an equal volume of CAMHB was displaced from the chemostat into a waste reservoir, simulating a monoexponential pharmacokinetic process and producing the desired t1/2 of the antibiotic.
Bacteria.
Two clinical isolates of P. aeruginosa (PSA M29899-1 and PSA H23259-1), kindly provided by R. Rapp, University of Kentucky, were studied. As determined by an accredited clinical laboratory, MICs of ciprofloxacin, piperacillin/tazobactam, imipenem, and tobramycin were >4, >128/4, 16, and >16 mg/liter, respectively, for both organisms. The MICs for PSA M29899-1 and PSA H23259-1 of ceftazidime were >32 and 32 mg/liter, respectively. Prior to concentration-time-kill curve experiments, several colonies of the isolate were grown overnight in 50 ml of cation-adjusted Mueller-Hinton broth (CAMHB) and were then diluted 1:5 in fresh CAMHB approximately 1 h prior to the experiment to allow the organisms to attain exponential growth. Bacterial isolates were grown to a 0.5 McFarland standard in CAMHB. A volume equal to 1/100 of the flask volume was added to the chemostat. The resultant starting bacterial inoculum was approximately 106 CFU/ml.
Susceptibility testing.
Susceptibility to colistin was tested in quadruplicate for each isolate prior to concentration-time-kill experiments and for isolates retrieved at 24 or 48 h postexposure. Susceptibility testing was performed by broth microdilution in CAMHB with an inoculum of approximately 106 CFU/ml. All isolates were subcultured onto fresh blood agar plates on at least three consecutive days prior to susceptibility testing.
Antibiotics.
Stock solutions of colistin (Sigma-Aldrich, St. Louis, Mo.), ceftazidime (Sigma-Aldrich), and ciprofloxacin (Bayer, New Haven, Conn.) were prepared by using the appropriate amount of sterile distilled water and kept at 4°C until needed for individual experiments. Antibiotics were administered as bolus infusions at either 12- or 24-h intervals or as a constant infusion over 24 or 48 h.
Pharmacokinetics.
Three groups of experiments were performed. The t1/2 simulated for colistin in each of the experiments was 3 h. In group 1, colistin was given alone over 24 h in one or two doses against PSA M29899-1. In the single-dose experiments, targeted peak drug concentrations (Cmax) were 0.5, 3, 6, 18, 36, 72, and 200 mg/liter. In experiments where colistin was given as two doses over 24 h, peaks of either 6 or 18 mg/liter were targeted. These Cmaxs, as well as the t1/2 of colistin, were chosen because they represent clinically relevant concentrations after standard intramuscular and intravenous doses (10). While the exact magnitude of colistin protein binding is not known, we are confident that with the wide range of concentrations studied, we have simulated the appropriate free drug concentration. In group 2, colistin was given every 24 h to achieve a Cmax of either 6 or 18 mg/liter along with a ceftazidime loading dose and constant infusion to keep the ceftazidime concentration at steady state (CSS) of 50 mg/liter for the 48-h duration of the experiment with PSA M29899-1 and PSA H23259-1. In group 3, colistin was given every 24 h to achieve a peak of either 6 or 18 mg/liter along with ciprofloxacin given every 12 h to achieve a peak of 5 mg/liter against PSA M29899-1. The simulated t1/2 for both ceftazidime and ciprofloxacin was 3 h.
HPLC.
The concentrations of colistin were determined from batched stored samples (frozen at −80°C) in Mueller-Hinton broth by using high-performance liquid chromatography (HPLC) according to previously described methods (15). Slight modifications in sample preparation were performed. Briefly, a 1.0-ml sample (diluted up to fivefold with Mueller-Hinton broth) was added to 1.0 ml of Millipore water and 0.2 ml of 3 M perchloric acid. This sample was vortexed for 10 s, and 1.4 ml was placed in a 16- by 100-mm silanized glass tube to which 0.28 ml of 1.5 M potassium hydroxide, 0.2 ml of 0.1 M hydrochloric acid, 0.1 ml of 9% sodium carbonate, and 0.4 ml of dansyl chloride (20 mg/ml) were added. The sample was vortexed after each addition. The sample was covered with Parafilm and heated in a covered 57°C water bath for 1 h. After 5 min, 0.1 ml of proline (300 mg/ml) was added. This sample was vortexed, covered with Parafilm, and again placed in the 57°C water bath for 1 h. After cooling, 2.0 ml was transferred to a 13- by 100-mm screw-cap glass tube, and 1.0 ml of ethyl acetate was added. The tube was covered with foil, capped, and placed on a Roto-torque rotator (model 7637; Cole-Palmer, Chicago, Ill.) for 2 min at setting 5. Samples were spun in a table-top centrifuge (Marathon 26 KMR) for 10 min at 3,000 rpm, and 0.16 ml was carefully removed from the top layer and placed in an autosampler vial with an insert for injection. Colistin standards were prepared in Mueller-Hinton broth between 5 and 50 μg/ml, and two controls were analyzed with the set of unknowns. By using log-transformed height data from the major colistin peak with DeltaGraph software on a Macintosh IIsi (Apple Computer, Cupertino, Calif.), a linear equation (r ≥ 0.9997) was obtained.
