Abstract
The pharmacokinetics and placental transfer of acyclovir and zidovudine monotherapies and acyclovir-zidovudine combination therapy were compared in the pregnant rat. Timed-pregnancy Sprague-Dawley rats were used for the study. Doses of 60 mg of each drug/kg of body weight in monotherapy and in combination therapy were given by intravenous bolus, and samples of maternal plasma, amniotic fluid, fetal tissue, and placental tissue were collected over a period of 8 h postdose. Concentrations of each drug in the various matrices were measured by high-performance liquid chromatography. All data were analyzed by using WinNonlin. A one-compartment model with first-order elimination was used to fit the AZT plasma data from the combination therapy rats, but the plasma data from the other groups were fit to a two-compartment model. Tissue data were analyzed by noncompartmental analysis to generate area-under-the-concentration-time-curve values. Implementation of the combination therapy altered the pharmacokinetics of each drug compared to its monotherapy pharmacokinetics. The combination of these two drugs may potentiate fetal and amniotic fluid exposures to each drug.
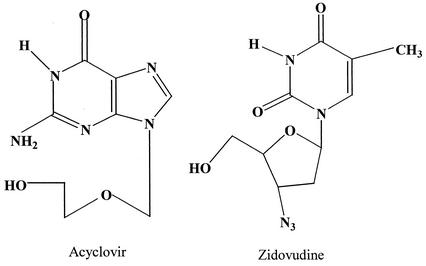
Acyclovir (9-[{2-hydroxyethoxy}-methyl]-guanosine [ACV]), an acyclic analog of the natural nucleoside 2′-deoxyguanosine (Fig. 1), is active against the members of the herpes group of DNA viruses (14, 42). For over 2 decades, ACV has been considered the first choice of treatment for herpes simplex virus types 1 and 2 (HSV-1 and -2), but it has also been shown to effectively treat varicella-zoster virus and provide protection from cytomegalovirus in immunosuppressed patients receiving transplants (12, 32). The success of ACV in treating HSV has prompted the synthesis of several structural analogs, but none has shown to be as tolerable as and have shown to have such a high therapeutic index as ACV (13, 35, 40). Zidovudine (3′-azido-3′-deoxythymidine [AZT]) (Fig. 1) is the premier reverse transcriptase inhibitor released for the treatment of human immunodeficiency virus (HIV). A therapy involving the combination of ACV and AZT is not uncommon to help suppress symptoms in patients who are both HIV positive and HSV-2 positive. These drugs, both in monotherapy and in combination, have been used to prevent vertical (mother-to-child) transmission of HSV-2 and HIV.
FIG. 1.
Structures of ACV and AZT.
The Acyclovir in Pregnancy Registry has compiled a large amount of case study information regarding the relative safety and efficacy of ACV use in HSV-2-positive pregnant women (1). Although a great deal is known about the pharmacokinetic properties of ACV, little work has been done to characterize the placental transfer of ACV in vivo, because pregnant women are routinely excluded from clinical trials. Pharmacokinetic parameters may be altered during pregnancy due to the increase in body fat content, cardiac output, and total body water seen in pregnant women (15, 41, 48). There may also be changes in plasma albumin concentration and protein binding affinities (25, 39). The perfused human placenta model has been used on occasion in attempts to characterize the placental transfer of ACV (21, 24). However, this type of model does not mimic the dynamic among fetus, amniotic fluid, and placenta that exists in the whole animal. Unlike ACV, AZT is approved by the Food and Drug Administration for use during pregnancy. To date, several groups have investigated the placental transfer of AZT monotherapy by using animal or in vitro models (20, 22, 26, 30, 38). Huang et al. developed a compartmental pharmacokinetic model for the pregnant rat that described AZT distribution in all matrices associated with pregnancy (maternal plasma, amniotic fluid, placenta, and fetal tissue) (26). The consensus concerning AZT behavior in pregnancy is that it readily crosses the placenta via passive diffusion (20, 22, 26, 38).
The pregnant rat model has been used successfully in the study of the placental transfer of many compounds, including nucleoside analogs (2-4, 9, 11, 18, 23, 26-28, 36, 37, 46). The hemodynamic changes present in the pregnant rat are similar to those seen in a human pregnancy (3, 16). The pregnant rat model is also ideal for pharmacokinetic studies because of the short gestation time and the containment of each fetus, placenta, and amniotic fluid in individual fetal sacs that allows for concurrent serial sampling of the pups.
To date, the effect of pregnancy on the placental transfer of ACV has not been investigated and limited data are available on the influence of pregnancy on the pharmacokinetics of ACV (29). Although antiviral combinations are often administered to pregnant women, the pharmacokinetic changes associated with each individual drug have not been studied under these circumstances. A study of the safety and efficacy of AZT with and without ACV found no changes in the efficacy of the drugs and no indication of renal dysfunction or hepatotoxicity associated with the drugs given in combination (10). Cooper et al. also found that, when ACV and AZT were given together, the HIV-induced cytopathic effect was increased two- to threefold over that found in AZT monotherapy (10). Mamede et al. showed that this drug combination did not lower the birth weights of rats but rather that the combination showed a protective effect against the low birth weights seen in ACV monotherapy (31). This study examines the pharmacokinetics of ACV and AZT monotherapy and ACV-AZT combination therapy during pregnancy by using the same doses as did Mamede et al. (31).
