Abstract
The phenothiazines chlorpromazine (CPZ) and thioridazine (TZ) have equal in vitro activities against antibiotic-sensitive and -resistant Mycobacterium tuberculosis. These compounds have not been used as anti-M. tuberculosis agents because their in vitro activities take place at concentrations which are beyond those that are clinically achievable. In addition, chronic administration of CPZ produces frequent severe side effects. Because CPZ has been shown to enhance the killing of intracellular M. tuberculosis at concentrations in the medium that are clinically relevant, we have investigated whether TZ, a phenothiazine whose negative side effects are less frequent and serious than those associated with CPZ, kills M. tuberculosis organisms that have been phagocytosed by human macrophages, which have nominal killing activities against these bacteria. Both CPZ and TZ killed intracellular antibiotic-sensitive and -resistant M. tuberculosis organisms when they were used at concentrations in the medium well below those present in the plasma of patients treated with these agents. These concentrations in vitro were not toxic to the macrophage, nor did they affect in vitro cellular immune processes. TZ thus appears to be a serious candidate for the management of a freshly diagnosed infection of pulmonary tuberculosis or as an adjunct to conventional antituberculosis therapy if the patient originates from an area known to have a high prevalence of multidrug-resistant M. tuberculosis isolates. Nevertheless, we must await the outcomes of clinical trials to determine whether TZ itself may be safely and effectively used as an antituberculosis agent.
The in vitro activities of phenothiazines against Mycobacterium tuberculosis have been known for many decades (28, 29, 31). Since the introduction of the phenothiazine chlorpromazine (CPZ) in 1953 by Rhône-Poulenc (9), sporadic reports have indicated that CPZ has in vitro activity similar to those of other phenothiazines in vivo (15, 30, 24). Nevertheless, CPZ has not been considered for use in the management of pulmonary tuberculosis due to the severe side effects associated with its chronic use (25, 26) as well as the fact that the in vitro activity of this compound takes place at concentrations that are well beyond those that can be safely achieved in patients (3, 27, 28, 29, 31). However, in 1992, Crowle et al. (10) demonstrated that CPZ could inhibit the growth of M. tuberculosis that had been phagocytosed by human macrophages when CPZ was present in the medium at concentrations ranging from 0.23 to 3.6 mg/liter.
The ability of the macrophage to concentrate CPZ had previously been established (20, 23), and Crowle et al. (10) postulated that the intracellular killing activity of CPZ is due to this concentrating ability of the macrophage. At that time (the early 1990s) the problem of tuberculosis (TB) was not significant in most Western countries. Therefore, CPZ, which produces serious side effects, was not considered for use in the management of TB.
In the early 1990s, New York City became aware that its annual case load of TB had increased dramatically (13). Moreover, the increase was accompanied by a large number of M. tuberculosis isolates resistant to one or more commonly used antibiotics (13). Soon thereafter, similar observations were made in the major urban centers of Europe (7, 34). A 1997 World Health Organization report (38) demonstrated the worldwide frequency of both pulmonary TB and the antibiotic resistance of the causative organism, M. tuberculosis. By that time TB was seen as endemic in many parts of the world and epidemic in still others.
The antibiotic resistance of M. tuberculosis is attributed to many factors and will not be discussed here. Because the rates of resistance of M. tuberculosis to both isoniazid (INH) and rifampin (RIF) (multidrug resistance [MDR]), the two most effective anti-TB agents, were increasing at alarming rates, the American Thoracic Society and the Centers for Disease Control and Prevention issued guidelines for the therapy of newly diagnosed pulmonary TB (6). Nevertheless, in areas of the world such as Portugal, where the rate of resistance of M. tuberculosis to as many as four of the frontline drugs used to treat pulmonary TB exceeds 5% (37), the guidelines were not as effective as they proved to be in the United States (16). The need for new and effective anti-TB drugs is recognized (35), and it is this need which has spurred our interest in pursuing the question of whether another less toxic phenothiazine may be exploited as an anti-TB agent.
Thioridazine (TZ), a sister phenothiazine of CPZ used for the treatment of psychosis, is equal to CPZ with respect to its in vitro antimycobacterial properties (1, 4, 8, 37). Because this compound is concentrated by human macrophages (10, 20, 23) and is active against intracellular Staphylococcus aureus, regardless of its susceptibility or resistance to methicillin (32), we have repeated the latter studies with M. tuberculosis in place of staphylococci. The results of this study provide strong direct evidence that TZ, a neuroleptic that is milder and less toxic than CPZ, kills intracellular M. tuberculosis isolates that are resistant to two or more antibiotics when the concentration of TZ in the medium approaches that in the plasma of a patient chronically managed with this compound.
