Abstract
The new quinolone garenoxacin (BMS-284756), which lacks a C-6 fluorine, was examined for its ability to block the growth of Staphylococcus aureus. Measurement of the MIC and the mutant prevention concentration (MPC) revealed that garenoxacin was 20-fold more potent than ciprofloxacin for a variety of ciprofloxacin-susceptible isolates, some of which were resistant to methicillin. The MPC for 90% of the isolates (MPC90) was below published serum drug concentrations achieved with recommended doses of garenoxacin. These in vitro observations suggest that garenoxacin has a low propensity for selective enrichment of fluoroquinolone-resistant mutants among ciprofloxacin-susceptible isolates of S. aureus. For ciprofloxacin-resistant isolates, the MIC at which 90% of the isolates tested were inhibited was below serum drug concentrations while the MPC90 was not. Thus, for these strains, garenoxacin concentrations are expected to fall inside the mutant selection window (between the MIC and the MPC) for much of the treatment time. As a result, garenoxacin is expected to selectively enrich mutants with even lower susceptibility.
The fluoroquinolones are potent antibacterial agents that have been used successfully to control a variety of bacterial pathogens. Unfortunately, Staphylococcus aureus readily develops resistance to derivatives such as ciprofloxacin (1, 3, 18). In some surveys of hospital-acquired infection, more than 90% of the S. aureus isolates were found to be ciprofloxacin-resistant (1, 22). Nevertheless, many community-acquired infections due to S. aureus are still susceptible (8, 14, 17, 20), raising the possibility that new quinolones may be active enough to treat S. aureus infections successfully.
Fluoroquinolone activity can be measured in a variety of ways. For susceptible cells, activity is generally assessed in terms of the MIC, the concentration that prevents growth when 104 to 105 cells are applied to drug-containing agar. When large bacterial and patient populations are considered, activity can be assessed by the mutant prevention concentration (MPC) (5) to take into account subpopulations of resistant mutants that may be present prior to antimicrobial treatment. The MPC is defined as the MIC of the least susceptible single-step mutant (12, 24). For bacterial populations that already contain mutations that lower susceptibility, the MPC is equivalent to the MIC of the least-susceptible next single-step mutant. The MPC is approximated by the concentration that prevents growth when at least 1010 cells are applied to agar plates (when 109 to 1010 bacteria are treated with fluoroquinolone, the recovery of mutants is progressively reduced by increasing the drug concentration, as expected for concentrations that approach the MIC of the least susceptible mutant).
In principle, the interval between the lowest concentration that blocks wild-type growth and the MPC represents a concentration window in which resistant mutants are selected (26, 27). The properties of the selection window, when combined with drug pharmacokinetics for relevant tissues, provide a framework that reflects the potential of a compound to selectively enrich resistant mutants.
In the present work, we used a small but genetically diverse set of clinical isolates of S. aureus, as assessed by spa typing (23), to compare a new quinolone, garenoxacin (BMS-284756), with the older fluoroquinolone ciprofloxacin. Garenoxacin lacks the C-6 fluorine characteristic of fluoroquinolones, but it has a difluoro methoxyl moiety attached to the C-8 position. Earlier work showed that C-8 methoxyl and halogen moieties enhance the bacteriostatic and bactericidal activities of fluoroquinolones against S. aureus, particularly with resistant mutants (16, 28). Thus, we measured the MPC of garenoxacin to determine if this in vitro parameter would be below garenoxacin concentrations in serum for most of the dosing interval for ciprofloxacin-susceptible isolates. Since previous work (6, 9, 21, 25) had shown good activity as measured by standard MIC-based criteria and since guarded optimism had been expressed for using the compound against ciprofloxacin-resistant S. aureus (4, 6, 9, 25), the MPC was also measured for ciprofloxacin-resistant isolates. For ciprofloxacin-susceptible isolates, garenoxacin concentrations reported in the published literature were above the MPC determined in vitro; for ciprofloxacin-resistant isolates, they fell inside the mutant selection window.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Laboratory strain RN450 is a methicillin-susceptible, erythromycin-susceptible derivative of S. aureus strain 8325-4 (10). Strain 450 M is a methicillin-resistant transformant of RN450 (10) for which donor DNA was obtained from strain COL (2). Clinical isolates of S. aureus, obtained from a variety of sources, represented a wide variety of genotypes. Cells were cultured (19) with vigorous shaking at 37°C in CY medium (1% Casamino Acids, 1% yeast extract, 0.1 M NaCl, 0.5% glucose, and 0.05 M sodium glycerophosphate [pH 7.2]), and colonies were grown on GL agar (0.3% Casamino Acids, 0.3% yeast extract, 0.1 M NaCl, 0.2% sodium lactate, 0.1% glycerol, and 1.5% agar [pH 7.8]) incubated at 37°C.
