Abstract
To investigate lymphocyte participation in wound healing, the migration of T lymphocyte subsets into healing wounds and subcutaneously implanted polyvinyl alcohol sponges was studied. Frozen sections of 5-, 7-, and 10-day-old incisional wounds and sponges from Lewis rats were stained with mouse anti-rat monoclonal antibodies. Cellular staining to OX1 (all leucocyte), W3/25 (helper/effector T lymphocytes), and OX8 (suppressor/cytotoxic T lymphocytes) was quantitated in two arbitrarily defined areas based on maximal cellular infiltration: the superficial wound, down to and including the papillary dermis, and the deep wound, the reticular dermis. Five-day wounds were significantly more cellular than 10-day wounds in the deep portion (p less than 0.05) and somewhat more cellular in the superficial section (p less than 0.10). Approximately 2:1 W3/25 to OX8 ratios were noted for wound strips on all days. At 5 and 10 days there are twice as many W3/25 and OX8 labeled cells in the deep wound as in the superficial portion. At 7 days there is a peak in surface W3/25 and OX8 lymphocytes, whereas the deep population remains constant. Seven- and 10-day sponge granulomas demonstrate ratios similar to the wound strips (5-day sponge lymphocytic infiltration was insufficient to count). The data demonstrate that lymphocyte subpopulation participation in wound healing is a dynamic and distinctive process.
Full text
PDF
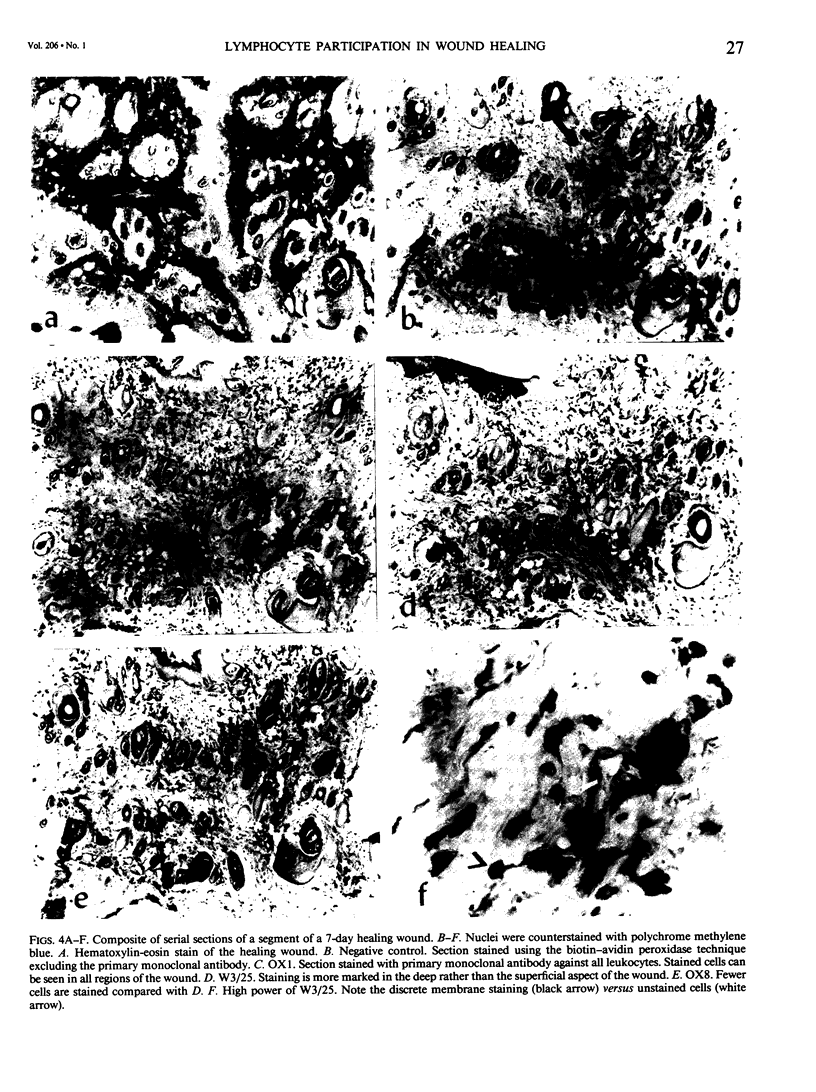
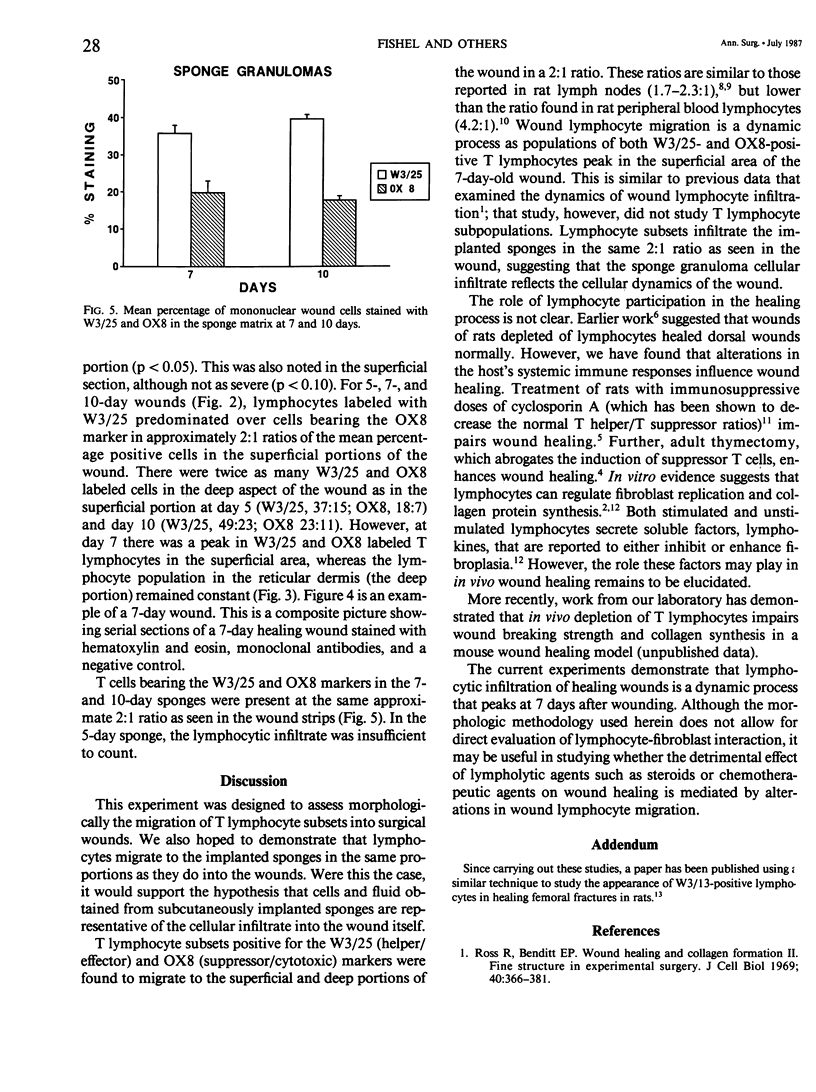

