Abstract
Using the standard Craig and Gudmundsson method (W. A. Craig and S. Gudmundsson, p. 296-329, in V. Lorian, ed., Antibiotics in Laboratory Medicine, 1996) as a guideline for determination of postantibiotic effects (PAE), we studied a large series of growth curves for two strains of Legionella pneumophila. We found that the intensity of the PAE was best determined by using a statistically fitted line over hours 3 to 9 following antibiotic removal. We further determined the PAE duration by using a series of observations of the assay interval from hours 3 to 24. We determined that inoculum reduction was not necessarily the only predictor of the PAE but that the PAE was subject to the type and dose of the drug used in the study. In addition, there was a variation between strains. Only levofloxacin at five and ten times the minimum inhibitory concentration (MIC) resulted in a PAE duration of 4 to 10 h for both strains of L. pneumophila tested. Ciprofloxacin at five and ten times the MIC and azithromycin at ten times the MIC caused a PAE for one strain only. No PAE could be demonstrated for either strain with erythromycin or doxycycline. Using the presently described method of measuring PAE for L. pneumophila, we were able to detect differences in PAE which were dependent upon the L. pneumophila strain, the antibiotic tested, and the antibiotic concentration. We suggest the use of mathematically fitted curves for comparison of bacterial growth in order to measure PAE for L. pneumophila.
Postantibiotic effect (PAE) is the continued suppression of bacterial growth after exposure of the bacteria to an antimicrobial agent and removal of this agent from the environment (3-5, 11, 13, 21-23). In vitro PAE is determined by applying methods which estimate the regrowth of antibiotic-exposed bacteria (2, 3, 13). It was previously demonstrated that pretreatment of Legionella pneumophila with antibacterial agents prior to the ingestion by mononuclear phagocytes caused a delay in the intracellular regrowth of the organism (18). Results of previous studies have suggested differences in the PAE among classes of anti-Legionella drugs (6, 8, 9, 15; J. Dubois and C. St. Pierre, Program Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 938, 1989). We designed a series of experiments to demonstrate the effects of different classes of drugs by using two L. pneumophila isolates and three classes (five antibiotics) of anti-Legionella compounds.
In this study, we designed experiments and performed the assays (the Q method) which lead to estimates of the PAEs of levofloxacin, ciprofloxacin, erythromycin, azithromycin, and doxycycline against L. pneumophila. We analyzed detailed growth curves of L. pneumophila to determine methods of estimating the PAE. We defined measurements of both the intensity and the duration of the PAE against L. pneumophila. We also calculated PAE durations by using the standard method of Craig and Gudmundsson (C&G method) (3).
MATERIALS AND METHODS
Bacterial strains.
L. pneumophila L-1033 and L-1043, serogroup 1, isolated from the sputa of patients with pneumonia, were obtained from the Wadsworth Center for Laboratories and Research, New York State Department of Health, Albany. The isolates were kept frozen in skim milk at −70°C prior to the experiments. They were then subcultured on buffered charcoal yeast extract (BCYE) agar supplemented with 0.1% α-ketoglutarate (BBL; Microbiology Systems, Cockeysville, Md.) and incubated at 35°C. Prior to each experiment, three to four colonies from a 48-h culture were subcultured from BCYE agar into buffered yeast extract (BYE) broth supplemented with 0.1% α-ketoglutarate and incubated for 18 h at 35°C in a shaking water bath.
Antimicrobial agents.
Levofloxacin was a gift from R.W. Johnson Research Institute, Princeton, N.J. Ciprofloxacin, azithromycin, erythromycin, and doxycycline were obtained from Sigma, St. Louis, Mo. All antibiotic solutions were made fresh for each experiment in accordance with the suppliers' instructions. By using the macrodilution technique, the minimum inhibitory concentration (MIC) (μg/ml) for each drug was determined in BYE broth (12, 17). For L. pneumophila L-1033, the MICs of levofloxacin, ciprofloxacin, erythromycin, azithromycin, and doxycycline were 0.03, 0.03, 0.5, 1.0, and 1.0 μg/ml, respectively; for L. pneumophila L-1043, they were 0.06, 0.004, 0.25, 0.25, and 1.0 μg/ml, respectively. All antimicrobials were tested for PAEs at 0.01, 0.1, 1.0, 5.0, and 10.0 times the MIC for each organism.