To assay the selection of fluoroquinolone-resistant mutants with laboratory strains, S. aureus was grown to stationary phase, harvested by centrifugation, and resuspended in 25 to 50 volumes of fresh growth medium. Incubation was continued for 5 to 6 h, producing a culture containing 1010 to 1011 CFU/ml. Cells were diluted and applied at various volumes and concentrations to quinolone-containing agar at a maximum of 2 × 1010 CFU per 150-mm-diameter plate. Drug-containing plates were incubated at 37°C for 72 to 96 h to allow time for slow-growing colonies to be recovered. After colonies were counted, they were confirmed to be composed of resistant mutants by regrowth on agar containing quinolone at the concentration used to select the colonies.
Fluoroquinolones.
Fluoroquinolones were obtained from Bristol-Myers Squibb (gatifloxacin and garenoxacin), Sigma (ciprofloxacin), and R. W. Johnson Pharmaceutical Research Institute (levofloxacin). Ciprofloxacin was dissolved in sterile water to give a final concentration of 10 mg/ml. Levofloxacin and gatifloxacin stock solutions were prepared similarly, except that about a 1/10 volume of 1 M NaOH was added to help dissolve both compounds. Garenoxacin was dissolved in 0.001 M acetic acid as a 5-mg/ml stock solution. Stock solutions were divided into 1-ml aliquots and stored at −80°C. Dilution series were prepared with autoclaved water. Solutions were occasionally stored at −20°C for several weeks.
Measurement of MIC and MPC.
The MIC was determined by placing duplicate aliquots (10 or 20 μl) of serial dilutions of bacterial cultures onto GL agar plates containing various concentrations of quinolone. Colonies were counted after overnight incubation. For laboratory strains, preliminary determinations with twofold dilutions of the drug provided an approximate MIC. This measurement was followed by a second measurement, plus a replicate, that utilized linear drug concentration increments that did not exceed 50% per sequential increase. The MIC was defined as the minimal drug concentration that inhibited growth by 99% (MIC99). For clinical isolates, twofold dilutions of the drug were used, and the MIC was taken as the lowest drug concentration that prevented the growth of 104 to 105 cells.
The MPC was defined as the lowest drug concentration that prevented recovery of colonies when more than 1010 cells, obtained as described above, were tested. For determination of the MPC, high-density cultures were prepared from overnight cultures grown in liquid medium followed by a 10-fold dilution and 4 h of incubation with shaking at 37°C. Cells were concentrated twofold by centrifugation. Preliminary determinations with twofold dilutions of the drug provided an approximate MPC. This measurement was followed by a second measurement, plus a replicate, that utilized linear drug concentration increments that did not exceed 50% per sequential increase.
Nucleotide sequence determination.
The nucleotide sequences of the quinolone resistance-determining regions of gyrA and parC were determined after amplification of the respective DNA fragments from S. aureus chromosomal DNA templates by using PCR as previously described (14). Primers SA-parCseq (5′-ACG TCG TAT TTT ATA TGC AA-3′) and SA-gyrAfwd (5′-AGA TTA TGC GAT GAG TGT TAT CGT TGC-3′) were used for sequencing after PCR amplification of DNA fragments with primers SA-parCfwd (5′-TGA TGA GGA GGA AAT CTA GTG-3′), SA-parCrev (5′-GGA AAT CTT GAT GGC AAT AC-3′), SA-gyrAfwd (defined above), and SA-gyrArev (5′-TAG TCA TAC GCG CTT CAG TAT AAC GCA-3′). spa typing, which involves DNA sequence analysis of the variable number tandem repeats in the protein A (spa) gene, followed the protocol described previously by Shopsin et al. (23).