MATERIALS AND METHODS
Reagents and chemicals.
Analytical standards of ACV, ganciclovir, and AZT were obtained from Sigma (St. Louis, Mo.). 3′-Azido-2′,3′-dideoxyuridine (AZDU), one of the internal standards used, was synthesized as previously described (8). Lamivudine (3TC), also an internal standard, was recrystallized from Epivir tablets. Reagent-grade citric acid was acquired from Sigma. Reagent-grade ammonium acetate and reagent-grade octanesulfonic acid were bought from Aldrich (Milwaukee, Wis.). High-performance liquid chromatography (HPLC)-grade acetonitrile and methanol were purchased from Fisher Scientific (Fair Lawn, N.J.). Sep-Pak Vac 1-ml C18 cartridges were purchased from Waters (Milford, Mass.). The deionized water used was generated from a Continental Deionized Water System (Natick, Mass.).
Animal study.
The use of animals for this study was approved by the University of Georgia Animal Use and Care Committee and was conducted in accordance with the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The rats were housed one animal per cage in the University of Georgia College of Pharmacy Association for Assessment of Laboratory Animal Care-accredited animal facility. The living environment of the animals was controlled, with 14 h of light per day, a constant temperature of 20 to 22°C, daily feedings of standard chow, and water ad libitum.
Timed-pregnancy Sprague-Dawley rats (Harlan, Indianapolis, Ind.) with an average weight of 331 ± 35 g were used on day 19 of pregnancy for the study. The anesthesia (ketamine:acepromazine, 75:2.5 mg/kg of body weight, intramuscularly given) was given in conjunction with subcutaneous atropine (0.5 mg/kg). Subsequent doses of anesthesia were administered as needed every 2 to 3 h. Body temperature was monitored with a Cooper Instrument Corporation temperature probe (model TC 100A; Cooper, Middlefield, Conn.) and maintained with heated surgical pads and incandescent lights. Prior to dosing, a laporatomy was performed and a small incision was made in the uterine wall to allow for sampling of the pups and a cannula was surgically implanted in the right jugular vein. The blood supply to the individual fetus was tied off prior to removal to minimize bleeding. Intravenous bolus doses (60 mg/kg) of each therapy group were prepared in 0.1 M NaOH in physiological saline (pH 7.4) and were administered via the jugular cannula followed by 1 ml of physiological saline (pH 7.4) to rinse the cannula. Three dosing groups were used to complete the study: (i) ACV monotherapy (60 mg/kg) (n = 6), (ii) AZT monotherapy (60 mg/kg) (n = 3), and (iii) ACV-AZT combination therapy (60 mg/kg each) (n = 7). Pups were harvested at 5, 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420, and 480 min. Blood samples of 150 to 250 μl were collected at 2, 5, 10, 15, 20, 30, 45, 60, 90, 120, 180, 240, 300, 360, and 480 min into heparinized tubes and were centrifuged for 10 min at 16,000 × g using a Biofuge Pico Microcentrifuge (Heraeus Instruments, Hanau, Germany) to allow for collection of the plasma. Amniotic fluid samples were pulled from the fetal sacs and deposited into clean Eppendorf tubes. Placental and fetal tissues were homogenized in 2 volumes of deionized water (wt/vol) by using a Tekmar tissue grinder (model SDT-1810; Tekmar, Cincinnati, Ohio). All samples were stored at −20°C until analysis.
HPLC analysis and ACV monotherapy.
The plasma and amniotic fluid samples were prepared by acid protein precipitation by adding 10 μl (50-μl amniotic fluid sample) or 20 μl (100-μl plasma sample) of 2 M perchloric acid. Placental and fetal tissue homogenates were processed by solid-phase extraction by using Waters Sep-Pak Vac C18 SPE cartridges. The solid-phase extraction procedure included a conditioning step with methanol and the mobile phase, followed by the sample load and a wash of the sample with deionized water and finally an elution with methanol. The internal standard, ganciclovir, was also spiked into each sample to yield a final ganciclovir concentration of 10 μg/ml in the sample. Calibration curves were generated by using samples from spiked blank matrix to yield final calibration points of 0.1, 0.5, 1, 5, 10, 50, and 100 μg/ml.
The chromatographic system consisted of a Hewlett-Packard (Agilent) 1100 Series HPLC with a quaternary pump, degasser, autosampler, and variable-wavelength UV detector (Palo Alto, Calif.). Chromatographic separations were achieved by using an Agilent Eclipse XDB C-8 column (150 by 2.1 mm, 5 μm) with a Phenomenex Security Guard C18 guard column (Torrance, Calif.).