Study design. (i) Growth.
The growth (measured in log10 CFU/ml) of L. pneumophila L-1033 was performed in BYE broth. The growth curve of the control was compared with those of organisms grown in broth containing levofloxacin or ciprofloxacin at 0.01, 0.1, 1, 5, and 10 times the MIC for 2 h. From hour 3, sampling was performed at 1- to 2-h intervals for hours 3 to 24 of the assay, then at 4-h intervals for hours 24 to 32 of the assay, and lastly at 48 h. Aliquots containing 0.1 ml were subcultured by using serial dilutions in duplicate on BCYE agar, and colonies were counted at 48 h.
(ii) PAE determinations.
For PAE determinations (3), L. pneumophila cultures prepared in BYE broth were diluted five times by using the twofold serial dilution technique in BYE broth and incubated overnight at 35°C. Following the incubation, the culture suspension with an optical density reading at 580 nm closest to, but not surpassing, 0.2 was selected as the inoculum. One milliliter of this bacterial suspension was added to 9 ml of previously prepared antibiotic-containing medium and to a drug-free control. The final inoculum was 106 to 107 CFU/ml. Control tubes and antibiotic-containing tubes were incubated in a 35°C shaking water bath. After 2 h of incubation, tubes were centrifuged (2,000 × g) for 10 min and the supernatants were removed. The bacterial pellets were washed in warmed BYE broth and resuspended to a volume of 10 ml. This procedure was repeated once. Thereafter, 100-μl samples were removed at indicated times and quantitatively plated in duplicate onto the surfaces of BCYE agar plates. The plates were incubated at 35°C for 48 h, and colonies were counted by using an electronic colony counter.
The period of antibiotic exposure was from 0 (inoculation) to 2 h. This was followed by a 1-h period for antibiotic removal and reinoculation for the determination of PAE. Observations for PAE determination continued from 3 to 48 h into the assay for each isolate. Measurements for PAE were made by using data obtained from hours 3 to 24 of the assay time. Three measurements were included, namely, average increase per hour in log10 CFU/ml, T log, and PAE duration (hours). A total of two to five assays were performed for each combination of antibiotic and L. pneumophila strain. A total of 276 individual growth curves were used for this study.
Statistical methods. (i) Measurement of immediate effect.
The log10 CFU/ml at 3 h was used to measure the immediate effect of the exposure of L. pneumophila to the antibiotic during hours 0 to 2 of the assay.
(ii) Measurement of PAE during hours 3 to 24. (a) Average increase per hour in log10 CFU/ml over hours 3 to 9.
By using the method of least squares (19), a linear regression line was fitted to the log10 (CFU/ml) values observed over the period from hours 3 to 9 of the assay. The average increase per hour in log10 CFU/ml over hours 3 to 9 of the assay was the slope of the fitted line. The period from hours 3 to 9 was chosen because it satisfied the following criteria. This period immediately followed removal of the antibiotic (i.e., assay hour 3); it was of long enough duration to provide several reasonably spaced observations (at least three observations with at least 1 h between consecutive readings); and it was a period of short enough duration so that a straight line could be used to approximate the relationship between log10 CFU/ml and assay time (hours) during this period. This last criterion required a period occurring before the bacterium entered a phase of faster or slower growth because of a return from suppressed to normal growth or from normal to maximum sustainable growth.
(b) Q T log.
A second-degree polynomial was fitted by the method of least squares (19) to the log10 CFU/ml values over hours 3 to 24 of the assay. The value for Q T log was the x value (hour) on the polynomial corresponding to the y value (log10 CFU/ml) on the polynomial which was one log greater than the y value on the polynomial at T = 3 h.
(c) Q PAE duration.
The duration of PAE by the Q method was calculated as Q T logtreated − Q T logcontrol, where Q T logtreated and Q T logcontrol were calculated as specified above for Q T log. The Q PAE duration is measured in hours.
(d) C&G PAE duration.
The duration of PAE by the C&G (3) method was calculated as C&G T logtreated − C&G T logcontrol, where C&G T log is calculated as specified below and by Craig and Gundmundsson (3). The C&G PAE duration is measured in hours.