RESULTS
Bacteriostatic activities of fluoroquinolones against laboratory strains of S. aureus.
To assess the ability of garenoxacin to block the growth of S. aureus, we measured the MIC99 and compared it to similar measurements for ciprofloxacin, levofloxacin, and gatifloxacin. Garenoxacin was 9- to 15-fold more active than ciprofloxacin and levofloxacin and 4-fold more active than gatifloxacin against a pair of laboratory strains (Table 1). Garenoxacin was also more active when the MPC was measured to assess the inhibition of mutant subpopulations (Table 1), but the differences were less than those measured with the MIC99.
TABLE 1.
Effect of mecA+ on MICs and MPCs for S. aureus
| Fluoroquinolone | MIC99 (μg/ml) for:
|
MPC (μg/ml) for:
|
||
|---|---|---|---|---|
| Strain RN450 | Strain 450 M (mecA+) | Strain RN450 | Strain 450 M (mecA+) | |
| Garenoxacin | 0.02 | 0.01 | 0.3 | 0.2 |
| Ciprofloxacin | 0.3 | 0.14 | 4 | 3 |
| Levofloxacin | 0.18 | 0.12 | 2 | 2 |
| Gatifloxacin | 0.08 | 0.04 | 0.5 | 0.5 |
Since isolates of methicillin-resistant S. aureus (MRSA) frequently exhibit fluoroquinolone resistance (22), the possibility exists that enrichment of fluoroquinolone-resistant mutants is intrinsically elevated for MRSA strains. The data in Table 1 can be used to argue against this hypothesis: the MICs and the MPCs of several fluoroquinolones for strain 450 M, a mecA+ (methicillin-resistant) derivative of strain RN450, were not higher than those observed for strain RN450.
Recovery of fluoroquinolone-resistant mutants.
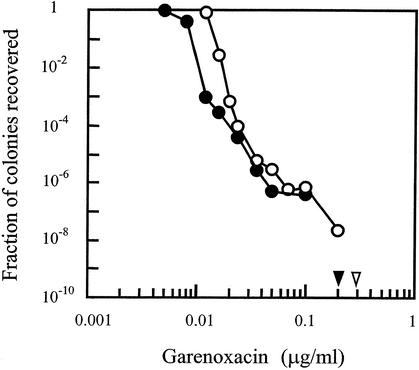
Strains RN450 and 450 M were also used for the selection of resistant mutants on agar plates containing various concentrations of quinolone. As shown in Fig. 1, the presence of mecA reduced the MIC99 of garenoxacin by about 50% but in general the recoveries of mutants were similar for the two strains. The concentration at which no colony was recovered when 1010 cells were applied to agar plates was not higher for the mecA+ strain. Similar results were obtained with ciprofloxacin and levofloxacin (data not shown). These data further demonstrate that mecA does not contribute directly to a high prevalence of ciprofloxacin resistance among MRSA isolates.
FIG. 1.
Selection of resistant S. aureus mutants. Cells from S. aureus strain RN450 (open circles) or strain 450 M (filled circles) were applied to agar plates containing the indicated concentrations of garenoxacin as described in Materials and Methods. Concentrations at which no colony was recovered when 1010 cells were applied to agar are indicated by arrowheads.
DNA was extracted from three resistant mutants of strain RN450 obtained from agar plates containing garenoxacin at concentrations near that represented by the inflection point in Fig. 1 (0.1 μg/ml). Nucleotide sequence analysis of the quinolone resistance-determining regions of gyrA and parC revealed that each mutant contained a Ser-to-Leu change at codon 84 in the GyrA protein (data not shown). No change was found in parC. These results support the conclusion that gyrase is the primary target of garenoxacin in S. aureus (4). In contrast to a previous study (4), we were able to obtain gyrase mutants in a single selection step.
Effects of ciprofloxacin and garenoxacin on ciprofloxacin-susceptible clinical isolates.
