Abstract
We investigated a possible synergistic effect of a macrolide and β-lactams against Streptococcus pneumoniae strains with different resistance profiles. Checkerboard and time-kill assays of erythromycin combined with penicillin or cefotaxime essentially showed indifference, suggesting that these antibiotics in combinations in vitro act substantially as individuals in their activity against S. pneumoniae.
In the United States, 4 million cases of community-acquired pneumonia (CAP) occur annually, and about one-fourth of the patients who require hospitalization die (9). Empirical treatment of seriously ill persons with CAP consists of combination antibiotic therapy with a broad-spectrum cephalosporin and a macrolide (3, 10). In two recent studies of bacteremic Streptococcus pneumoniae CAP among adults, combined antibiotic therapy with a macrolide and a penicillin or cephalosporin resulted in lower case fatality rates than treatment with a broad-spectrum cephalosporin alone (7, 13).
Several investigators tested different combination antibiotic regimens in vitro for antimicrobial activity against penicillin-susceptible (PSSP) and penicillin-resistant S. pneumoniae (PRSP), but only one group of investigators reported on the interaction of penicillin and a macrolide in time-kill assays, and their results uniformly showed antagonism (2, 4-6; J. Haynes, P. M. Hawkey, and E. W. Williams, Letter, J. Antimicrob. Chemother. 18:426-428, 1986). Since clinical studies showed a beneficial effect of combination antibiotic therapy in the treatment of bacteremic pneumococcal CAP, we investigated the interactions of erythromycin and penicillin and of erythromycin and cefotaxime in vitro to determine whether these combinations exhibit synergy against several invasive PSSP and PRSP isolates.
For our studies, we selected 11 invasive S. pneumoniae isolates based on resistance profiles for penicillin and cefotaxime (MIC determined by E-test) and to erythromycin (disk diffusion) (Table 1) (8). Isolates were thawed at 37°C in a water bath, plated overnight on blood agar, and diluted according to the individual protocols for checkerboard microdilution and time-kill assays.
TABLE 1.
In vitro activities of penicillin and erythromycin individually and in combination against S. pneumoniae strains of different serotypes and resistance profiles
| Serotype (isolate no.)a | MIC (μg/ml)
|
FICI | Interactive category | ||
|---|---|---|---|---|---|
| Penicillin | Erythromycin | Penicillin-erythromycin combination | |||
| Penicillin susceptible, erythromycin susceptible | |||||
| 1 (364.0344) | 0.016 | 0.125 | 0.016/0.125 | 2 | Indifference |
| 12 (005.0358) | 0.016 | 0.250 | 0.016/0.250 | 2 | Indifference |
| 12 (1014) | 0.016 | 0.062 | 0.016/0.125 | 3 | Indifference |
| Penicillin resistant, erythromycin susceptible | |||||
| 9 (0566) | 0.500 | 0.125 | 0.500/0.125 | 2 | Indifference |
| 9 (3848) | 0.250 | 0.125 | 0.250/0.125 | 2 | Indifference |
| 14 (039.0723) | 0.250 | 0.125 | 0.500/0.125 | 2 | Indifference |
| 19 (2660) | 0.125 | 0.250 | 0.125/0.250 | 2 | Indifference |
| 19 (4320) | 0.500 | 0.250 | 0.500/0.125 | 1.5 | Indifference |
| Penicillin susceptible, erythromycin resistant | |||||
| 7 (315.0353) | 0.016 | 8.000 | 0.016/8.0 | 2 | Indifference |
| 38 (0865) | 0.016 | 4.000 | 0.016/4.0 | 2 | Indifference |
| Penicillin resistant, erythromycin resistant | |||||
| 6 (4828) | 0.125 | 4.000 | 0.125/4.0 | 2 | Indifference |
Serotype was determined by Quellung procedures.
The checkerboard method used a 96-well plate. The first four columns of wells served as controls for S. pneumoniae growth and plate sterility. Eight twofold dilutions of antibiotics were made with Todd Hewitt yeast medium in the grid of eight rows by eight columns. One antibiotic was placed in the wells of eight rows in descending concentrations starting at two times the MIC and ending at zero MIC. The other antibiotic was similarly distributed among the eight columns. Thus, each of the 64 wells held a unique combination of concentrations of the two antibiotics. An inoculum of 100 μl of S. pneumoniae per well was used at a concentration of about 5 × 105 CFU/ml. The plates were incubated overnight, and the MIC was read as the last dilution without any turbidity. A fractional inhibitory concentration index (FICI) was used to interpret the results (D. M. Johnson and R. N. Jones, Letter, J. Antimicrob. Chemother. 42:555-557, 1998).
