Abstract
A new gene, sul3, which specifies a 263-amino-acid protein similar to a dihydropteroate synthase encoded by the 54-kb conjugative plasmid pVP440 from Escherichia coli was characterized. Expression of the cloned sul3 gene conferred resistance to sulfamethoxazole on E. coli. Two copies of the insertion element IS15Δ/26 flanked the region containing sul3. The sul3 gene was detected in one-third of the sulfonamide-resistant pathogenic E. coli isolates from pigs in Switzerland.
Sulfonamides have been widely used to treat bacterial and protozoal infections ever since their clinical introduction in 1935. To overcome the rapid emergence of resistance, sulfonamides have generally been combined since the 1970s with diaminopyrimidines (5, 6). The combination trimethoprim-sulfamethoxazole, for instance, is still commonly used in human medicine for the treatment of urinary tract infections. In veterinary medicine, sulfonamides alone or in combination with other antimicrobial compounds are widely used to prevent and treat diarrhea and other infectious diseases in intensive animal husbandry.
Sulfonamides compete with the structural analog p-aminobenzoic acid for binding to dihydropteroate synthase (DHPS), a catalytic enzyme in the folic acid biosynthesis pathway, thus inhibiting the formation of dihydrofolic acid (17). Resistance to sulfonamides in Escherichia coli can result from mutations in the chromosomal DHPS gene (folP) (20, 23) or more frequently from the acquisition of an alternative DHPS gene (sul) (14, 19), whose product has a lower affinity for sulfonamides (21).
Only two alternative sulfonamide resistance DHPS genes (sul1 and sul2) in gram-negative bacteria have been described to date (17). sul1 and sul2 from E. coli share 57% DNA identity, and their origin remains unknown, as their sequences are clearly distinct from all the known chromosomal DHPS genes from E. coli and other bacteria (14). Both genes seem to be distributed at equal frequency among sulfonamide-resistant E. coli isolates of clinical origin (15).
Pathogenic E. coli isolates from cases of neonatal and postweaning diarrhea and edema diseases in pigs are frequently resistant to sulfonamides. In a recent study on pathogenic E. coli from animals in Switzerland, only 70% of the sulfonamide resistance in isolates from pigs could be explained by the presence of sul1 or sul2 genes (8). The mechanism of sulfonamide resistance was unexplained for the remaining 30% of the isolates. The enterotoxigenic E. coli rl0044 strain (LT, STb, and K88 positive), isolated from a case of diarrhea in a pig, was chosen to characterize the determinant responsible for sulfonamide resistance in these isolates. The name sul3 has been inappropriately used for a DHPS gene found in both Mycobacterium fortuitum (9) and Corynebacterium striatum (GenBank accession no. AJ294721) whose protein is virtually identical to that of sul1, with the exception of four additional amino acids at its N terminus (6). Therefore, we chose to consider the new DHPS gene described below the third alternative DHPS gene conferring sulfonamide resistance in E. coli and named it sul3.
Analysis of the chromosomal DHPS (FolP) gene.
To determine whether mutations in the folP gene were responsible for sulfonamide resistance, the folP gene of E. coli rl0044 was amplified by PCR and sequenced as described previously (8). The DNA sequence was identical to that described by Lanz et al. (8) (GenBank accession no. AF483270). The amino acid sequence showed 100% identity to FolP of the sulfonamide-susceptible strain E. coli K-12 MG1655 (GenBank accession no. AE000398), confirming that the resistance was not due to a mutated folP gene.
Conjugal transfer of plasmid pVP440.