We examined two groups of bacterial isolates that were susceptible to ciprofloxacin. One comprised methicillin-susceptible S. aureus (MSSA) isolates collected from dialysis patients while the other comprised MRSA isolates from various sources. The isolates were genetically diverse, as indicated by DNA sequencing of the protein A gene repeats (spa typing). Little difference in the MICs was found for the two groups of strains (the MIC90 of garenoxacin was about 0.03 μg/ml, a value that is similar to that reported in other studies [15, 21]). However, the MPC was slightly higher for MRSA than for MSSA (the modal MPC of garenoxacin was 0.2 μg/ml for MSSA and between 0.3 and 0.4 μg/ml for MRSA). For susceptible clinical isolates, the MPC of ciprofloxacin for 90% of the isolates (MPC90) was 8 μg/ml; the modal MPC and the MPC range were 3 to 4 and 2 to 12 μg/ml, respectively. These values are roughly 10- to 20-fold higher than those observed with garenoxacin. MPCs for isolates collected before quinolones were in widespread clinical use (isolates numbered 948 and 912) were the lowest (ciprofloxacin, 2 μg/ml; garenoxacin, 0.07 and 0.1 μg/ml, respectively), but the MICs were similar to those for the more recent isolates (ciprofloxacin, 0.3 and 0.4 μg/ml, respectively; garenoxacin, 0.015 μg/ml). These data are consistent with the extensive use of fluoroquinolones causing susceptibility as measured by the MPC to gradually decrease.
Effect of garenoxacin on ciprofloxcin-resistant clinical isolates.
To assess the potency of garenoxacin against ciprofloxacin-resistant isolates, we measured the MICs and the MPCs for a diverse panel of isolates comprising 18 MRSA and 4 MSSA isolates. The MIC ranged between 0.04 and 4.8 μg/ml, the modal MIC was between 0.8 and 1.2 μg/ml, and the MIC90 was 3.2 μg/ml, similar to data in one report (15) and slightly higher than that in another (21). The range of MPCs was 3.2 to >29 μg/ml, the MPC90 was >19.6 μg/ml, and the modal MPC ranged between 9.6 and 12.8 μg/ml. These values, which are listed in Table 2, are 30- to 100-fold higher than those observed for ciprofloxacin-susceptible isolates.
TABLE 2.
Activities of ciprofloxacin and garenoxacin against clinical isolates of S. aureus
| Test compound | Ciprofloxacin phenotype of isolates | No. of isolates | No. of spa types | MIC (μg/ml)
|
MPC (μg/ml)
|
MPC/MIC ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 90% | Mode | Range | 90% | Mode | Range | |||||
| Ciprofloxacin | Susceptible | 20 | 15 | 0.6 | 0.4 | 0.2-0.6 | 8 | 3-4 | 2-12 | 13 |
| Garenoxacin | Susceptible | 20 | 15 | 0.03 | 0.015 | 0.005-0.03 | 0.4 | 0.3-0.4 | 0.07-0.8 | 13 |
| Resistant | 22 | 10 | 3.2 | 0.8-1.2 | 0.04-4.8 | >19.6 | 9.6-12.8 | 3.2->28.8 | >6 | |
DISCUSSION
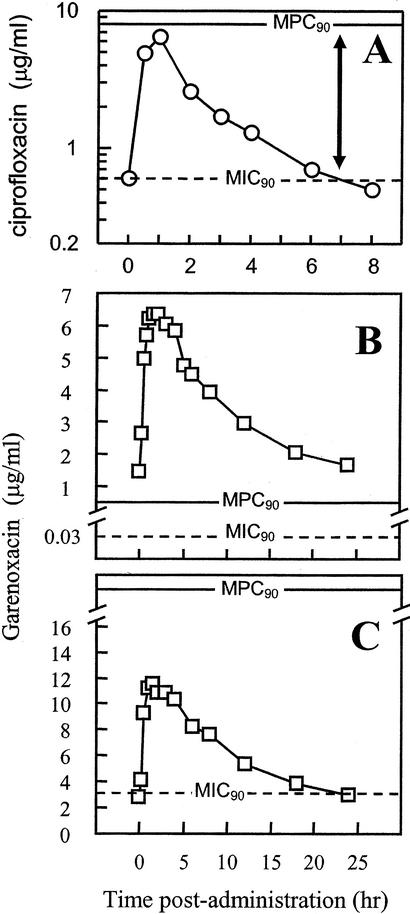
The increasing prevalence of antimicrobial resistance makes it important that new agents be considered for their propensity to selectively enrich resistant mutants. It has been suggested that mutant enrichment occurs when the concentration of the antimicrobial agent is high enough to block the growth of susceptible cells (above the MIC) but not that of resistant mutants (below the MPC) (26, 27). Compounds that can be dosed so that relevant concentrations at the site of infection exceed the MPC are expected to enrich fewer mutants than those whose concentrations do not exceed the MPC, provided that mutation frequencies are similar (26, 27). For example, ciprofloxacin, a compound that readily selects resistant S. aureus mutants (1, 3), has an in vitro MPC90 of about 8 μg/ml when the value is determined for a small but genetically