Pharmacodynamics.
At predetermined timed intervals, samples of CAMHB were removed from the model for quantification of the bacterial density by a saline dilution technique. Antibiotic carryover was minimized by saline serial diluting. The number of predetermined timed intervals for sampling was dependent on the frequency of antibiotic administration; however, a minimum of nine samples were removed in the 24-h experiments, and a minimum of 13 samples were removed in the 48-h experiments. Bacterial counts were determined by a 1:10 serial dilution of 100 μl of medium into saline that was plated onto Trypticase soy agar supplemented with 5% sheep blood.
After incubation for 18 to 24 h at 37°C, the numbers of CFU on each plate were counted visually. The theoretical lower limit of bacterial counting accuracy was 300 CFU/ml. Concentration-time-kill curves were constructed by plotting the log10 CFU per milliliter versus time.
The change in log10 colony count from the starting inoculum at each sampling point was calculated after the starting inoculum had been standardized to 106 CFU/ml by adding or subtracting the necessary fraction of the log10 colony count. This was done so that the change in log10 colony count could be compared between experiments. As our lower limit of detection is 2.48 log10 CFU/ml, the maximum reduction in log10 colony count was 3.52 log10 CFU/ml. Synergy was defined as a ≥2-log10 decrease in colony count relative to the count obtained with the most active of the two antibiotics at 24 h. Additivity was defined as a 0- to 1.9-log10 decrease at 24 h.
RESULTS
MICs.
The MIC of colistin for each strain was 0.125 mg/liter, despite the organisms' being intermediate or resistant to other antibiotics studied.
Pharmacokinetics.
Comparing colistin levels attained in the model, determined by HPLC, to the expected levels helped verify the attained pharmacokinetic parameters (t1/2 and Cmax). The colistin levels did not vary significantly from the expected levels (paired t test P value, 0.581), with an average (mean) difference of 14.84% and a range of 0.69 to 37.5%. Calculated t1/2, using the actual concentrations, ranged from 2.2 to 3.8 h. All HPLC samples were run on the same day, under limit controls described earlier (15).
Time-kill kinetics.
Colistin showed rapid bactericidal activity in single-dose studies where Cmax was at least 3 mg/liter, evident by a 3-log reduction in colony counts at or before 3 h (Table 1). The extent of killing at the sampling times was greater as initial colistin concentration increased from 0.5 to 18 mg/liter, indicating concentration-dependent activity. Colistin may have concentration-dependent activity above this level, though we were unable to detect this in our experiments because bacterial counts dropped rapidly below our limit of detection. At 24 h, regrowth was evident in all single-dose colistin experiments, though the viable colony count decreased with increasing colistin exposure up to 200 mg/liter, which is consistent with concentration-dependent activity.
TABLE 1.
Changes in P. aeruginosa M29899-1 colony counts in initial-inoculum, single- and multiple-dose colistin experiments
| Time (h) | Change in colony count (log10 CFU/ml) with Cmax (mg/liter) of:a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 3 | 6 | 18 | 36 | 72 | 200 | 6 (BID) | 18 (BID) | |
| 0.5 | ND | ND | ND | ND | ND | ND | ≥−3.52 | ND | ND |
| 1 | −1.14 | −2.30 | −2.62 | −3.48 | ND | ≥−3.52 | ≥−3.52 | −2.34 | −2.66 |
| 2 | −1.13 | −2.87 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −3.10 | ≥−3.52 |
| 3 | −1.33 | −3.31 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 |
| 4 | −1.43 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 |
| 5.5 | −1.34 | −3.37 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 |
| 7 | −0.69 | −2.80 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ND | ND |
| 12 | 1.49 | −0.97 | −2.34 | −3.41 | −2.70 | −2.94 | ≥−3.52 | ≥−3.52 | ≥−3.52 |
| 13 | ND | ND | ND | ND | ND | ND | ND | −3.42 | ≥−3.52 |
| 14 | ND | ND | ND | ND | ND | ND | ND | −3.16 | ≥−3.52 |
| 24 | 1.68 | 1.53 | 0.94 | −0.05 | −0.64 | −0.86 | −1.02 | −0.79 | −2.16 |
Cmax was achieved at time zero only, except where noted otherwise. The time of sampling varied slightly in certain experiments at 5.5, 7, and 12 h, but the variation was within 15 to 90 min of the stated sampling times. BID, twice a day; ND, sample not taken.