(e) C&G T log.
The assay time (in hours) at which a 1-log10 increase in log10 CFU/ml (relative to the log10 CFU/ml at 3 h) was observed is defined as C&G T log. When the 1-log10 increase occurred between two observed time points, a straight line increase in log10 CFU/ml was assumed between the two time points of observation. The C&G T log was read from this line.
(iii) An additional variable to characterize growth curves.
The increase per hour in log10 CFU/ml over hours 12 to 20 of the assay was also used to characterize the growth curves of L. pneumophila strain L-1033. By using the method of least squares (19), a linear regression line was fitted to the log10 (CFU/ml) values observed over the 12 to 20 h period of the assay. The increase per hour in the log10 CFU/ml, hours 12 to 20, was the slope of the fitted line.
The average increase in log10 CFU/ml per hour over hours 3 to 9 of the assay, the Q T log, and the Q PAE duration are the variables which were used in this study to measure the intensity and the duration of PAE. The C&G variables are included as the recognized standard.
Statistical analysis of the study variables was done by using the analysis of variance methodology (20). Comparison of the PAE durations calculated by the Q and by the C&G methods was made by using Pearson's X2 goodness-of-fit test (20). The level of significance was 0.05.
RESULTS
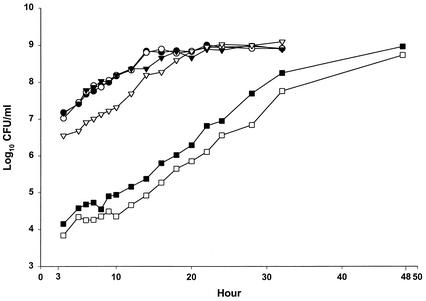
Figure 1 demonstrates the growth curve (log10 CFU/ml) of L. pneumophila L-1033 by using sequential sampling data from the assays done every 1 to 2 h during hours 3 to 24, at 4-h intervals during hours 24 to 32, and at 48 h. Growth curves where PAE is or is not exhibited are included. Over the period of the assay from hours 3 to 24, the observed CFU/ml grew exponentially and the observed log10 CFU/ml were well described by a second-degree polynomial (Fig. 1) (R2 range, 0.97 to 0.99). For the period from hours 3 to 9 of the assay, the observed log10 CFU/ml were approximated by a straight line (Fig. 1) (R2 range, 0.71 to 0.98) whose slope (average increase in log10 CFU/ml per hour) was used as a measure of the effect of the antibiotic on L. pneumophila growth in the early hours of the assay.
FIG. 1.
Log growth of L. pneumophila L-1033 over hours 3 to 48. Microbial growth was measured every 1 to 2 h over the period from hours 3 to 24, every 4 h over the period from hours 24 to 32, and at 48 h. Symbols represent the control (•) and organisms grown with levofloxacin at 0.01 (○), 0.1 (▾), 1 (▹), 5 (▪), and 10 (□) times the MIC. Data are the results of one assay.
L. pneumophila strain L-1033 was treated with levofloxacin at 0, 0.01, 0.1, 1, 5, and 10 times the MIC of levofloxacin. As seen in Fig. 1 and Table 1, no levofloxacin effect was detected at 0.01 or 0.1 times MIC. At the MIC, there was a significant (P < 0.01) reduction of the inoculum at hour 3, but the rate of regrowth was similar to that of the control (0.12 log10 CFU/ml/h), and no PAE was detected. In contrast, at levofloxacin concentrations of five and ten times the MIC, the regrowth from hours 3 to 9 was significantly lower (P < 0.05) (Table 1, Col. 3) than that of the control, and thus a PAE could be calculated. In addition, at these levofloxacin concentrations, the hourly increase in log10 CFU/ml over hours 12 to 20 was similar to that of the control during hours 3 to 9. In contrast, for control and levofloxacin at concentrations of 0.01 and 0.1 times the MIC, the increase in log10 CFU/ml per hour over hours 12 to 20 was much slower (0.05 log10 CFU/ml/h) as it approached stationary phase. As seen in Table 1, the Q T log for the control and for L. pneumophila with levofloxacin concentrations of 0.01, 0.1, and 1.0 times the MIC were similar (10.3, 8.8, 9.5, and 11.5 h, respectively). However, for the higher concentrations, the Q T log values (12.3 and 13.0 h) were significantly (P < 0.01) greater than those of the control. In contrast, the T log value estimated by the C&G method was not significantly different from that of the control at any concentration of levofloxacin tested (Table 1, col. 6). Of note was our observation that the intra-assay variability (standard deviation) was 3.0 h for the C&G method compared to 1.2 h for the Q T log in this study. When ciprofloxacin was used as the test antimicrobial at similar concentrations against L. pneumophila L-1033, the results were similar to those described for levofloxacin (data not shown).