diverse set of ciprofloxacin-susceptible S. aureus (Table 2) isolates. Pharmacokinetic measurements reported in the published literature indicate that ciprofloxacin concentrations in serum do not exceed the MPC (Fig. 2A). The MPC90 of garenoxacin was lower (0.4 μg/ml). Pharmacokinetic measurements with garenoxacin indicate that human serum drug concentrations will exceed this value for the entire dosing period (24 h) at the recommended 400-mg once-daily dosage (Fig. 2B), even if 75% of the agent is considered unavailable due to protein binding (11). We emphasize that the MPC has not been measured in vivo; determining whether concentrations that exceed the MPC measured in vitro are sufficient to block the development of resistance requires clinical studies.
FIG. 2.
Quinolone pharmacodynamics with S. aureus. (A) Ciprofloxacin and susceptible S. aureus. The MIC90 and the MPC90 were obtained from the data in Table 2; pharmacokinetic data were determined for 400-mg doses of ciprofloxacin administered intravenously three times per day (13). (B) Garenoxacin and susceptible S. aureus. The MIC90 and the MPC90 were obtained from the data in Table 2; pharmacokinetic data were determined for 400-mg doses of garenoxacin administered orally once daily (7). (C) Garenoxacin and ciprofloxacin-resistant S. aureus. The MIC90 and the MPC90 were obtained from the data in Table 2; pharmacokinetic data were determined for 600-mg doses of garenoxacin administered orally once daily (P. Hale, personal communication).
Another strategy for restricting the development of resistance is to find compounds that have very narrow mutant selection windows (MPC/MIC99 = 1). With S. aureus, fluoroquinolone structure affects this ratio (27). In the present work, the ratios of the MPC of ciprofloxacin to the MIC99 of ciprofloxacin for laboratory strains RN450 and 450 M were 13 and 21, respectively. The ratios of the MPC of garenoxacin to the MIC99 of garenoxacin were similar (15 and 20 for RN450 and 450 M, respectively). Average values for clinical isolates, which were expected to be lower due to the use of the standard MIC rather than the MIC99, were about 13 for both ciprofloxacin and garenoxacin (Table 2). Thus, the mutant selection window for garenoxacin, which is about twice that of gatifloxacin (Table 1), is not unusually narrow.
Many S. aureus isolates that exhibit resistance to methicillin are also ciprofloxacin resistant. Since selection of resistance by garenoxacin produced similar results with mecA+ and mecA mutant laboratory strains (Fig. 1) and with MRSA and MSSA clinical isolates (MPCs were similar [data not shown]), it is likely that factors other than mecA are involved in the fluoroquinolone resistance of MRSA. The association between MRSA and ciprofloxacin resistance may stem from the more frequent exposure of MRSA to fluoroquinolones and to the dissemination of ciprofloxacin-resistant strains in institutional settings.
Isolates recovered before quinolone use was widespread exhibited susceptibilities (as measured by the MICs) that were similar to those observed for other ciprofloxacin-susceptible isolates. However, the MPCs were lower. This result is consistent with the recent accumulation of mutations that have little effect on the growth of the bulk population in the presence of quinolone but increase the contribution of resistant subpopulations to growth at high drug concentrations. A similar phenomenon has been seen with Escherichia coli (29). In that case, a parC (DNA topoisomerase IV) mutation had no effect on the MIC but dramatically increased the recovery of gyrA (gyrase) mutants.