Similar to the results in single-dose experiments, colistin was not able to prevent regrowth when given every 12 h. However, when colistin was given to achieve a peak of 18 mg/liter every 12 h, the colony count at 24 h was lower than in any of the single-dose experiments.
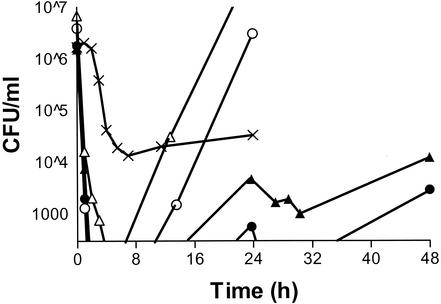
At 24 h, the combinations of colistin (Cmax = 6 or 18 mg/liter) with constant ceftazidime infusion (CSS = 50 mg/liter) showed synergistic activity against PSA M29899-1 (differences in colony count compared with colistin alone at the same Cmax, 3.45 and 2.70 log10 CFU/ml, respectively [Tables 1 and 2 ]). The combination of ceftazidime infusion and colistin given every 24 h had excellent activity against both P. aeruginosa strains (Fig. 1). Although the higher colistin dose had slightly more activity, the difference in log10 colony count was never greater than 0.70 CFU/ml at any time point for either strain. Despite the fact that ceftazidime was given by continuous infusion at 1.5 times the MIC, the drug alone showed marginal activity and did not produce 3-log killing (Table 2).
TABLE 2.
Changes in P. aeruginosa colony counts in initial-inoculum, single-dose colistin experiments
| Time (h) | Change in colony count (log10 CFU/ml) with colistin at indicated Cmax (mg/liter) and/or indicated druga
|
||||||
|---|---|---|---|---|---|---|---|
| 6, +CTZb | 18, +CTZb | 6, +CTZc | 18, +CTZc | CTZ aloneb | 6, +CIPb,d | 18, +CIPb,d | |
| 1 | −2.30 | −2.96 | ≥−3.52 | −3.39 | 0.08 | −2.99 | −2.76 |
| 2 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −0.01 | ≥−3.52 | ≥−3.52 |
| 3 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −0.63 | ≥−3.52 | ≥−3.52 |
| 4 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −1.60 | −3.41 | ≥−3.52 |
| 5.5 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −1.94 | −2.37 | ≥−3.52 |
| 7 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −2.10 | −1.90 | ≥−3.52 |
| 12 | ≥−3.52 | ≥−3.52 | ≥−3.52 | ≥−3.52 | −1.92 | −1.08 | −1.86 |
| 24 | −2.51 | −2.75 | −2.77 | −2.84 | −1.70 | 1.78 | 0.08 |
| 26 | −2.96 | ≥−3.52 | −3.43 | ≥−3.52 | |||
| 28 | −2.90 | ≥−3.52 | −3.45 | ≥−3.52 | |||
| 30 | −3.17 | ≥−3.52 | −3.03 | ≥−3.52 | |||
| 48 | −2.09 | −2.79 | −2.09 | −2.25 | |||
Colistin Cmax was achieved at time zero only. Ceftazidime (CTZ) concentration was maintained as a constant infusion. The time of sampling varied slightly in certain experiments between 5.5 and 30 h, but the variation was within 15 to 60 min of the stated sampling times. CTZ, ceftazidime.
P. aeruginosa M29899-1.
P. aeruginosa H23259-1.
Ciprofloxacin (CIP) was given to achieve a Cmax of 5 mg/liter at 0 and 12 h.
FIG. 1.
Time-kill curve of P. aeruginosa M29899-1 with colistin with Cmaxs of 6 mg/liter (▵) and 18 mg/liter (○), the combination of ceftazidime and colistin as a ceftazidime infusion at 50 mg/liter and colistin with Cmaxs of 6 mg/liter (▴) and 18 mg/liter (•), and ceftazidime infusion (CSS = 50 mg/liter) alone (×). The ceftazidime-alone experiment was conducted for only 24 h.