The mobile phase used for the plasma and amniotic fluid matrices was a 10 mM acetate-citrate buffer:3.7 mM aqueous octanesulfonic acid (87.5:12.5 [vol/vol]) adjusted to pH 3.08 with phosphoric acid. Under these conditions, ganciclovir eluted at ∼8 min and ACV eluted at ∼11 min. The mobile phase used for the placental and fetal tissue samples was a 30 mM acetate-citrate buffer with 5 mM octanesulfonic acid (pH 3.08) and acetonitrile (99:1 [vol/vol]). Under these conditions, ganciclovir eluted at ∼10 min and ACV eluted at ∼12 min. All flow rates were kept at a constant 0.200 ml/min, the injection volume used was 10 μl, and the detection wavelength was fixed at 254 nm. This method has been previously validated to show acceptable precision and accuracy for the quantitation of ACV in the range of 0.1 to 100 μg/ml (6).
HPLC analysis and AZT monotherapy and ACV-AZT combination therapy.
Sample preparation for plasma and all tissues is as described above. 3TC was spiked into each plasma and amniotic fluid sample (25 μg/ml) to serve as an internal standard. Because of the chromatographic interference of endogenous peaks, 3TC could not be used as an internal standard for the placental and fetal tissues and was replaced by AZDU. AZDU was spiked into each placenta and fetal tissue sample at a level of 10 μg/ml. Calibration curves were generated by using a spiked blank matrix to yield final calibration points of 0.1, 0.5, 1, 5, 10, 50, and 100 μg/ml.
The HPLC system used in this assay is the same as described above. Because of the relative differences in the polarities of ACV and AZT, a gradient elution technique had to be utilized for timely analysis. The mobile phase consisted of a 30 mM acetate-citrate buffer at pH 3.08 (component A) and methanol (component B). Under these conditions, ACV eluted at 7.6 min, AZT at 15.9 min, and 3TC at 10.9 min in the plasma and amniotic fluid. In the fetus and placental tissues, ACV eluted at 7.4 min, AZT at 19.8 min, and AZDU at 18.4 min. This assay was validated to ensure both precision and accuracy in accordance with the Food and Drug Administration guidelines for bioanalytical method validation (43). The assay showed acceptable reproducibility (per cent relative standard deviation < 15%) and accuracy (per cent error < 15%) over the calibration range of 0.1 to 100 μg/ml (7).
Data analysis.
When WinNonlin was used, the plasma data from all rats were subjected to compartmental analysis. A two-compartment intravenous bolus model with first-order elimination was used to fit the plasma data generated from AZT monotherapy-dosed rats and ACV for both monotherapy and combination therapy animals. However, the plasma distribution phase for AZT in the combination therapy animals was not observed; therefore, a one-compartment intravenous bolus model with first-order elimination was used to fit the AZT plasma data from the combination therapy animals. A 1/y weighting scheme was used throughout the analysis. Amniotic fluid, fetus, and placenta were subjected to noncompartmental analysis, and the area under the concentration-time curve (AUC) was truncated at 8 h due to the inability of calculate an accurate elimination half-life for all tissues and amniotic fluid. To express relative exposure to each matrix, the AUC values for the individual tissues were compared to the AUC (0 to 8 h) values for the corresponding plasma data. The pharmacokinetic parameters generated for each dosing group and the relative exposure numbers were compared by using the unpaired t test (P < 0.05) to detect statistically significant differences.
RESULTS AND DISCUSSION
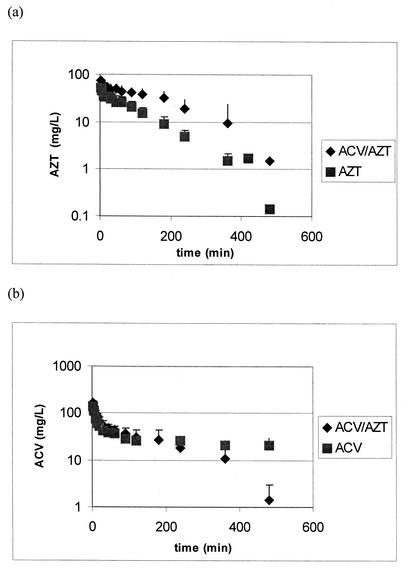
The fitted concentration in plasma-time profiles from a representative animal for AZT and ACV are shown in Fig. 2. The pharmacokinetic parameters generated from the compartmental analysis of the plasma data are presented in Table 1. Coadministration of ACV resulted in a 60% decrease in total clearance of AZT. Considering that renal excretion is the major route of elimination for both drugs in rats and that ACV and AZT are both transported by the organic anion transporter, this decrease in clearance is probably due to the inhibition of active tubular secretion in the kidney (35, 37). A significant increase in both half-life and AUC for AZT was also seen when ACV is coadministered. These differences result from the decrease in AZT clearance. No statistically significant changes can be noted in the volume of distribution or maximum concentration of drug in serum (Cmax) of AZT when given in the combination therapy.
FIG. 2.
Concentration in plasma (mean plus standard deviation) versus time profiles for AZT alone and in combination with ACV (a) and ACV alone and in combination with AZT (b).
TABLE 1.