Conjugations using E. coli rl0044 as a donor and E. coli JF33 (a rifampin-resistant E. coli K-12 derivative [2]) as a recipient strain were performed as described previously (1). The transconjugants were selected on Mueller-Hinton agar plates containing 50 μg of rifampin and 200 μg of sulfamethoxazole per ml. A single 54-kb plasmid (pVP440) conferring sulfonamide resistance was transferred into the recipient strain at a frequency of 10−4 transconjugants per donor. Plasmid DNA was isolated from the transconjugants on Nucleobond AX cartridges by using the low-copy-number-plasmid extraction protocol recommended by the supplier (Macherey-Nagel GmbH & Co. KG, Düren, Germany). The size of plasmid pVP440 was determined by restriction endonuclease analysis using SphI and HindIII. Small fragments were separated by classical agarose gel electrophoresis, and large fragments were separated by pulsed-field gel electrophoresis at 12°C in 1% SeaKem Gold agarose gel (BioWhittaker Molecular Applications Inc., Rockland, Maine) for 15 h at 200 V with pulse time ramping from 0.5 to 3.5 s in a CHEF-DR III electrophoresis unit (Bio-Rad Laboratories Inc., Hercules, Calif.). Plasmid pVP440 contained six SphI fragments with calculated sizes of 1.0, 2.0, 4.5, 6.0, 7.2, and 33 kb for a total size of 53.7 kb and seven HindIII fragments of 1.3, 1.6, 2.2, 9.1, 9.5, 14.1, and 15.8 kb for a total size of 53.6 kb. The size of pVP440 was estimated to be 54 kb.
Characterization of the sul3 gene.
Plasmid pVP440 was digested with the restriction enzyme ClaI, and the resulting fragments were randomly cloned into the vector pBluescript II SK(−) (Stratagene, La Jolla, Calif.). The ligated DNA was transformed into E. coli DH5α (Life Technologies, Gaithersburg, Md.) by heat shock, and the transformants were selected on Mueller-Hinton agar containing 200 μg of sulfamethoxazole per ml. The sulfonamide-resistant transformants harbored plasmid pBVP44, which was generated by the insertion of a 3-kb ClaI fragment from pVP440 into pBluescript II SK(−). The 3-kb DNA insert was sequenced and found to contain a 789-bp gene (sul3) that specifies a putative 263-amino-acid protein (Sul3) with a calculated molecular mass of 28.9 kDa. Putative −35 and −10 promoter sequences exist within the 136 bp preceding the ATG start codon at positions 2841 and 2862, respectively. Similarity searches of protein data banks using BLAST (National Center for Biotechnology Information) and LALIGN (4) revealed evident homologies of Sul3 with DHPSs. There were amino acid identities of 50.4% overall to Sul2 from Salmonella enterica subsp. enterica plasmid pHCM1 (12), 40.6% to Sul2 from E. coli plasmid RSF1010 (16), and 40.9% to Sul1 from E. coli plasmid R388 (19). Based on amino acid homology (Fig. 1) and phenotype, Sul3 was considered a new sulfonamide-resistant DHPS.
FIG. 1.
Alignment of the amino acid sequence of the DHPSs Sul1, Sul2, and Sul3, conferring sulfonamide resistance. Sul1, DHPS of E. coli plasmid R388 (GenBank accession no. AF071413); Sul2, DHPS of S. enterica subsp. enterica plasmid pHCM1 (AL513383); Sul3, DHPS of E. coli plasmid pVP440 (AJ459418). The identical and similar amino acids are shaded black and gray, respectively.
Phenotypic expression of sul3.
The region containing only the sul3 gene and its putative promoter sequence was amplified by PCR from plasmid pVP440 by using Taq DNA polymerase (Roche Diagnostics AG, Basel, Switzerland) and an annealing temperature of 51°C. XbaI and PstI restriction sites were incorporated into forward (sul3PF, 5′-CATTCTAGAAAACAGTCGTAGTTCG [positions 2780 to 2797 in AJ459418]) and reverse (sul3R, 5′-CATCTGCAGCTAACCTAGGGCTTTGGA [positions 3770 to 3753]) primers to facilitate directional cloning into pUC19. In the resulting plasmid, pUVP4401, sul3 is placed in the direction opposite that of the lacZ promoter of pUC19 and is under the control of its own promoter. MICs were determined by broth microdilution tests according to NCCLS guidelines (11). The expression of sul3 on plasmid pUVP4401 conferred increased resistance to sulfamethoxazole in E. coli JF33, with the MIC being higher than 1,024 μg/ml, whereas JF33 alone or harboring the vector pUC19 remained susceptible to sulfamethoxazole, with the MIC being 8 μg/ml. MICs higher than 1,024 μg of sulfamethoxazole per ml were also observed for the wild-type strain E. coli rl0044 and the JF33 transconjugant harboring the sulfonamide resistance plasmid pVP440. No chloramphenicol resistance was observed (MIC ≤ 2 μg/ml) for E. coli JF33 and JF33 harboring plasmid pVP440, confirming the nonfunctionality of the truncated cmlA1 gene (3) on plasmid pVP440 (see below).