TABLE 1.
Microbial growth of L. pneumophila L-1033 following exposure to levofloxacin at 0, 0.01, 0.1, 1, 5 and 10 times the MIC, expressed as log10 CFU/ml at 3 h, and average increase in log10 CFU/ml per hour over the intervals from hours 3 to 9 and 12 to 20a
| MIC multiple | Log10 CFU/ml at 3 h (SD = 0.11 log10 CFU/ml) | Avg increase in log10 CFU/ml per hour (SD, 0.03) |
Q T log (h), hrs 3 to 24 (SD, 1.2) | C&G T log (h), hrs 3 to 24 (SD, 3.0) | |
|---|---|---|---|---|---|
| Hrs 3 to 9 | Hrs 12 to 20 | ||||
| 0 | 7.18 | 0.14 | 0.05 | 10.3 | 11.8 |
| 0.01 | 7.02 | 0.18 | 0.05 | 8.8 | 8.9 |
| 0.1 | 7.17 | 0.15 | 0.05 | 9.5 | 11.8 |
| 1 | 6.55** | 0.12 | 0.13** | 11.5 | 11.7 |
| 5 | 4.15** | 0.10* | 0.15** | 12.3** | 10.8 |
| 10 | 3.84** | 0.09* | 0.16** | 13.0** | 11.5 |
Asterisks denote statistical difference from the control, 0.01 and 0.1 times the MIC.
P < 0.05.
P < 0.01.
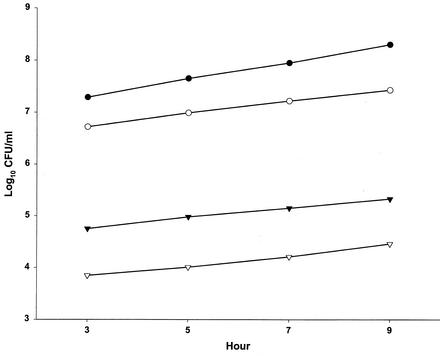
Figure 2 depicts the lack of influence of the inoculum size on the hourly increase in log10 CFU/ml of L. pneumophila isolate L-1043 during assay hours 3 to 9. It is evident that L-1043 grows more slowly (P < 0.01) than L-1033 (0.12 log10 CFU/ml/h versus 0.17 log10 CFU/ml/h).
FIG. 2.
Linear log growth over the period from hours 3 to 9 in controls (no antibiotics). Symbols represent L. pneumophila L-1033 at a 1:1 dilution (•) and L-1043 at dilutions of 1:1 (○), 1:100 (▾), and 1:1,000 (▿). Data are the results of 5 to 24 assays; standard deviations were 0.06 and 0.02 log10 CFU/ml per hour for L-1033 and L-1043, respectively.
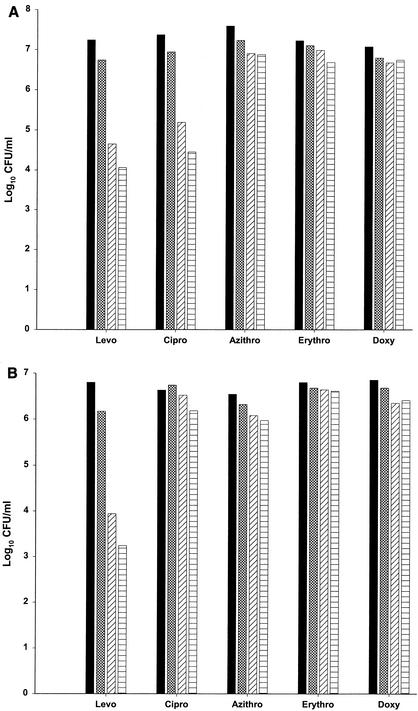
Table 2 and Fig. 3A and B present the log10 CFU/ml of L. pneumophila L-1033 (Fig. 3A) and L-1043 (Fig. 3B) at 3 h following exposure to five antimicrobials (levofloxacin, ciprofloxacin, azithromycin, erythromycin, and doxycycline) at 1, 5, and 10 times the MIC of each drug. Levofloxacin and ciprofloxacin caused a dose-dependent decrease in colony counts at 3 h, reaching 3 log10 CFU/ml for L-1033 (P < 0.01). There was a difference in the effect of these two fluoroquinolones for L-1043; levofloxacin suppressed the log10 CFU/ml at hour 3 more than did ciprofloxacin (P < 0.01). In contrast, azithromycin, erythromycin, and doxycycline had little or no effect on colony counts at 3 h.