Pharmacokinetic parameters (mean plus or minus standard deviation) generated from the compartmental analysis of plasma data collected from pregnant ACV monotherapy, AZT monotherapy, and ACV-AZT combination therapy rats (60 mg/kg)a
| Drug therapy | Half-life (h) | AUC (h · mg/liter) | Clearance (liter/h · kg) | Vss (liter/kg) | Cmax (mg/liter) |
|---|---|---|---|---|---|
| ACV | 9.12 ± 1.1 | 467 ± 183 | 0.14 ± 0.05 | 1.61 ± 0.26 | 148.4 ± 88 |
| ACV-AZT | 2.48 ± 1.9* | 241 ± 165* | 0.34 ± 0.2* | 0.80 ± 0.1* | 196.7 ± 27 |
| AZT | 1.39 ± 0.3 | 80 ± 21 | 0.78 ± 0.2 | 1.52 ± 0.42 | 72.1 ± 14 |
| AZT-ACV | 2.69 ± 1.2* | 240 ± 130 | 0.31 ± 0.2* | 1.02 ± 0.2 | 59.9 ± 10 |
* indicates a significant difference between monotherapy and combination therapy (P < 0.05). Vss, volume of distribution at steady state.
The decrease in the clearance of AZT in the combination therapy group is coupled with a 60% increase in ACV clearance. Also noted is a 50% decrease in the volume of distribution of ACV when administered with AZT. It is unlikely that this can be attributed to a change in plasma protein binding, for ACV inherently has a low affinity for plasma protein binding sites (4.4 to 15.4% bound) (33). An increase in uptake of ACV by the fetus in the combination therapy may help explain this change in volume. There is a threefold increase in the amount of ACV (expressed as a percentage of dose) taken up into the fetal compartment when ACV is coadministered with AZT. The decrease in half-life of ACV in the combination therapy rats results from a decrease in volume of distribution and an increase in clearance.
Duplicate and triplicate pups were sampled at the same time point from individual pregnant rats throughout the study to ensure that each fetal sac (placenta, fetus, and amniotic fluid) had similar concentrations at any given time. No corrections were made for metabolic differences between male and female pups; however, this was not of great concern considering that neither ACV nor AZT is extensively metabolized in the rat. Low coefficients of variation were observed among fetal sacs removed at the same time point in individual dams (7.4% in fetal tissue, 6.8% in placenta, and 4.3% in amniotic fluid), indicating good reliability for this data.
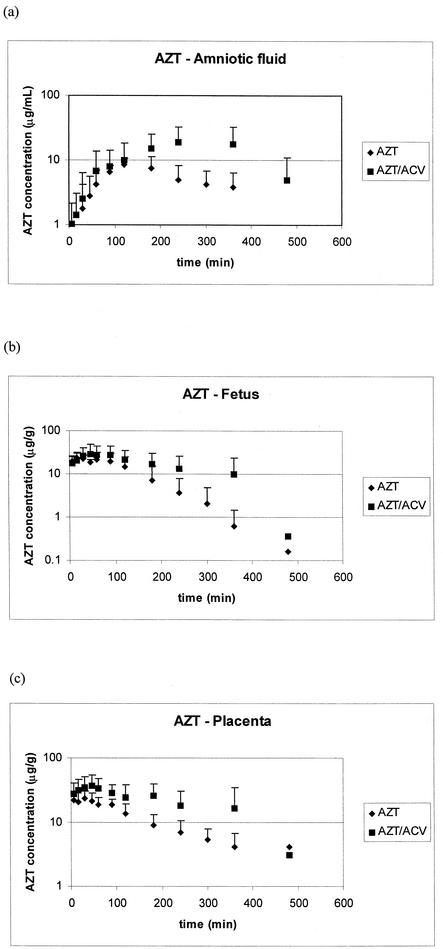
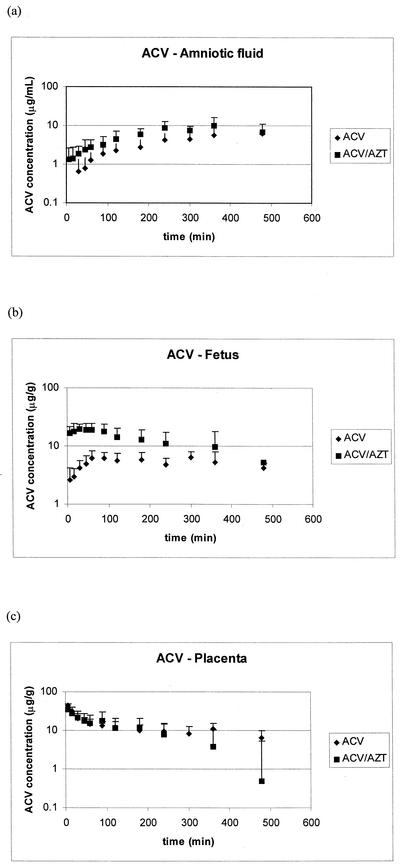
The concentration-time profiles of the two drugs in amniotic fluid, placenta, and fetus are shown in Fig. 3 (AZT) and 4 (ACV), and the pharmacokinetic parameters for these matrices generated from noncompartmental analysis are tabulated in Tables 2, 3, and 4. In the rats receiving AZT alone or in combination with ACV, the initial rate of uptake in amniotic fluid and fetus is similar; however, Cmax, the time to maximum concentration of drug in serum (Tmax), and the AUC are higher in these tissues with the animals receiving combination therapy. A similar pattern can be seen in the placental tissue of the AZT group: a longer Tmax, a higher AZT Cmax, and a larger AUC are seen in the combination therapy group. For ACV (Fig. 4), no significant differences are noted in the placental concentration versus time profiles. However, both amniotic fluid and fetal exposures to ACV are much lower when ACV is given alone. An increase in the Cmaxs of ACV in the amniotic fluid (threefold) and the fetus (twofold) is demonstrated in the combination therapy animals. This is coupled with a shorter Tmax in both of these tissues due to a higher rate of uptake of ACV for the combination therapy group. An increase in the overall exposure of the fetal compartment to ACV in the ACV-AZT-dosed group is indicated by the twofold increase in AUC in both amniotic fluid and fetal tissue.