MATERIALS AND METHODS
Materials.
Balanced salt solution, phosphate-buffered saline, Hanks' balanced salt solution, RPMI medium, Ficoll, and l-glutamine were purchased from Gibco, Paisley, United Kingdom. CPZ, TZ, paraformaldehyde, sodium dodecyl sulfate (SDS), Triton X-100, sodium azide, naphthol blue-black, and trypan blue were purchased from Sigma Aldrich Quimica SA, Madrid, Spain. Middlebrook 7H11 solid medium was purchased from Difco, Detroit, Mich. The materials and equipment used for antibiotic susceptibility testing of M. tuberculosis with the BACTEC 460-TB system were purchased from Becton Dickinson Diagnostic Instrument Systems, Sparks, Md., and prepared according to the recommendations of the manufacturer. Microwell tissue culture plates were purchased from Nalgene, Rochester, N.Y. All phenothiazine solutions were prepared in distilled, sterile water on the day of the experiment.
Bacterial strains.
M. tuberculosis H37Rv (ATCC 27294), which is susceptible to RIF, INH, streptomycin (SM), and ethambutol (EMB), served as the absolute control; and five clinical strains (strains A to E) that have been maintained in the Unit of Mycobacteriology, Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa, Lisbon, Portugal, and characterized for their susceptibility and resistance to the antibiotics listed above with the BACTEC 460-TB system were also used (1, 8, 36). Strains A and B are susceptible to all four drugs. Strain C is resistant to all four drugs. Strains D and E, besides being resistant to INH and RIF, are also resistant to SM. Strain D was isolated from a patient involved in an outbreak caused by a highly virulent MDR M. tuberculosis strain (cluster A or cluster Lisbon) in a Lisbon Prison Hospital in 1997 and has been typed by IS6110-based restriction fragment length polymorphism analysis as a member of this cluster (33).
Methods. (i) Determination of MICs and MBCs.
The MICs of CPZ and TZ were determined individually by the BACTEC 460-TB method as described previously (1, 8, 36). Briefly, carefully prepared stock solutions containing various amounts of each compound were separately prepared in 12B BACTEC 460 medium, and 0.1-ml aliquots of each were added to 12B BACTEC 460 vials. The final concentrations of CPZ and TZ in the BACTEC 460 vials were 0, 1, 5, 10, 15, 20, 30, 40, 50, and 60 mg/liter. Each vial received 0.1 ml of 12B BACTEC 460 medium containing an adjusted concentration of mycobacteria corresponding to approximately 105 to 106 CFU of organisms identified as M. tuberculosis by rRNA-DNA probe hybridization (AccuProbe; Gen-Probe-BioMerieux, Lyon, France) (36). The minimum bactericidal concentrations (MBCs) of CPZ and TZ were determined by extending the MIC curves well beyond each MIC, and 10-μl aliquots of the BACTEC 12B cultures at zero time and those after 30 days that showed no evidence of growth in the BACTEC 12B vials were subjected to determination of the numbers of CFU (36). The MIC and MBC determinations were repeated three times, and the values obtained did not differ significantly.
(ii) Macrophage cell line THP-1 and isolation of human PBMDMs.
The THP-1 macrophage cell line was kindly provided in 1998 by Hazel Dockrell, London School of Hygiene and Tropical Medicine, London, United Kingdom, and has been maintained in the Unit of Mycobacteriology, Instituto de Higiene e Medicina Tropical, since that time. Human peripheral blood monocyte-derived macrophages (PBMDMs) from 50 ml of whole blood donated by healthy subjects were isolated by the Ficoll sedimentation method described previously (32).
(iii) Short-term cultures.
The THP-1 macrophage cell line or PBMDM cell cultures containing 1.0 × 105 cells/ml were added in triplicate to 24- or 96-well microplates, the plates were incubated at 37°C with 5% CO2 for 5 days, and the wells were washed with Hanks' balanced salt solution for removal of nonadherent cells (32). Adherent cells were removed for counting with 1.0 ml of 0.01% SDS, and the numbers of adherent cells in triplicate wells did not vary by more than 3%.