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbul A., Damewood R. B., Wasserkrug H. L., Penberthy L. T., Efron G. Fluid and mononuclear cells from healing wounds inhibit thymocyte immune responsiveness. J Surg Res. 1983 Jun;34(6):505–509. doi: 10.1016/0022-4804(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Britton S., Palacios R. Cyclosporin A--usefulness, risks and mechanism of action. Immunol Rev. 1982;65:5–22. doi: 10.1111/j.1600-065x.1982.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Falini B., Taylor C. R. New developments in immunoperoxidase techniques and their application. Arch Pathol Lab Med. 1983 Mar;107(3):105–117. [PubMed] [Google Scholar]
- Fernandez-Cruz E., Gilman S. C., Feldman J. D. Altered levels of mononuclear leukocytes in tumor-bearing rats: decrease of helper T lymphocytes and increase of suppressor cells. J Immunol. 1982 Sep;129(3):1324–1328. [PubMed] [Google Scholar]
- Fishel R., Barbul A., Wasserkrug H. L., Penberthy L. T., Rettura G., Efron G. Cyclosporine A impairs wound healing in rats. J Surg Res. 1983 Jun;34(6):572–575. doi: 10.1016/0022-4804(83)90112-9. [DOI] [PubMed] [Google Scholar]
- Korotzer T. I., Page R. C., Granger G. A., Rabinovitch P. S. Regulation of growth of human diploid fibroblasts by factors elaborated by activated lymphoid cells. J Cell Physiol. 1982 Jun;111(3):247–254. doi: 10.1002/jcp.1041110305. [DOI] [PubMed] [Google Scholar]
- Nielson E. G., Phillips S. M., Jimenez S. Lymphokine modulation of fibroblast proliferation. J Immunol. 1982 Mar;128(3):1484–1486. [PubMed] [Google Scholar]
- Ross R., Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969 Feb;40(2):366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. M., Levenson S. M. Effect of the inflammatory reaction on subsequent wound healing. Surg Forum. 1966;17:484–485. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]