TABLE 2.
Microbial growth of L. pneumophila L-1033 and L-1043 following exposure to levofloxacin, ciprofloxacin, azithromycin, erythromycin, and doxycycline at various concentrationsa
| MIC multiple | Log10 CFU/ml at 3 h with: |
||||
|---|---|---|---|---|---|
| Levofloxacin | Ciprofloxacin | Azithromycin | Erythromycin | Doxycycline | |
| L. pneumophila L-1033 (SD, 0.29 log10 CFU/ml) | |||||
| 0 | 7.24 | 7.37 | 7.59 | 7.22 | 7.07 |
| 1 | 6.74** | 6.94** | 7.23** | 7.10 | 6.79 |
| 5 | 4.65** | 5.19** | 6.90** | 6.98 | 6.67 |
| 10 | 4.06** | 4.45** | 6.87** | 6.68 | 6.74 |
| L. pneumophila L-1043 (SD, 0.18 log10 CFU/ml) | |||||
| 0 | 6.80 | 6.63 | 6.54 | 6.80 | 6.85 |
| 1 | 6.17** | 6.74 | 6.32 | 6.68 | 6.68 |
| 5 | 3.94** | 6.52 | 6.08** | 6.64 | 6.35** |
| 10 | 3.24** | 6.18** | 5.97** | 6.61 | 6.41** |
Three to five assays were performed for strain L-1033; two to three assays were performed for strain L-1043. Asterisks denote statistical difference from the control (0 times the MIC).
P < 0.01.
FIG. 3.
Log10 CFU/ml of L. pneumophila L-1033 (A) and L. pneumophila L-1043 (B) at hour 3 following exposure to levofloxacin, ciprofloxacin, azithromycin, erythromycin, and doxycycline at 0 (▪), 1 (▩), 5 (▨), or 10 (▤) times the MIC. Data are the results of 3 to 5 assays (L-1033) and 2 to 3 assays (L-1043); standard deviations were 0.29 and 0.18 log10 CFU/ml for L-1033 and L-1043, respectively.
Table 3 demonstrates the effects of five antimicrobials on the hourly log10 CFU/ml growth of L. pneumophila strains L-1033 and L-1043 over the first 6 h following removal of the drugs at hour 3. The recovery of the microorganisms depended on the concentration of the fluoroquinolones used during the first 2 h. For both L. pneumophila strains, there was a significant slowing of hourly growth of log10 CFU/ml (P < 0.01) when 5 or 10 times the MIC of levofloxacin was used. Effects of exposure to ciprofloxacin differed for the two strains; a significant (P < 0.01) effect was observed for strain L-1033 but not for L-1043. Little if any effect could be demonstrated with either fluoroquinolone at a drug concentration of 1 times the MIC. Increasing either fluoroquinolone concentration from 5 to 10 times the MIC had no significant effect. Significant suppression of hourly growth of log10 CFU/ml was demonstrable at 10 times the MIC with azithromycin for strain L-1033 but not for strain L-1043 (P < 0.01). No significant inhibition of growth was observed upon exposure to erythromycin or doxycycline at any of these drug concentrations studied for either L. pneumophila strain.
TABLE 3.