(iv) Toxic activity of TZ against THP-1 and PBMDMs.
Because CPZ has been shown to have toxicity against macrophages (17) as well as to inhibit phagocytic processes (14), CPZ and TZ at concentrations that ranged from 0.001 to 0.5 mg/liter were tested for these in vitro activities against THP-1 and PBMDMs by the naphthol blue-black and trypan blue methods and the annexin V-binding method with flow cytometry. Processing for the annexin V-binding method was as described in the instructions accompanying the annexin V-fluorescein isothiocyanate kit (Research and Development Systems, Abingdon, United Kingdom), and the results were analyzed with a Cytron absolute flow cytometer (Ortho-Clinical Diagnostics, Raritan, N.J.) (32).
(v) Effects of CPZ and TZ on percent annexin V binding by subpopulations of cells present in Ficoll preparations of whole blood (PBMCs) from healthy donors.
Mononuclear cells (peripheral blood monocyte cells [PBMCs]) present in the Ficoll preparation were suspended in medium to yield a concentration of 106 cells/ml, and aliquots of 0.1 ml were transferred to microplate wells.
A total of 0.01 ml of freshly made stock solutions of CPZ or TZ with final concentrations that ranged from 0 to 5.0 mg/liter were added to the wells; other wells received 0.01 ml of approximately 104 live M. tuberculosis organisms (H37Rv [ATCC 27294]). The microplates were incubated at 37°C for 3 days, and the cells were harvested and stained with fluorescent antibodies against alpha-beta T-cell receptor-positive (TCR+), gamma-delta TCR+, and CD14+ macrophages in combination with annexin V. The cells were analyzed with an Ortho absolute flow cytometer. The results are expressed as the mean ± standard deviation percent annexin V binding in lymphocytes. Annexin V binding in excess of 10% is an indication of early apoptosis (32). Previously obtained results demonstrated that either CPZ or TZ produces some toxicity against macrophages derived from monocyte sources when the drug concentrations exceed 0.5 mg/liter (32). These experiments were conducted four separate times, and each experiment involved cells obtained from whole blood from different donors.
(vi) Phagocytosis and killing activities of M. tuberculosis strains by THP-1 and PBMDMs.
After 18 h of culture of PBMDMs (1.0 × 106 cells/ml) in RPMI medium containing 2 mM l-glutamine and autologous human serum, approximately 10% of the PBMDMs that adhere to the bottom of the wells are monocytes (32). A bacterial suspension of 1.0 × 105 cells in 0.010 ml, which had previously been found to result in optimum phagocytosis (11), was added to monolayer cultures of monocyte-derived macrophages or THP-1 macrophage cell lines, and the mixtures were incubated at 37°C for 30 min. Two consecutive washes with RPMI removed the extracellular bacteria. The washings were then pooled and subjected to counting of the numbers of CFU in order to determine the efficiency of phagocytosis. A third wash was performed, and the washings were subjected to counting of the numbers of CFU for verification of the complete absence of nonphagocytosed bacteria. The cultures of adhered cells-phagocytosed bacteria were incubated at 37°C for 0 to 3 days and lysed by the addition of 1.0 ml of 0.01% SDS at the end of each incubation period. Aliquots of 0.1 ml of the lysed cultures were serially diluted with saline to 10−4, 0.1-ml aliquots of each dilution were plated onto Middlebrook 7H11 solid medium, the plates were incubated at 37°C for 4 weeks, and the colonies were counted (CFU determination) (36).
(vii) Effects of CPZ and TZ on killing activities of THP-1 and PBMDMs.
A total of 105 macrophages in the microplate wells were prepulsed with either CPZ or TZ at nontoxic concentrations of 0.01 and 0.1 mg/liter for 1 h, and M. tuberculosis was then added to the cultures at a ratio of 1 CFU/macrophage for 30 min. The addition of these concentrations of CPZ or TZ to a macrophage culture prior to infection with S. aureus does not significantly affect the ability of the macrophage to phagocytose the bacteria (32).
These cultures were then washed with medium in order to remove any unphagocytosed bacteria; fresh medium was added; and the cultures were incubated for 0, 1, 2, and 3 days at 37°C. At the end of each culture period, 0.01% SDS was added and the number of viable bacterial cells present in the supernatants of the lysed cultures was determined by counting of the numbers of CFU. All experiments were conducted in triplicate and were performed at least twice.
Statistical analysis.