For the isolates that were already resistant to ciprofloxacin, the MIC90 of garenoxacin was 3.2 μg/ml, roughly eight times higher than the MPC90 for the susceptible isolates. This observation suggests that multiple mutations were present in some of the resistant strains, as has been documented by other studies (15, 21). Since the MIC for resistant isolates is below achievable serum drug concentrations (Fig. 2C), it is conceivable that treatment of ciprofloxacin-resistant S. aureus with garenoxacin might sometimes cure infection. However, the MPC90 of the isolates already resistant to ciprofloxacin was >19.6 μg/ml, which is well above the serum drug levels achieved with garenoxacin, even if daily doses are raised to 600 mg (Fig. 2C). Indeed, the MPC-based pharmacodynamics of garenoxacin and the mutants (Fig. 2C) are similar to those of ciprofloxacin and fully susceptible isolates (Fig. 2A). Since ciprofloxacin selected resistant mutants rapidly (1, 3), we predict that additional mutations will be fixed in S. aureus if garenoxacin is used against ciprofloxacin-resistant strains. Those mutations would then preclude the use of garenoxacin in combination therapy.
Acknowledgments
We thank M. Gennaro for critical comments on the manuscript and X. Li for technical assistance in PCR and DNA sequencing.
This work was supported by grants from the NIH (AI35257) and Bristol-Myers Squibb.
REFERENCES
- 1.Acar, J., and F. Goldstein. 1997. Trends in bacterial resistance to fluoroquinolones. Clin. Infect. Dis. 24:S67-S73. [DOI] [PubMed]
- 2.Archer, G. L., D. Niemeyer, J. Thanassi, and M. Pucci. 1994. Dissemination among staphylococci of DNA sequences associated with methicillin resistance. Antimicrob. Agents Chemother. 38:447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg, H. M., D. Rimland, D. J. Carroll, P. Terry, and I. K. Wachsmuth. 1991. Rapid development of ciprofloxacin resistance in methicillin-susceptible and -resistant Staphylococcus aureus. J. Infect. Dis. 1 63:1279-1285. [DOI] [PubMed] [Google Scholar]
- 4.Discotto, L. F., L. Lawrence, K. Denbleyker, and J. F. Barrett. 2001. Staphylococcus aureus mutants selected by BMS-284756. Antimicrob. Agents Chemther. 45:3273-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, Y., X. Zhao, J. Domagala, and K. Drlica. 1999. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fung-Tomc, J., L. Valera, B. Minassian, D. Bonner, and E. Gradelski. 2001. Activity of the novel des-fluoro(6) quinolone BMS-284756 against methicillin-susceptible and -resistant staphylococci. J. Antimicrob. Chemother. 48:735-748. [DOI] [PubMed] [Google Scholar]
- 7.Gajjar, D., S. Sukoneck, A. Bello, Z. Ge, L. Christopher, and D. Grasela. 2002. Effect of a high-fat meal on the pharmacokinetics of the des-F(6)-quinolone BMS-284756. Pharmacotherapy 22:160-165. [DOI] [PubMed] [Google Scholar]
- 8.Gorak, E., S. Yamada, and J. Brown. 1999. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin. Infect. Dis. 29:797-800. [DOI] [PubMed] [Google Scholar]
- 9.Jones, R., M. Pfaller, M. Stilwell, and the SENTRY Antimicrobial Surveillance Program Participants Group. 2001. Activity and spectrum of BMS 284756, a new des-F (6) quinolone, tested against strains of ciprofloxacin-resistant Gram-positive cocci. Diagn. Microbiol. Infect. Dis. 39:133-135. [DOI] [PubMed] [Google Scholar]
- 10.Kornblum, J. S. 1987. Some findings concerning methicillin resistance in S. aureus and analysis of countertranscript RNAs of several pT181 copy mutants. Ph.D. thesis. New York University, New York.