Average increase in log10 CFU/ml per hour during hours 3 to 9a
| MIC multiple | Log10 CFU/ml per hour with: |
||||
|---|---|---|---|---|---|
| Levofloxacin | Ciprofloxacin | Azithromycin | Erythromycin | Doxycycline | |
| L. pneumophila L-1033 (SD, 0.06 log10 CFU/ml per hour) | |||||
| 0 | 0.18 | 0.17 | 0.16 | 0.16 | 0.16 |
| 1 | 0.18 | 0.13* | 0.18 | 0.19 | 0.10 |
| 5 | 0.09** | 0.06** | 0.17 | 0.16 | 0.13 |
| 10 | 0.08** | 0.09** | 0.08** | 0.15 | 0.10 |
| L. pneumophila L-1043 (SD, 0.02 log10 CFU/ml per hour) | |||||
| 0 | 0.14 | 0.14 | 0.12 | 0.12 | 0.10 |
| 1 | 0.11 | 0.12 | 0.10 | 0.10 | 0.10 |
| 5 | 0.01** | 0.12 | 0.10 | 0.10 | 0.10 |
| 10 | 0.01** | 0.12 | 0.10 | 0.10 | 0.10 |
Three to five assays were performed for strain L-1033; two to three assays were performed for strain L-1043. Asterisks denote statistical difference from the control (0 times the MIC).
P < 0.05
P < 0.01.
Table 4 depicts the PAE measurements (hours) for three antibiotics by using the Q PAE and C&G PAE durations for L. pneumophila L-1033 and L-1043. The Q PAE durations are measurable (P < 0.01) for levofloxacin at 5 and 10 times the MIC for L-1033 and L-1043, for ciprofloxacin at 5 and 10 times the MIC for L-1033, and for azithromycin at 10 times the MIC for L-1033. Q PAE was not present for either strain for other fluoroquinolone or azithromycin concentrations or for any concentration of erythromycin or doxycycline. In contrast, C&G PAE measurements are detectable (P < 0.01) for levofloxacin at 5 and 10 times the MIC against L-1043 only. No other C&G PAEs could be detected against either strain with the other antimicrobials studied at any concentration. In 128 pairs of estimates of Q PAE and C&G PAE, 89 (70%) of the pairs exhibited a Q PAE larger than the corresponding C&G PAE. This percentage is significantly (P < 0.01) different from 50%.
TABLE 4.
PAE duration by the Q and C&G methodsa
| MIC multiple | Q PAE (h) with: |
C&G PAE (h) with: |
||||
|---|---|---|---|---|---|---|
| Levofloxacin | Ciprofloxacin | Azithromycin | Levofloxacin | Ciprofloxacin | Azithromycin | |
| L. pneumophila L-1033b | ||||||
| 1 | 0.1 | 1.1 | −1.7 | −0.7 | −0.5 | −2.5 |
| 5 | 3.9** | 4.9** | −1.3 | 1.7 | 1.9 | −2.9 |
| 10 | 5.8** | 4.3** | 4.6** | 4.0 | 2.1 | 1.5 |
| L. pneumophila L-1043c | ||||||
| 1 | 1.1 | 1.0 | 0.7 | 0.2 | 1.0 | 0.2 |
| 5 | 10.5** | 1.5 | 1.2 | 8.1** | 1.5 | 0.6 |
| 10 | 10.4** | 0.9 | 0.9 | 8.4** | 1.0 | 0.2 |
Three to five assays were performed for strain L-1033; two to three assays were performed for strain L-1043. Asterisks denote statistical difference from the control (0 times the MIC).
P < 0.01.
SD for Q and C&G PAE, 2.5 and 3.9 h, respectively.
SD for Q and C&G PAE, 2.0 and 1.8 h, respectively.