Time-kill assays were performed by incubating S. pneumoniae isolates with antibiotic concentrations of four times the MIC of penicillin and cefotaxime and one-half times the MIC of erythromycin individually and as combinations of erythromycin and penicillin or cefotaxime (4, 11). These mixtures were incubated in 50 ml of Todd Hewitt yeast medium in a 125-ml Erlenmeyer flask at 35°C in an incubator-shaker over a 6-h period (5, 6). The final concentration of each S. pneumoniae isolate at time zero was about 1.4 × 106 CFU/ml. Viable plate counts were done after 0, 3, and 6 h of incubation, because S. pneumoniae undergoes autolysis by 12 to 24 h (4, 6). Aliquots of 100 μl were removed from the flasks and inoculated onto blood agar plates. After overnight incubation, CFU were counted, and the least acceptable concentration was 30 CFU/ml. Drug carryover effects were not assessed. Synergy was defined as at least a 100-fold decrease in CFU with the antibiotics in combination compared with the lowest CFU with antibiotics used singly. Antagonism was defined as a level of CFU 100-fold higher in the antibiotics used in combination compared to the CFU of the antibiotics used singly (4).
By checkerboard microdilution, all 11 S. pneumoniae isolates tested representing four resistance profiles against erythromycin and penicillin showed indifference against this antibiotic combination (Table 1). The combined FICIs ranged between 1.5 and 3. Of the seven isolates tested representing three resistance profiles against erythromycin and cefotaxime, six showed indifference and one showed antagonism (Table 2). The combined FICI of the isolate that showed antagonism was 4, and those for the other isolates ranged from 1.25 to 3.
TABLE 2.
In vitro activities of cefotaxime and erythromycin individually and in combination against S. pneumoniae
| Serotype (isolate no.) | MIC (μg/ml)
|
FICI | Interactive category | ||
|---|---|---|---|---|---|
| Cefotaxime | Erythromycin | Cefotaxime-erythromycin combination | |||
| Cefotaxime susceptible, erythromycin susceptible | |||||
| 1 (364.0344) | 0.016 | 0.0625 | 0.016/0.0625 | 3 | Indifference |
| 12 (005.0358) | 0.016 | 0.1250 | 0.016/0.250 | 4 | Antagonism |
| 12 (1014) | 0.016 | 0.0625 | 0.016/0.125 | 3 | Indifference |
| Cefotaxime susceptible, erythromycin resistant | |||||
| 7 (315.0353) | 0.016 | 8.000 | 0.016/8.000 | 2.0 | Indifference |
| 38 (0865) | 0.062 | 2.000 | 0.016/2.000 | 1.25 | Indifference |
| 6 (4828) | 0.125 | 4.000 | 0.125/8.000 | 3 | Indifference |
| Cefotaxime resistant, erythromycin sensitive | |||||
| 19 (2660) | 8.000 | 0.250 | 4.00/0.250 | 1.5 | Indifference |
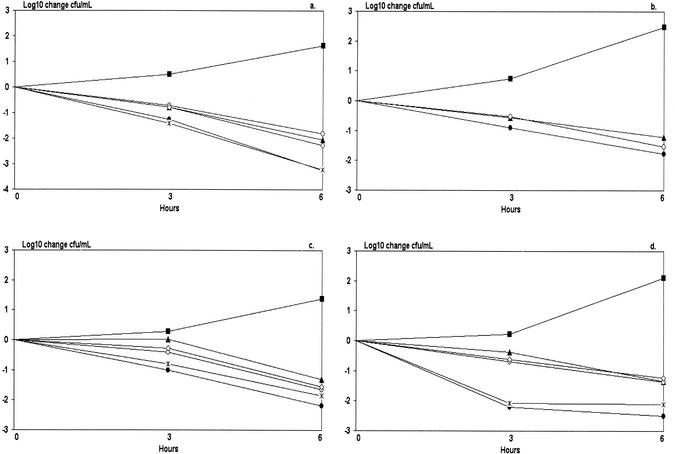
All 11 S. pneumoniae isolates were tested with time-kill assays, and the results of four representative assays each of a different antibiotic resistance profile are shown in Fig. 1. Figure 1a shows a pneumococcus susceptible to all test antibiotics. As single antibiotics, penicillin and cefotaxime resulted in a 1,000-fold decrease in CFU after 6 h, and erythromycin resulted in a 100-fold decrease in CFU in 6 h, but both the erythromycin-penicillin and erythromycin-cefotaxime combinations did not differ significantly from the least-effective singly used antibiotic. Figure 1b shows an isolate resistant to penicillin but susceptible to erythromycin and cefotaxime. Erythromycin and penicillin alone and in combination showed similar results: a 100-fold decrease in CFU after 6 h. Figure 1c shows an isolate resistant to erythromycin only, and Fig. 1d shows an isolate resistant to erythromycin and penicillin but susceptible to cefotaxime. The time-kill assays for these two isolates showed no significant differences between antibiotics used in combination and those used singly.
FIG. 1.
Four time-kill kinetic curves of S. pneumoniae strains incubated with erythromycin, penicillin, or cefotaxime, alone or in combination. (a) Serotype 12 (1014), fully susceptible. (b) Serotype 14 (039.0723), penicillin resistant. (c) Serotype 7 (315.0353), erythromycin resistant. (d) Serotype 6 (4828), both erythromycin and penicillin resistant. •, penicillin (four times the MIC); ▴, erythromycin (one-half times the MIC);  , cefotaxime (four times the MIC); ○, erythromycin with penicillin; ◊, erythromycin with cefotaxime; ▪, control.