All data obtained were subjected to nonparametric statistical evaluation with the aid of the Mann-Whitney U test.
Ethical permission.
The blood samples used in this study were obtained from healthy donors in Portugal after the donors granted written permission. This study was conducted subsequent to approval by the ethical committees at the Hospital Egas Moniz, Lisbon, Portugal, and the Instituto de Higiene e Medicina Tropical.
RESULTS
MICs and MBCs of CPZ and TZ for antibiotic-susceptible and MDR M. tuberculosis strains.
The average MICs and MBCs of CPZ and TZ for three antibiotic-susceptible strains (including strain ATCC 27294) and three antibiotic-resistant M. tuberculosis strains are summarized in Table 1. Briefly, the MBCs of CPZ and TZ were significantly greater than the respective MICs for antibiotic-susceptible and -resistant strains of M. tuberculosis. The activities of TZ against each of the strains were greater than those of CPZ. Lastly, MDR M. tuberculosis strains were significantly more resistant to the actions of CPZ and TZ than the antibiotic-susceptible strains were. It is noteworthy that because the MICs and MBCs were not determined by the broth dilution method and, indeed, were the precise concentrations used against a constant standardized inoculum, the differences noted above are deemed significant.
TABLE 1.
MICs and MBCs of CPZ and TZ for antibiotic-sensitive and MDR strains of M. tuberculosisa
| Drug | Susceptible strains
|
Resistant strains
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 27294
|
A
|
B
|
C
|
D
|
E
|
|||||||
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |
| CPZ | 10 | 20 | 10 | 20 | 10 | 20 | 30 | 40 | 20 | 30 | 20 | 30 |
| TZ | 15 | 30 | 15 | 30 | 15 | 30 | 30 | 50 | 20 | 30 | 20 | 30 |
The individual MICs of RIF, INH, SM, EMB, CPZ and TZ, were determined by the BACTEC 460-TB method, as described previously (1, 8, 36). Stock solutions containing various amounts of each compound were freshly prepared in BACTEC 460 medium, and 0.1-ml aliquots of each solution were added to BACTEC 460 vials. The final concentrations of CPZ and TZ in the BACTEC 460 vials were 0, 1, 5, 10, 15, 20, 30, 40, 50, and 60 mg/liter. A total of 0.1 ml of BACTEC 460 medium containing an adjusted concentration of mycobacteria corresponding to approximately 105 to 106 CFU was added to each vial (36). The MBCs of CPZ and TZ were determined by extending the MIC curves well beyond each MIC, and 10-μl aliquots of the 10-ml cultures at zero time and those after 30 days that showed no evidence of growth in the BACTEC 12B vials were subjected to counting of the numbers of CFU (36). The MIC and MBC determinations were repeated three times, and the values obtained did not differ significantly. Strains ATCC 27294, A, and B are susceptible to INH, RIF, SM, and EMB. Strain C is resistant to all four drugs. Strains D and E, besides being resistant to INH and RIF, are also resistant to SM.
Toxicities of CPZ and TZ against THP-1 cells, PBMDMs, and subsets of the Ficoll preparation.
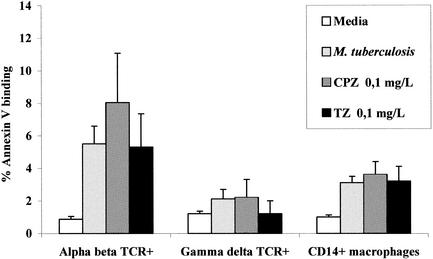
The potential toxicities of CPZ or TZ against THP-1 cells and PBMDMs were evaluated by three distinct methods in a previous study, and 50% of the full toxicity was shown to take place at concentrations that exceeded 3.0 mg/liter (32). Similar results were obtained in this study (data not shown). The toxicities of the phenothiazines at concentrations below those anticipated to be present in the plasma of a patient treated with 600 mg of either phenothiazine per day (5) against subsets of the Ficoll monocyte preparation were examined in the present study. Briefly, as shown in Fig. 1, the concentrations of CPZ and TZ that correspond to those readily evident during initial therapy of a patient, i.e., 0.1 mg/liter, had a nominal effect on annexin V binding, determination of which is the method of choice for the demonstration of apoptosis (32). The percentage of annexin V binding indicative of early apoptosis is 10% for alpha-beta and gamma-delta T cells and CD14+ macrophages (32). The data presented in Fig. 1 also show that the presence of live mycobacteria during a culture period of 3 days did not increase the percent annexin V binding by the various monocyte subsets evaluated. Significant toxicity against THP-1 cells, PBMDMs, or lymphocytes was evident with concentrations of either phenothiazine that exceeded 0.5 mg/liter (data not shown).