DISCUSSION
Methods for estimating the results of a defined period of antibiotic exposure on behavior of bacteria and fungi after removal of the organisms from the environment containing anti-infective agents have been used since the early work of Eagle (7). Previous investigators have reviewed the PAE measurement as a function of the growth curve of the test organism (2, 3, 13, 14, 16). Initially, PAE estimates were based on the portion of the growth curve which can best be described as stationary growth (7). Organisms differ in the rate of resumption of more rapid growth, and different drugs can cause different effects. This must be taken into consideration when comparisons of growth rates are made after antimicrobial treatment. The method of C&G takes these factors into account by measuring the time necessary for treated organisms, as a population, to enter logarithmic-phase growth. The choice of a 1-log increase was felt to be appropriate based on evaluation of large numbers of growth curves (in vitro) for organisms such as Escherichia coli and Staphylococcus aureus. Based upon their own observations of Bacteroides fragilis, E. coli, and Staphylococcus epidermidis as well as literature reports on S. aureus, Brunden et al. proposed a two-phase model (1, 2). The model specified a parallel control and antibiotic log growth composed of an initial no-growth phase (constant log count over time) followed by a positive linear log growth. The assay time at the point of intersection of the two phase lines was the time point of interest. Although the methods proposed by C&G and by Brunden et al. have merit, neither one could be applied in toto in our study of L. pneumophila. However, elements of both were adapted to our study of PAE on L. pneumophila.
In evaluating the growth characteristics of the organism immediately after and long after drug exposure, we identified the period from hours 3 to 24 (interval after drug removal) as the period during which estimates of PAE with L. pneumophila should be calculated. We determined that for all classes of drugs tested, the intensity and the duration of the PAE could be measured for L. pneumophila. To do this for two strains of L. pneumophila, we utilized the average hourly change in log10 CFU/ml immediately after antibiotic removal (hours 3 to 9) to determine the intensity of the residual effect of the antibacterial agent. The duration of the effect (PAE duration) is determined by measuring the rates of growth of treated and control bacteria from hours 3 to 24, with a resultant estimate of the additional time (hours) needed to attain a 1-log increase in growth of treated bacteria. The initial growth of the log10 CFU/ml of L. pneumophila cannot be assumed to be similar from strain to strain. The measurements are not affected by the initial inocula.
Previous estimates of PAE for a variety of drug classes (quinolones, macrolides, azalides) against L. pneumophila have been published (6, 8, 9, 15; Dubois and St. Pierre, 39th ICAAC). Variations in the assay methodology preclude comparisons among studies. We observed that there was dose dependency when a drug was found to be effective in causing a PAE of measurable duration. In addition, although we tested each of the strains at identical relative (multiple of MIC) drug concentrations, we observed strain differences within the same class of anti-infectives. Ciprofloxacin was less likely to produce a PAE than was levofloxacin, and azithromycin affected only one of the two strains. By using a single drug concentration at four times the MIC, one study found the average PAE for azithromycin at 1.65 h (10), while another study found the PAE to be at 2.91 ± 3 h and 2.16 ± 1 h for erythromycin-sensitive and -resistant strains (6). The method used in our study led to measurements of PAE duration which were longer than those reported by others for L. pneumophila (6, 10; Dubois and St. Pierre, 39th ICAAC) and, within this study, usually longer than those measured by the C&G method.
In summary, for PAE determination we utilized a method which employed an observation period from hours 3 to 24. We were able to determine both the intensity and the duration of PAE for L. pneumophila. Levofloxacin was the only agent which demonstrated a PAE against both strains tested. Studies reporting the determination of PAE should define the method used. Furthermore, not all methods can be applied to all bacteria.
Acknowledgments
This work was supported by the Ortho McNeil Pharmaceuticals and, in part, by the U.S. Department of Veterans Affairs.
We thank Stephanie Brennan for excellent secretarial assistance.