FIG. 3.
Concentration (mean plus standard deviation) versus time profiles of AZT monotherapy and in combination with ACV from amniotic fluid (a), fetus (b), and placenta (c).
TABLE 2.
Pharmacokinetic parameters for amniotic fluid generated using noncompartmental analysisa
| Drug therapy | Cmax (mg/liter) | Tmax (h) | AUC (h · mg/liter) |
|---|---|---|---|
| ACV | 4.99 ± 2.7 | 6.20 ± 2.5 | 18.6 ± 9.3 |
| ACV-AZT | 10.5 ± 4.4 | 4.00 ± 1.2 | 39.6 ± 16 |
| AZT | 8.57 ± 2.0 | 2.33 ± 0.58 | 35.7 ± 18 |
| AZT-ACV | 19.6 ± 13 | 3.67 ± 0.52* | 74.1 ± 48 |
* indicates a significant difference between monotherapy and combination therapy (P < 0.05).
TABLE 3.
Pharmacokinetic parameters for placenta generated by using noncompartmental analysis
| Drug therapy | Cmax (μg/g) | Tmax (h) | AUC (h · μg/g) |
|---|---|---|---|
| ACV | 34.6 ± 16 | 0.26 ± 0.3 | 82.0 ± 42 |
| ACV-AZT | 38.5 ± 19 | 0.12 ± 0.07 | 81.9 ± 62 |
| AZT | 24.2 ± 8.5 | 0.36 ± 0.3 | 72.9 ± 23 |
| AZT-ACV | 40.8 ± 17 | 0.68 ± 0.5 | 139 ± 62 |
TABLE 4.
Pharmacokinetic parameters for fetus generated using noncompartmental analysisa
| Drug therapy | Cmax (μg/g) | Tmax (h) | AUC (h · μg/g) |
|---|---|---|---|
| ACV | 7.89 ± 1.4 | 2.50 ± 2.0 | 37.4 ± 10 |
| ACV-AZT | 24.5 ± 4.7* | 0.65 ± 0.3 | 88.1 ± 27 |
| AZT | 28.9 ± 5.3 | 0.50 ± 0.4 | 59.4 ± 32 |
| AZT-ACV | 32.7 ± 15 | 1.2 ± 0.5 | 108 ± 64 |
* indicates a significant difference between monotherapy and combination therapy (P < 0.05).
FIG. 4.
The concentration (mean plus standard deviation) versus time profile of ACV monotherapy and in combination with AZT from amniotic fluid (a), fetus (b), and placenta (c).
Relative exposure numbers are shown in Table 5. This table shows the ratios of extrapolated AUC values for each tissue to plasma AUC. For AZT, decreases in exposure to all three tissues were seen in the presence of ACV (16 to 24% decrease). This decrease suggests saturation of uptake into the fetal compartment (placenta, amniotic fluid, and fetus). On the other hand, ACV showed a threefold increase in drug exposure in amniotic fluid and fetal tissue with the combination therapy. No change in placental exposure was seen for ACV in the two therapy groups. Previous reports indicate that ACV accumulates in the amniotic fluid (17, 29). Although this may not be obvious from the concentration-versus-time profiles of ACV, the accumulation of ACV in the amniotic fluid is apparent from the prolonged half-life in this tissue (5.93 ± 3.9 h) compared to that in plasma.
TABLE 5.
Relative exposure (AUCtissue/AUCplasma) data (mean plus or minus standard deviation) from amniotic fluid, fetal tissue, and placental tissue generated from the noncompartmental analysis of tissue data collected from pregnant ACV monotherapy, AZT monotherapy, and ACV-AZT combination therapy rats (60 mg/kg)a
| Tissue | AUCtissue/AUCplasma
|
|||
|---|---|---|---|---|
| ACV | ACV-AZT | AZT | AZT-ACV | |
| Fetus | 0.20 ± 0.05 | 0.56 ± 0.2* | 0.72 ± 0.2 | 0.59 ± 0.2 |
| Placenta | 0.42 ± 0.1 | 0.43 ± 0.3 | 1.00 ± 0.5 | 0.76 ± 0.2 |
| Amniotic fluid | 0.091 ± 0.02 | 0.26 ± 0.1* | 0.48 ± 0.3 | 0.40 ± 0.2 |
* indicates a significant difference between monotherapy and combination therapy.