- 11.Lawrence, L., M. Frosco, B. Ryan, S. Chaniewski, H. Yang, D. Hooper, and J. Barrett. 2002. Bactericidal activities of BMS-284756, a novel des-F(6)-quinolone, against Staphylococcus aureus strains with topoisomerase mutations. Antimicrob. Agents Chemother. 46:191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, X., X. Zhao, and K. Drlica. 2002. Selection of Streptococcus pneumoniae mutants having reduced susceptibility to levofloxacin and moxifloxacin. Antimicrob. Agents Chemother. 46:522-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippman, J., J. Scribante, A. G. Gous, H. Hon, and S. Tshukutsoane. 1998. Pharmacokinetic profiles of high-dose intravenous ciprofloxacin in severe sepsis. Antimicrob. Agents Chemother. 42:2235-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lister, P. 2000. Emerging resistance problems among respiratory tract pathogens. Am. J. Managed Care 6(8 Suppl.):S409-S418. [PubMed]
- 15.Low, D. E., M. Muller, C. Duncan, B. Willey, J. C. deAzavedo, A. McGeer, B. Kreiswirth, S. Pong-Porter, and D. Bast. 2002. Activity of BMS-284756, a novel des-fluoro(6) quinolone, against Staphylococcus aureus, including contributions of mutations to quinolone resistance. Antimicrob. Agents Chemother. 46:1119-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, T., X. Zhao, X. Li, A. Drlica-Wagner, J.-Y. Wang, J. Domagala, and K. Drlica. 2001. Enhancement of fluoroquinolone activity by C-8 halogen and methoxy moieties: action against a gyrase resistance mutant of Mycobacterium smegmatis and a gyrase-topoisomerase IV double mutant of Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma, X., T. Ito, C. Tienasitorn, M. Jamklang, P. Chongtrakool, S. Boyle-Vavra, R. Daum, and K. Hiramatsu. 2002. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 46:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neu, H. 1992. The crisis in antibiotic resistance. Science 257:1064-1073. [DOI] [PubMed] [Google Scholar]
- 19.Novick, R. P., and R. Brodsky. 1972. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J. Mol. Biol. 68:285-302. [DOI] [PubMed] [Google Scholar]
- 20.Schito, G., E. Debbia, A. Pesce, and the Alexander Project Collaborative Group. 1996. Susceptibility of respiratory strains of Staphylococcus aureus to fifteen antibiotics: results of a collaborative surveillance study (1992-1993). J. Antimicrob. Chemother. 38(Suppl. A):97-106. [DOI] [PubMed] [Google Scholar]
- 21.Schmitz, F.-J., M. Boos, S. Mayer, H. Jagusch, and A. Fluit. 2002. Increased in vitro activity of the novel des-fluoro(6) quinolone BMS-284756 against genetically defined clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 49:283-287. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz, F.-J., A. Fluit, S. Brisse, J. Verhoef, K. Koher, and D. Milatovic. 1999. Molecular epidemiology of quinolone resistance and comparative in vitro activities of new quinolones against European Staphylococcus aureus isolates. FEMS Immunol. Med. Microbiol. 26:281-287. [DOI] [PubMed] [Google Scholar]
- 23.Shopsin, B., M. Gomez, S. Montgomery, D. H. Smith, M. Waddington, D. E. Dodge, D. Bost, M. Riehman, S. Naidich, and B. N. Kreiswirth. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sindelar, G., X. Zhao, A. Liew, Y. Dong, J. Zhou, J. Domagala, and K. Drlica. 2000. Mutant prevention concentration as a measure of fluoroquinolone potency against mycobacteria. Antimicrob. Agents Chemother. 44:3337-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller, T., J. Andrews, G. Jevons, and R. Wise. 2002. The in vitro activity of BMS-2847676, a new des-fluorinated quinolone. J. Antimicrob. Chemother. 49:177-184. [DOI] [PubMed] [Google Scholar]
- 26.Zhao, X., and K. Drlica. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl. 3):S147-S156. [DOI] [PubMed]
- 27.Zhao, X., and K. Drlica. 2002. Restricting the selection of antibiotic-resistant mutants: measurement and potential uses of the mutant selection window. J. Infect. Dis. 185:561-565. [DOI] [PubMed] [Google Scholar]
- 28.Zhao, X., J.-Y. Wang, C. Xu, Y. Dong, J. Zhou, J. Domagala, and K. Drlica. 1998. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob. Agents Chemother. 42:956-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]