REFERENCES
- 1.Brunden, M. N., B. H. Yagi, M. S. Lajiness, and G. E. Zurenko. 1991. Estimating the postantibiotic effect: a two-phase mathematical model. J. Pharmacokin. et Biopharm. 19:457-468. [DOI] [PubMed] [Google Scholar]
- 2.Bundtzen, R. W., A. U. Gerber, D. L. Cohn, and W. A. Craig. 1981. Postantibiotic suppression of bacterial growth. Rev. Infect. Dis. 3:28-37. [DOI] [PubMed] [Google Scholar]
- 3.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 4.Craig, W. A. 1993. Post-antibiotic effects in experimental infection models: relationship to in-vitro phenomena and to treatment of infections in man. J. Antimicrob. Agents Chemother. 3l(Suppl. D):149-158. [DOI] [PubMed]
- 5.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Dubois, J., and C. St. Pierre. 2000. Comparative in-vitro activity and post-antibiotic effect of gemifloxacin against Legionella spp. J. Antimicrob. Chemother. 45(Suppl. 1):41-46. [DOI] [PubMed] [Google Scholar]
- 7.Eagle, H. 1949. The recovery of bacteria from the toxic effects of penicillin. J. Clin. Investig. 28:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelstein, P. H., M. A. C. Edelstein, J. Weidenfeld, and M. B. Dorr. 1990. In vitro activity of sparfloxacin (CI-978, At-4140) for clinical legionella isolates, pharmacokinetics in guinea pigs, and use to treat guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 34:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelstein, P. H., M. A. Edelstein, J. Ren, R. Polzer, and R. P. Gladue. 1996. Activity of trovafloxacin (CP-99,219) against Legionella isolates: in vitro activity, intracellular accumulation and killing in macrophages, and pharmacokinetics and treatment of guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 40:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Lus, R., F. Adrián, R. del Campo, P. Gómez-Lus, S. Sánchez, C. García, and M. C. Rubio. 2001. Comparative in vitro bacteriostatic and bactericidal activity of trovafloxacin, levofloxacin and moxifloxacin against clinical and environmental isolates of Legionella spp. Internat. J. Antimicrob. Agents 18:49-54. [DOI] [PubMed] [Google Scholar]
- 11.Jason, A. C., F. M. MacKenzie, D. Jason, and I. M. Gould. 1994. Automatic procedures for measuring post-antibiotic effect and determining random errors. J. Antimicrob. Chemother. 34:669-678. [DOI] [PubMed] [Google Scholar]
- 12.Liebers, D. M., A. L. Baltch, R. P. Smith, M. C. Hammer, and J. V. Conroy. 1989. Susceptibility of Legionella pneumophila to eight antimicrobial agents including four macrolides under different assay conditions. J. Antimicrob. Chemother. 23:37-41. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie, F. M., and I. M. Gould. 1993. The post-antibiotic effect. J. Antimicrob. Chemother. 32:519-537. [DOI] [PubMed] [Google Scholar]
- 14.MacKenzie, F. M., I. M. Gould, D. G. Chapman, and D. Jason. 1994. Comparison of methodologies used in assessing the postantibiotic effect. J. Antimicrob. Chemother. 34:223-230. [DOI] [PubMed] [Google Scholar]
- 15.Martin, S. J., and S. L. Pendland. 1998. Bactericidal activity and postantibiotic effect of clarithromycin and 14-hydroxyclarithromycin, alone, and in combination, against Legionella pneumophila. J. Antimicrob. Chemother. 41:643-648. [DOI] [PubMed] [Google Scholar]
- 16.Meng, X., C. H. Nightingale, and K. R. Sweeney. 1991. Quantification of in-vitro post-antibiotic effect based on the mean recovery-time. I. Theoretical perspectives and a practical procedure. J. Antimicrob. Chemother. 28:505-514. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1990. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A2. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 18.Smith, R. P., A. L. Baltch, M. Franke, W. Hioe, W. Ritz, and P. Michelsen. 1997. Effect of levofloxacin, erythromycin or rifampin pretreatment on growth of Legionella pneumophila in human monocytes. J. Antimicrob. Chemother. 40:673-678. [DOI] [PubMed] [Google Scholar]
- 19.Stuart, A., and J. K. Ord. 1991. Kendall's advanced theory of statistics, vol. 2, p. 707-708. Oxford University Press, New York, N.Y.
- 20.Stuart, A., and J. K. Ord. 1991. Kendall's advanced theory of statistics, vol.2, p. 1101-1153, 1161. Oxford University Press, New York, N.Y.
- 21.Vogelman, B. S., and W. A. Craig. 1985. Postantibiotic effects. J. Antimicrob. Chemother. 15:37-46. [DOI] [PubMed] [Google Scholar]
- 22.Wilson, D. A., and G. N. Rolinson. 1979. The recovery period following exposure of bacteria to penicillins. Chemotherapy 25:14-22. [DOI] [PubMed] [Google Scholar]
- 23.Zhanel, G. G., D. J. Hoban, and G. K. M. Harding. 1991. The postantibiotic effect: a review of in vitro and in vivo data. DICP Ann. Pharmacother. 25:153-163. [DOI] [PubMed] [Google Scholar]