The disposition of AZT and ACV in the pregnant rat was significantly altered when the two drugs were coadministered. The changes noted in the placenta and fetus suggest that transporters, in addition to passive diffusion, play a role in the uptake of both ACV and AZT in these tissues. Nucleoside, organic cation, and organic anion transporters could possibly contribute to the placental transfer of these two compounds (19, 45, 47). Interestingly, the uptake of ACV into the placenta was not affected by coadministration of AZT. However, the fetal and amniotic fluid ACV exposure was increased approximately threefold. The increase in plasma clearance coupled with an increase in fetal uptake suggests up-regulation of a transport process when ACV and AZT are coadministered.
The driving force behind the “protective effect” of AZT against ACV fetal toxicity proposed by Mamede et al. is not apparent from this study (31). The exposures to fetus and amniotic fluid of ACV are increased dramatically in the presence of AZT. However, this is not of extreme concern, considering that ACV has been shown to exhibit no detrimental effects on the fetus when used during pregnancy (1, 5, 17, 29, 34, 44). The theory that ACV potentiates the actions of AZT when given in combination is supported by this data. The significant increase in half-life and AUC coupled with the 60% decrease in clearance, which resulted in higher AZT concentrations observed in both the mother and the fetus, could support the observation of an increased activity of AZT when given in this combination. However, caution should be taken with direct extrapolation of these results to humans until further investigations have been conducted with in vitro human placentas or primates.
Both ACV and AZT are known to be safe and effective against protecting unborn children against their respective viruses. This combination of drugs could allow for a potentiated activity of AZT while increasing the exposure of the fetus to ACV. Although the pharmacokinetics of each drug is altered in the combinations, therapeutic levels in plasma of each can be maintained when they are given together. However, the higher levels achieved in the fetus and dam could also increase the toxicities associated with these agents.
Acknowledgments
We acknowledge the assistance of Warren Beach and Valeria Coscia for their help in extracting and recrystallizing some of the drugs used in this study from their respective pharmaceutical formulations.
REFERENCES
- 1.Andrews, E. B., B. C. Yankaskas, J. F. Cordero, K. Schoeffler, and S. Hampp. 1992. Acyclovir in pregnancy registry: six years' experience. Obstet. Gynecol. 79:7-13. [PubMed] [Google Scholar]
- 2.Beachy, J. C., and L. E. Weisman. 1993. Acute asphyxia affects neutrophil number and function in the rat. Crit. Care Med. 21:1929-1934. [DOI] [PubMed] [Google Scholar]
- 3.Boike, G. M., G. Deppe, J. D. Young, N. L. Gove, S. F. Bottoms, J. M. Malone, Jr., V. K. Malviya, and R. J. Sokol. 1989. Chemotherapy in a pregnant rat model. 1. Mitomycin-C: pregnancy-specific kinetics and placental transfer. Gynecol. Oncol. 34:187-190. [DOI] [PubMed] [Google Scholar]
- 4.Boike, G. M., G. Deppe, J. D. Young, J. M. Malone, Jr., V. K. Malviya, and R. J. Sokol. 1989. Chemotherapy in a pregnant rat model. 2. 5-Fluorouracil: nonlinear kinetics and placental transfer. Gynecol. Oncol. 34:191-194. [DOI] [PubMed] [Google Scholar]
- 5.Broussard, R. C., D. K. Payne, and R. B. George. 1991. Treatment with acyclovir of varicella pneumonia in pregnancy. Chest 99:1045-1047. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S. D., C. A. White, C. K. Chu, and M. G. Bartlett. 2002. Determination of acyclovir in maternal plasma, amniotic fluid, fetal and placental tissues by high-performance liquid chromatography. J. Chromatogr. B. 772:327-334. [DOI] [PubMed] [Google Scholar]
- 7.Brown, S. D., C. A. White, and M. G. Bartlett. 2002. HPLC determination of Acyclovir and Zidovudine in maternal plasma, amniotic fluid, fetal and placental tissues using ultra-violet detection. J. Liq. Chromatogr. Relat. Technologies 25:2855-2869. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y., J. G. Bauman, and C. K. Chu. 1992. Practical synthesis of AZT and AZDU from xylose-efficient deoxygenation via nucleoside 2′-xanthates. Nucleosides Nucleotides 11:693. [Google Scholar]
- 9.Clark, T. N., C. A. White, C. K. Chu, and M. G. Bartlett. 2001. Determination of 3′-azido-2′,3′-dideoxyuridine in maternal plasma, amniotic fluid, fetal and placental tissues by high-performance liquid chromatography. J. Chromatogr. B 755:165-172. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, D. A., C. Pederson, F. Aiuti, J. L. Vilde, M. Ruhnke, P. O. Pehrson, N. Clumeck, C. Farthing, R. Luthy, R. R. Doherty, and P. A. Cload. 1991. The efficacy and safety of zidovudine with or without acyclovir in the treatment of patients with AIDS-related complex. AIDS 5:933-943. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, B. V., P. D. Johnson, S. J. Goldberg, and J. B. Ulreich. 1990. Cardiac teratogenesis of trichloroethylene and dichloroethylene in a mammalian model. J. Am. Coll. Cardiol. 16:1304-1309. [DOI] [PubMed] [Google Scholar]
- 12.De Clercq, E. 1993. Antivirals for the treatment of herpesvirus infections. J. Antimicrob. Chemother. 32:121-132. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq, E. 1995. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev. Med. Virol. 5:149-164. [Google Scholar]
- 14.Elion, G. B., P. A. Furman, J. A. Fyfe, P. de Miranda, L. Beauchamp, and H. J. Schaeffer. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA 74:5716-5720.202961 [Google Scholar]
- 15.Elkayam, U., and N. Gleicher. (ed.). 1982. Cardiac problems in pregnancy. Alan R. Liss, New York, N.Y.