Sequence and structure of the sul3-flanking regions.
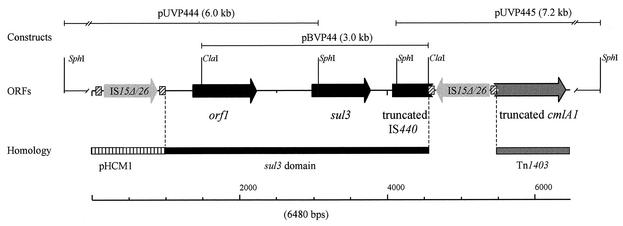
The regions flanking the left and the right sides of the sul3 gene were sequenced from two large SphI fragments from pVP440 cloned into pUC19 (pUVP444 and pUVP445) and overlapping with the ClaI insert of pBVP44 (Fig. 2). DNA analysis of the sul3-flanking regions revealed distinct domains with strong homologies to DNA sequences of diverse origins. A 3.6-kb domain contained the sul3 gene, a putative oxidoreductase gene (orf1), and the partial sequence of a new putative insertion element (IS440). This domain was embedded between two copies of the insertion element IS15Δ/26. These IS15Δ/26 copies separate the sul3 domain from DNA sequences almost identical (one single base change) to plasmid pHCM1 of the multidrug-resistant S. enterica subsp. enterica serovar Typhi strain CT18 (GenBank accession no. AL513383) (12) on the left side and from DNA sequences almost identical (one single base change) to Tn1403 from Pseudomonas aeruginosa (GenBank accession no. AF313472) (13) on the right side (Fig. 2). Mobile genetic elements containing antibiotic resistance genes flanked by two copies of IS15Δ/26 have already been described (10, 18). Two direct repeats of IS15Δ/26 flanking the kanamycin resistance transposon Tn2680 have been shown to mediate cointegration in the E. coli chromosome, generating an 8-bp target site duplication (7). This suggests that the sul3 gene could be borne on a composite element flanked by IS15Δ/26. However, the 8-bp direct repeats were not present outside the flanking IS15Δ/26.
FIG. 2.
Organization of the region from plasmid pVP440 containing the sulfonamide resistance gene sul3. The sul3 domain and the pHCM1-like and Tn1403-like sequences are labeled; the different domains are separated with vertical dotted lines. The open reading frames (ORFs) of the sul3 domain are represented by black arrows (orf1, putative oxidoreductase gene; sul3, sulfonamide-resistant DHPS gene, IS440, truncated putative insertion element) and the ORF of the truncated cmlA1 gene (chloramphenicol efflux gene) is represented by a gray arrow. The two copies of the insertion sequence IS15Δ/26 are represented by gray arrows, with inverted repeats indicated by hatched squares. The fragments of pVP440 that were cloned into pUC19 (pUVP444 and pUVP445) and into pBluescript II SK(−) (pBVP44) and used for sequence analysis are depicted by narrow lines.