Colistin alone had greater activity at 24 h than the combination of colistin and ciprofloxacin, though the colony count at this time was generally greater than the initial inoculum (Tables 1 and 2). In general, adding ciprofloxacin to colistin produced poorer killing at any time point. This was not unexpected, as the ciprofloxacin Cmax was 5 mg/liter and the MIC was at least 4 mg/liter.
DISCUSSION
Colistin may need to be used in patients with infections due to multiantibiotic-resistant Pseudomonas and Acinetobacter spp. and in those with multiple drug allergies. Despite the fact that the polymyxins have been available for over 40 years, relatively few pharmacokinetic and pharmacodynamic data regarding these agents are available.
Although studies have not been in agreement with regard to the rate of colistin-induced nephro- and neurotoxicity (3, 9, 12, 15), the drug has been associated with significant adverse events. Generally, toxicity was thought to be dose dependent (9), though recent data suggest that there is no clear relationship between concentration and toxicity (15). If such a relationship between dose and toxicity does exist, colistin-sparing antibiotic regimens that reduce the frequency of administration and/or the quantity of colistin exposure may offer an advantage over conventional dosing schemes.
Colistin binds tightly to tissue membranes and accumulates in tissue when given as a continuous infusion (7). This process is thought to be involved in the toxicity of the drug (7, 9). As is thought to be the case with aminoglycosides, the administration of colistin over extended intervals may lead to less tissue accumulation, with a possible reduction of adverse effects due to tissue interaction (16). In addition, larger doses of drug are thought to maximize the activity of concentration-dependent antibiotics (6). Here, colistin demonstrated concentration-dependent activity at concentrations between 0.5 and 18 mg/liter and was bactericidal above 0.5 mg/liter, which is in agreement with a recent study by Li et al. (13). The bacterial killing was so rapid that we were unable to detect any bacteria at our initial sampling point when the initial colistin concentration was greater than 18 mg/liter. Although some of our data suggest that the drug continues to be concentration dependent above 18 mg/liter, there was rapid bactericidal activity whenever the starting concentration was above 3 mg/liter. Typical intravenous doses of sulfomethyl colistin (2 mg/kg) generally produce colistin Cmaxs of at least 18 mg/liter (10, 15). The concentration-dependent activity of colistin may be exploited by giving relatively large doses of the drug less frequently, as is the case with aminoglycosides. Viewed from the clinical setting perspective, a once-a-day dosing scheme with colistin would expose pathogens to elevated concentrations of antibiotic followed by an antibiotic-free period that may minimize tissue accumulation and reduce drug-related adverse reactions.
Alternatively, the drug may be given with other agents to maximize activity while decreasing the total colistin daily exposure. In this study, the combination of colistin and ceftazidime was synergistic. Comparing the killing produced by colistin administered so as to achieve a Cmax of 18 mg/liter every 12 h alone and that produced by colistin achieving a Cmax of 6 mg/liter every 24 h with ceftazidime demonstrates a regimen that could reduce the overall daily exposure to colistin. In these experiments, the initial killing between 0 and 2 h was approximately the same, and the viable count at 24 h was actually less in the colistin-plus-ceftazidime experiments, despite the fact that 75% less colistin was used over 24 h. The ceftazidime was maintained at a constant infusion level that was 1.5 times the MIC. In contrast, the combination of ciprofloxacin and colistin produced poorer killing than colistin alone. The ciprofloxacin was administered twice daily, achieving peak/MIC ratios approximately equal to or less than 1. Secondary to the elevated P. aeruginosa ciprofloxacin MIC (and the resultant ratio of exposure to MIC), the poor activity seen with the colistin-ciprofloxacin combination was expected. This may be also be partially explained by binding between the two antibiotics or by the fact that colistin's mechanism of action, which is mediated by the binding of cations necessary to stabilize the bacterial membrane, may have been altered in the presence of the fluoroquinolone, which interferes with cations in solution (10, 14).
While these data suggest that novel dosing strategies that reduce colistin exposure may reduce colistin-related adverse drug reactions while maintaining comparable antibacterial activity against likely resistant pathogens, clinical trials involving such strategies are unlikely to be performed, as colistin is a generic drug. The question of whether regimens such as 2.5 mg/kg once daily or 5 mg/kg once daily in combination with a second agent such as a beta-lactam could serve as rational therapeutic options for multidrug-resistant Pseudomonas or Acinetobacter infections poses an interesting dilemma. The lower dose would potentially address drug-related toxicity via an overall reduction of daily dose and a more complete elimination, and the larger dose would take additional advantage of the agent's concentration-dependent activity, potentially preventing the development of resistance. Recent clinical data supporting the use of colistin in combination with other antipseudomonal antibiotics to which the isolate shows in vitro sensitivity have been reported (5). Additionally, some clinicians are beginning to incorporate dosing strategies that utilize high levels of colistin. At institutions in Ohio, Indiana, and Minnesota, clinicians have reverted to colistin daily doses ranging from 4 to 7.5 mg/kg (15; D. W. Smith, personal communication; J. C. Rotschafer, unpublished data). While at one of these institutions this high exposure is achieved by multiple doses per day because of tolerability issues (15), the other two institutions have used 5-mg/kg once-daily strategies.