- 16.Faber, J. J., and K. L. Thornburg. 1983. Placental physiology: structure and function of fetomaternal exchange. Raven, New York, N.Y.
- 17.Frenkel, L. M., Z. A. Brown, Y. J. Bryson, L. Corey, J. D. Unadkat, P. A. Hansleigh, A. M. Arvin, C. G. Prober, and J. D. Conner. 1991. Pharmacokinetics of acyclovir in the term human pregnancy and neonate. Am. J. Obstet. Gynecol. 164:569-576. [DOI] [PubMed] [Google Scholar]
- 18.Fujinaga, M., J. M. Baden, A. Suto, J. K. Myatt, and R. I. Mazze. 1991. Preventative effects of phenoxybenzamine on nitrous oxide-induced reproductive toxicity in Sprague-Dawley rats. Teratology 43:151-157. [DOI] [PubMed] [Google Scholar]
- 19.Ganapathy, V., P. D. Prasad, M. E. Ganapathy, and F. H. Leibach. 2000. Placental transporters relevant to drug distribution across the maternal-fetal interface. J. Pharmacol. Exp. Ther. 294:413-420. [PubMed] [Google Scholar]
- 20.Garland, M., H. H. Szeto, S. S. Daniel, P. J. Trooper, M. M. Myers, and R. I. Stark. 1998. Placental transfer and fetal metabolism of zidovudine in the baboon. Pediatr. Res. 44:47-53. [DOI] [PubMed] [Google Scholar]
- 21.Gilstrap, L. C., R. E. Bawdon, S. W. Roberts, and S. Sobhi. 1994. The transfer of the nucleoside analog acyclovir across the perfused human placenta. Am. J. Obstet. Gynecol. 170:967-973. [DOI] [PubMed] [Google Scholar]
- 22.Hankins, G. D. V., C. L. Lowery, R. T. Scott, W. R. Morrow, K. D. Carey, M. M. Leland, and E. V. Colvin. 1990. Transplacental transfer of zidovudine in the near-term pregnant baboon. Am. J. Obstet. Gynecol. 163:728-732. [DOI] [PubMed] [Google Scholar]
- 23.Heffez, D. S., J. Aryanpur, G. M. Hutchins, and J. M. Freeman. 1990. The paralysis associated with myleomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery 26:987-992. [PubMed] [Google Scholar]
- 24.Henderson, G. I., Z. Hu, R. F. Johnson, A. B. Perez, Y. Yang, and S. Schenker. 1992. Acyclovir transport by the human placenta. J. Lab. Clin. Med. 120:885-892. [PubMed] [Google Scholar]
- 25.Herngren, L., M. Ehrnebo, and L. O. Boreus. 1983. Drug binding to plasma proteins during human pregnancy and in the perinatal period. Studies on cloxacillin and alprenolol. Dev. Pharmacol. Ther. 6:110-124. [DOI] [PubMed] [Google Scholar]
- 26.Huang, C. S.-H., F. D. Boudinot, and S. Feldman. 1996. Maternal-fetal pharmacokinetics of zidovudine in rats. J. Pharm. Sci. 85:965-970. [DOI] [PubMed] [Google Scholar]
- 27.Huang, S. H., R. P. Remmel, and C. L. Zimmerman. 1991. The bioavailability and nonlinear clearance of (-)-carbovir in the rat. Pharm. Res. 8:739-743. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim, S. S., and F. D. Boudinot. 1989. Pharmacokinetics of 2′,3′-dideoxycytidine in rats: application to interspecies scale-up. J. Pharm. Pharmacol. 41:829-834. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin, D. F., S. Weller, R. J. Whitley, W. W. Andrews, J. C. Hauth, F. Lakeman, and G. Miller. 1998. Pharmacokinetics of oral valacyclovir and acyclovir in late pregnancy. Am. J. Obstet. Gynecol. 179:846-851. [DOI] [PubMed] [Google Scholar]
- 30.Little, B. B., R. E. Bawdon, J. T. Christmas, S. Sobhi, and L. C. Gilstrap. 1989. Pharmacokinetics of azidothymidine during late pregnancy in Long-Evans rats. Am. J. Obstet. Gynecol. 161:732-734. [DOI] [PubMed] [Google Scholar]
- 31.Mamede, J. A., M. D. J. Simoes, N. F. Nono, Y. Juliano, R. M. Oliveira-Fihlo, and L. Kulay. 1995. Chronic effects of azidothymidine and acyclovir on pregnant rats. Gen. Pharmacol. 26:523-526. [DOI] [PubMed] [Google Scholar]
- 32.Markham, A., and D. Faulds. 1994. Ganciclovir. An update of its therapeutic use in cytomegalovirus infection. Drugs 48:455-484. [DOI] [PubMed] [Google Scholar]