The putative oxidoreductase (orf1) of the sul3 domain showed the highest amino acid identity (45.5%) with the oxygenase-like protein Aur2G from Streptomyces aureofaciens (GenBank accession no. AY033994). The putative IS440, whose incomplete transposase shared 53% amino acid identity with that of IS406 from Burkholderia cepacia (GenBank accession no. P24575) (24), appeared to be interrupted by the insertion of an IS15Δ/26 element. From there, the DNA was 100% identical to the DNA of IS15Δ/26 from transposon Tn1525 (GenBank accession no. M12900) (22). A partial chloramphenicol resistance efflux protein CmlA1 (3) from Tn1403 lacking the first 86 amino acids was identified downstream of IS15Δ/26. Similar to the 3′ end of the putative IS440, the 5′ end of cmlA1 gene has apparently been truncated by the insertion of IS15Δ/26.
Detection and distribution of sul3 in the pig flora.
Twenty-seven sulfonamide-resistant E. coli isolates, from pigs, that did not hybridize with specific DNA probe for sul1 and sul2 (8) were examined for the presence of sul3. Twenty-five of them were enterotoxigenic strains from cases of neonatal and postweaning diarrhea, and two were verotoxigenic strains of serogroups O139 and O141 (rl0066 and rl0281) from cases of edema disease (8). The sul3 gene was detected in all 27 strains by PCR using two oligonucleotide primers specific to sul3 (sul3F, 5′-GAGCAAGATTTTTGGAATCG [positions 2981 to 3000 in AJ459418] and sul3R, 5′-CATCTGCAGCTAACCTAGGGCTTTGGA [positions 3770 to 3753]) and an annealing temperature of 51°C. The presence of sul3 in these 27 isolates was confirmed by dot blot DNA hybridization as described previously (8) using a PCR probe for sul3 labeled by random priming using the Dig-High Prime kit (Roche Diagnostics). E. coli strain rl0637 (laboratory collection), which harbors both sul1 and sul2, was used as a negative control for both PCR and dot blot hybridization analyses (data not shown).
Restriction analysis with SphI of the sul3-carrying conjugative plasmid of a second epidemiologically unrelated enterotoxigenic E. coli strain (rl0096) showed a profile identical to that of pVP440. This suggests either the presence of a pVP440-carrying enterotoxigenic E. coli clone in the pig population or the horizontal spread of pVP440 between E. coli strains. Additional conjugations were attempted with the verotoxigenic E. coli strains rl0066 and rl00281. E. coli rl0281 transferred a sul3-harboring plasmid (pVP2810) larger than pVP440 and different in its SphI restriction profile into the recipient strain JF33. No transconjugants were obtained with E. coli rl0066. However, DNA-DNA hybridization analyses showed that the sul3 gene from E. coli rl0066 was also located on a plasmid with a size similar to that of pVP2810 (data not shown).
In conclusion, our study describes a new sulfonamide resistance determinant called sul3, probably acquired by E. coli from a distantly related organism. The mobility of the sul3 element was not strictly demonstrated in the present study. However, the structure of the sul3 domain with its flanking IS15Δ/26 copies and the presence of sul3 on different plasmids in different E. coli clonal lineages suggest that this resistance determinant has a strong potential for a large diffusion within the bacterial population.
The discovery of the new antimicrobial resistance determinant sul3 demonstrates once more the wide ability of bacteria to adapt to hostile environments. The appearance of new resistance genes, even toward old antimicrobial agents like sulfonamides, on conjugative plasmids and transposable elements should stress the importance of the appropriate and more prudent use of antibiotics in both public health and agriculture.
Nucleotide sequence accession number.
The sul3 gene and the flanking regions have been assigned EMBL accession number AJ459418.
Acknowledgments
We thank Lorianne Fawer for technical assistance and Sarah Burr for assistance in editing of the manuscript.
This work was supported by the research grant of the Institute of Veterinary Bacteriology, University of Berne.