In conclusion, colistin demonstrated rapid, concentration-dependent bactericidal activity against these strains of P. aeruginosa in the in vitro model. Activity was only slightly enhanced when two doses were given over 24 h compared to a single dose. Colistin was synergistic when given with ceftazidime, and the combination of colistin with a Cmax of 18 mg/liter and ceftazidime was only slightly superior to the combination with colistin with a Cmax of 6 mg/liter. Whether these findings can be extrapolated to the clinical setting remains to be seen. Deviations from standard dosing methods should be evaluated on a patient-by-patient basis, and general guidance of this sort can only follow proper clinical study that is unlikely to be performed with colistin. The combination of colistin plus a beta-lactam may prove effective against P. aeruginosa isolates for which beta-lactam MICs are elevated, but the magnitudes of the required exposure(s) have not been tested clinically. As resistant-pathogen infections increase, more clinicians may need to resort to alternative unorthodox regimens, and a better understanding of colistin dosing and colistin combination therapy may be elucidated.
REFERENCES
- 1.Barnett, M., S. R. M. Bushby, and S. Wilkinson. 1964. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. 23:552-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beveridge, E. G., and A. J. Martin. 1967. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. 29:125-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosso, J. A., C. A. Liptak, D. K. Seilheimer, and G. M. Harrison. 1991. Toxicity of colistin in cystic fibrosis patients. DICP 25:1168-1170. [DOI] [PubMed] [Google Scholar]
- 4.Catchpole, C. R., J. M. Andrews, N. Brenwald, and R. Wise. 1997. A reassessment of the in-vitro activity of colistin sulphomethate sodium. J. Antimicrob. Chemother. 39:255-260. [DOI] [PubMed] [Google Scholar]
- 5.Conway, S. P., M. N. Pond, A. Watson, C. Etherington, H. L. Robey, and M. H. Goldman. 1997. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax 52:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., and C. M. Kunin. 1976. Significance of serum protein and tissue binding of antimicrobial agents. Annu. Rev. Med. 27:287-300. [DOI] [PubMed] [Google Scholar]
- 8.Eickhoff, T. C., and M. Finland. 1965. Polymyxin B and colistin: in vitro activity against Pseudomonas aeruginosa. Am. J. Med. Sci. 249:172-174. [PubMed] [Google Scholar]
- 9.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 10.Froman, J., L. Gross, and S. Curatola. 1970. Serum and urine levels following parenteral administration of sodium colistimethate to normal individuals. J. Urol. 103:210-214. [DOI] [PubMed] [Google Scholar]
- 11.Kucers, A., and N. M. Bennett. 1987. The use of antibiotics: a comprehensive review with clinical emphasis, 4th ed., p. 899-913. Lippincott, Philadelphia, Pa.
- 12.Levin, A. S., A. A. Barone, J. Penco, M. V. Santos, I. S. Marinho, E. A. Arruda, E. I. Manrique, and S. F. Costa. 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28:1008-1011. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., J. Turnidge, R. Milne, R. L. Nation, and K. Coulthard. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, R. C., D. E. Nix, and J. J. Schentag. 1994. Interaction between ciprofloxacin and metal cations: its influence on physicochemical characteristics and antibacterial activity. Pharm. Res. 11:917-920. [DOI] [PubMed] [Google Scholar]
- 15.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 16.Verpooten, G. A., R. A. Giuliano, L. Verbist, G. Eestermans, and M. E. De Broe. 1989. Once-daily dosing decreases renal accumulation of gentamicin and netilmicin. Clin. Pharmacol. Ther. 45:22-27. [DOI] [PubMed] [Google Scholar]
- 17.Zabinski, R. A., K. Vance-Bryan, A. J. Krinke, K. J. Walker, J. A. Moody, and J. C. Rotschafer. 1993. Evaluation of activity of temafloxacin against Bacteroides fragilis by an in vitro pharmacodynamic system. Antimicrob. Agents Chemother. 37:2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]