- 33.Miranda, P., S. S. Good, O. L. Laskin, H. C. Krasny, J. D. Conner, and P. S. Lietman. 1981. Disposition of intravenous radioactive acyclovir. Clin. Pharmacol. Ther. 30:662-672. [DOI] [PubMed] [Google Scholar]
- 34.Moore, H. L., G. M. Szczech, D. E. Rodwell, R. W. Kapp, P. de Miranda, and W. E. Tucker. 1983. Preclinical toxicology studies with acyclovir: teratologic, reproductive and neonatal tests. Fundam. Appl. Toxicol. 3:560-568. [PubMed] [Google Scholar]
- 35.O'Brien, J. J., and D. M. Campoli-Richards. 1998. Acyclovir: an updated review of its antiviral activity, pharmacokinetic properties, and therapeutic efficacy. Drugs 37:233-309. [DOI] [PubMed] [Google Scholar]
- 36.Ostrea, E. M., Jr., A. Romero, D. K. Knapp, A. R. Ostrea, J. E. Lucena, and R. B. Utarnachitt. 1994. Postmortem drug analysis of meconium in early-gestation human fetuses exposed to cocaine: clinical implications. J. Pediatr. 124:477-479. [DOI] [PubMed] [Google Scholar]
- 37.Patel, B. A., C. K. Chu, and F. D. Boudinot. 1989. Pharmacokinetics and saturable renal tubular secretion of zidovudine in rats. J. Pharm. Sci. 78:530-534. [DOI] [PubMed] [Google Scholar]
- 38.Patterson, T. A., Z. K. Binienda, G. W. Lipe, M. P. Gillam, W. Slikker, Jr., and J. A. Sandberg. 1997. Transplacental pharmacokinetics and fetal distribution of azidothymidine, its glucuronide, and phosphorylated metabolites in late-term rhesus macaques after maternal infusion. Drug Metab. Dispos. 25:453-459. [PubMed] [Google Scholar]
- 39.Perucca, E., and A. Crema. 1982. Plasma-protein binding of drugs in pregnancy. Clin. Pharmacokinet. 7:336-352. [DOI] [PubMed] [Google Scholar]
- 40.Posner, T. N. 1998. Herpes simplex. Routledge, New York, N.Y.
- 41.Ritschel, W. A., and G. L. Kerns. 1999. Drug disposition in pregnancy, p. 342-348. In J. I. Graubart (ed.), Handbook of basic pharmacokinetics, 5th ed. American Pharmaceutical Association, Washington, D.C.
- 42.Schaeffer, H. J., L. Beauchamp, P. de Miranda, G. B. Elion, D. J. Bauer, and P. Collins. 1978. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 272:583-585. [DOI] [PubMed] [Google Scholar]
- 43.Shah, V. P., K. K. Midha, S. Dighe, I. J. McGiveray, J. P. Skelly, A. Yacobi, T. Layloff, C. T. Vishwanathan, C. E. Cook, R. D. McDowall, K. A. Pittman, and S. Spector. 1992. Analytical method validation—bioavailability, bioequivalence and pharmacokinetic studies. Pharm. Res. 9:588-592. [DOI] [PubMed] [Google Scholar]
- 44.Spangler, J. G., J. K. Kirk, and M. P. Knudson. 1994. Uses and safety of acyclovir in pregnancy. J. Fam. Pract. 38:186-191. [PubMed] [Google Scholar]
- 45.Takeda, M., S. Khamdang, S. Narikawa, H. Kimura, Y. Kobayashi, T. Yamamoto, S. Cha, T. Sekine, and H. Endou. 2002. Human organic anion transporters and human organic cation transporters mediate renal antiviral transport. J. Pharmacol. Exp. Ther. 300:918-924. [DOI] [PubMed] [Google Scholar]
- 46.Unadkat, J. D., J. P. Wang, D. Pulham, and R. L. O. Semmes. 1989. Dose-ranging pharmacokinetics of zidovudine (azidothymidine) in the rat. Pharm. Res. 6:734-736. [DOI] [PubMed] [Google Scholar]
- 47.Wada, S., M. Tsuda, T. Sekine, S. H. Cha, M. Kimura, Y. Kanai, and H. Endou. 2000. Rat multispecific organic anion transporter 1(rOAT1) transports zidovudine:acyclovir, and other antiviral nucleoside analogs. J. Pharmacol. Exp. Ther. 294:844-849. [PubMed] [Google Scholar]
- 48.Ward, R. M. 1989. Maternal-placental-fetal unit: unique problems of pharmacologic study. Pediatr. Clin. N. Am. 36:1075-1088. [DOI] [PubMed] [Google Scholar]