REFERENCES
- 1.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey, J., P. Ghersa, P. G. Palacios, and M. Belet. 1986. Physical and genetic analysis of the ColD plasmid. J. Bacteriol. 166:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George, A. M., and R. M. Hall. 2002. Efflux of chloramphenicol by the CmlA1 protein. FEMS Microbiol. Lett. 209:209-213. [DOI] [PubMed] [Google Scholar]
- 4.Huang, X., and W. Miller. 1991. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 12:337-357. [Google Scholar]
- 5.Huovinen, P. 2001. Resistance to trimethoprim-sulfamethoxazole. Clin. Infect. Dis. 32:1608-1614. [DOI] [PubMed] [Google Scholar]
- 6.Huovinen, P., L. Sundström, G. Swedberg, and O. Sköld. 1995. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 39:279-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iida, S., B. Mollet, J. Meyer, and W. Arber. 1984. Functional characterization of the prokaryotic mobile genetic element IS26. Mol. Gen. Genet. 198:84-89. [DOI] [PubMed] [Google Scholar]
- 8.Lanz, R., P. Kuhnert, and P. Boerlin. 2003. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 91:73-84. [DOI] [PubMed]
- 9.Martin, C., J. Timm, J. Rauzier, R. Gomez-Lus, J. Davies, and B. Gicquel. 1990. Transposition of an antibiotic resistance element in mycobacteria. Nature 345:739-743. [DOI] [PubMed] [Google Scholar]
- 10.Naas, T., Y. Mikami, T. Imai, L. Poirel, and P. Nordmann. 2001. Characterization of In53, a class 1 plasmid- and composite transposon-located integron of Escherichia coli which carries an unusual array of gene cassettes. J. Bacteriol. 183:235-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed., vol. 17, no. 2. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 13.Partridge, S. R., G. D. Recchia, H. W. Stokes, and R. M. Hall. 2001. Family of class 1 integrons related to In4 from Tn1696. Antimicrob. Agents Chemother. 45:3014-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rådström, P., and G. Swedberg. 1988. RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob. Agents Chemother. 32:1684-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rådström, P., G. Swedberg, and O. Sköld. 1991. Genetic analyses of sulfonamide resistance and its dissemination in gram-negative bacteria illustrate new aspects of R plasmid evolution. Antimicrob. Agents Chemother. 35:1840-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholz, P., V. Haring, B. Wittmann Liebold, K. Ashman, M. Bagdasarian, and E. Scherzinger. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271-288. [DOI] [PubMed] [Google Scholar]
- 17.Sköld, O. 2000. Sulfonamide resistance: mechanisms and trends. Drug Resist. Updates 3:155-160. [DOI] [PubMed] [Google Scholar]
- 18.Sundström, L., C. Jansson, K. Bremer, E. Heikkilä, B. Olsson-Liljequist, and O. Sköld. 1995. A new dhfrVIII trimethoprim-resistance gene, flanked by IS26, whose product is remote from other dihydrofolate reductases in parsimony analysis. Gene 154:7-14. [DOI] [PubMed] [Google Scholar]
- 19.Sundström, L., P. Rådström, G. Swedberg, and O. Sköld. 1988. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol. Gen. Genet. 213:191-201. [DOI] [PubMed] [Google Scholar]
- 20.Swedberg, G., C. Fermér, and O. Sköld. 1993. Point mutations in the dihydropteroate synthase gene causing sulfonamide resistance. Adv. Exp. Med. Biol. 338:555-558. [DOI] [PubMed] [Google Scholar]
- 21.Swedberg, G., and O. Sköld. 1980. Characterization of different plasmid-borne dihydropteroate synthases mediating bacterial resistance to sulfonamides. J. Bacteriol. 142:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trieu-Cuot, P., and P. Courvalin. 1984. Nucleotide sequence of the transposable element IS15. Gene 30:113-120. [DOI] [PubMed] [Google Scholar]
- 23.Vedantam, G., G. G. Guay, N. E. Austria, S. Z. Doktor, and B. P. Nichols. 1998. Characterization of mutations contributing to sulfathiazole resistance in Escherichia coli. Antimicrob. Agents Chemother. 42:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood, M. S., A. Byrne, and T. G. Lessie. 1991. IS406 and IS407, two gene-activating insertion sequences for Pseudomonas cepacia. Gene 105:101-105. [DOI] [PubMed] [Google Scholar]