, cefotaxime (four times the MIC); ○, erythromycin with penicillin; ◊, erythromycin with cefotaxime; ▪, control.
Overall, combinations of erythromycin and penicillin or erythromycin and cefotaxime against 11 PSSP and PRSP isolates of different capsular serotypes showed indifference in checkerboard and time-kill assays, except for one isolate susceptible to erythromycin and cefotaxime, which showed antagonism by checkerboard assay, but indifference by time-kill assay. Johansen et al. found only antagonism between erythromycin and penicillin in combination in time-kill assays (6). Synergy was not detected in time-kill or checkerboard assays, although it was problematic in the checkerboard assay.
The worldwide increase in antibiotic-resistant isolates of S. pneumoniae and other bacterial pathogens of CAP has generated increased interest in new recommendations for empirical therapy of seriously ill persons with CAP, including treatment with a macrolide and a broad-spectrum cephalosporin (3, 10, 12). In invasive pneumococcal pneumonia treated with these two antibiotics, the case fatality rate was significantly lower than with conventional one-drug therapy of penicillin or cephalosporin (7, 13). However, the results of checkerboard and time-kill assays did not provide in vitro experimental support for these clinical findings. Because erythromycin is highly concentrated in leukocytes in vivo, its concentration at the site of an infection might be higher than the concentration used in our in vitro assays (1). Even so, pneumococci are not usually intracellular. The disparity between the clinical findings and the in vitro findings remains to be elucidated, and additional prospective clinical treatment studies might clarify it.
REFERENCES
- 1.Amsden, G. W. 1999. Pneumococcal macrolide resistance—myth or reality? J. Antimicrob. Chemother. 44:1-6. [DOI] [PubMed] [Google Scholar]
- 2.Bajaksouzian, S., M. A. Visalli, M. R. Jacobs, and P. C. Appelbaum. 1996. Antipneumococcal activities of cefpirome and cefotaxime, alone and in combination with vancomycin and teicoplanin, determined by checkerboard and time-kill methods. Antimicrob. Agents Chemother. 40:1973-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., R. F. Breiman, L. A. Mandell, and T. M. File, Jr. 1998. Community-acquired pneumonia in adults: guidelines for management. Clin. Infect. Dis. 26:811-838. [DOI] [PubMed] [Google Scholar]
- 4.Friedland, I. R., M. Paris, S. Shelton, and G. H. McCracken. 1994. Time-kill studies of antibiotic combinations against penicillin-resistant and -susceptible Streptococcus pneumoniae. J. Antimicrob. Chemother. 34:231-237. [DOI] [PubMed] [Google Scholar]
- 5.Gross, M. E., K. P. Giron, J. D. Septimus, E. O. Mason, Jr., and D. M. Musher. 1995. Antimicrobial activities of beta-lactam antibiotics and gentamicin against penicillin-susceptible and penicillin-resistant pneumococci. Antimicrob. Agents Chemother. 39:1166-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen, H. K., T. G. Jensen, R. B. Dessau, B. Lundgren, and N. Frimodt-Moller. 2000. Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo. J. Antimicrob. Chemother. 46:973-980. [DOI] [PubMed] [Google Scholar]
- 7.Mufson, M. A., and R. J. Stanek. 1999. Bacteremic pneumococcal pneumonia in one American city: a 20-year longitudinal study, 1978-1997. Am. J. Med. 107:34S-43S. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Niederman, M. S., J. B. Bass, Jr., G. D. Campbell, A. M. Fein, R. F. Grossman, L. A. Mandell, T. J. Marrie, G. A. Sarosi, A. Torres, and V. L. Yu. 1993. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. Am. Rev. Respir. Dis. 148:1418-1426. [DOI] [PubMed] [Google Scholar]
- 10.Niederman, M. S., L. A. Mandell, A. Anzueto, J. B. Bass, W. A. Broughton, G. D. Campbell, N. Dean, T. File, M. J. Fine, P. A. Gross, F. Martinez, T. J. Marrie, J. F. Plouffe, J. Ramirez, G. A. Sarosi, A. Torres, R. Wilson, and V. L. Yu. 2001. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 11.Saverino, D., E. A. Debbia, A. Pescue, A. M. Lepore, and G. C. Schito. 1992. Antibacterial profile of flurithromycin, a new macrolide. J. Antimicrob. Chemother. 30:261-272. [DOI] [PubMed] [Google Scholar]
- 12.Stanek, R. J., and M. A. Mufson. 1995. Emergence of penicillin-resistant invasive pneumococci in a single American community. Am. J. Med. Sci. 310:150-155. [DOI] [PubMed] [Google Scholar]
- 13.Waterer, G. W., G. W. Somes, and R. G. Wunderink. 2001. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch. Intern. Med. 161:1837-1842. [DOI] [PubMed] [Google Scholar]