FIG. 1.
Effects of CPZ and TZ on percent annexin V binding by monocyte subsets of Ficoll preparations of PBMCs. Subpopulations of the Ficoll preparation containing PBMCs from healthy donors were separately stimulated for 3 days with M. tuberculosis (H37Rv [ATCC 27294]) or with concentrations of CPZ and TZ that ranged from 0 to 0.5 mg/liter. After 3 days the cells were harvested and stained with fluorescent antibodies against alpha-beta TCR+, gamma-delta TCR+, and CD14+ macrophages in combination with annexin V. Cells were analyzed with an Ortho absolute flow cytometer. Results are expressed as the mean ± standard deviation percent annexin V binding in lymphocytes. Annexin V binding in excess of 10% is an indication of early apoptosis (32). The highest concentrations of CPZ and TZ that had a nominal effect on the percent annexin V binding were 0.1 mg/liter.
Effects of CPZ and TZ on killing of M. tuberculosis phagocytosed by PBMDMs or THP-1 macrophages.
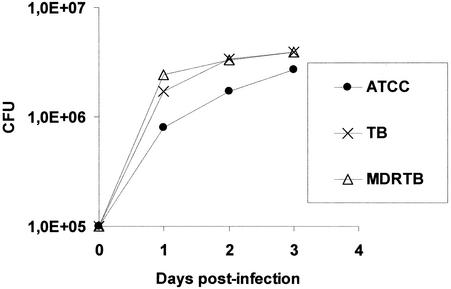
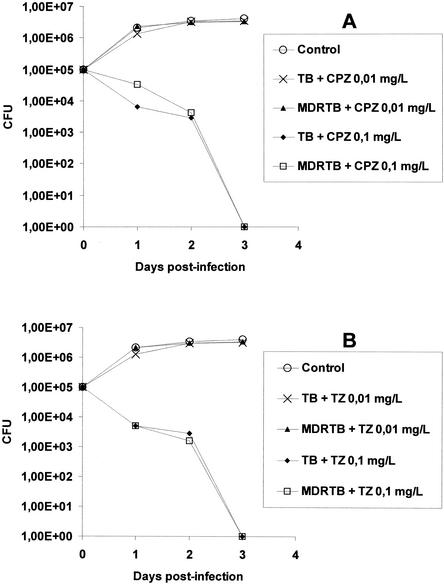
THP-1 macrophages and PBMDMs were selected for use in the assays of the phagocytosis of M. tuberculosis on the basis of the fact that these cells, unlike neutrophils, possess slight killing activities against bacteria (22, 32). This provides an understanding of the killing activities of agents against intracellular bacteria. As shown in Fig. 2, the phagocytosis of approximately 105 mycobacterial cells by an equal number of PBMDMs does not result in any effective killing for 3 days postinfection, regardless of the organism's susceptibility to INH, RIF, SM, and EMB (including strain H37Rv [ATCC 27294]) or resistance to INH and RIF (MDR M. tuberculosis). In contrast to this lack of killing, when CPZ or TZ was added to the infected macrophage cultures at 0.1 mg/liter, the killing of strain ATCC 27294 and the antibiotic-susceptible and MDR M. tuberculosis strains was enhanced within 1 day postinfection (Fig. 3A and B). Complete killing took place by the end of the third day postinfection (Fig. 3A and B). Lower concentrations of these phenothiazines were ineffective, and the rates of survival of all intracellular mycobacterial strains were indistinguishable from those for their controls (Fig. 3A and 3B). Although the results presented above were obtained by use of PBMDMs, similar results were obtained by use of the THP-1 macrophage cell line (data not shown).
FIG. 2.
Average killing activities of human PBMDMs against M. tuberculosis ATCC 27294 (ATCC), two antibiotic-susceptible strains (strains A and B [TB]), and three MDR M. tuberculosis strains (strains C, D, and E; resistant at least to RIF and INH [MDRTB]). The killing activities against the strains used were not significantly different.
FIG. 3.
Average killing activities of human PBMDMs against three MDR M. tuberculosis strains (resistant to RIF and INH) (MDRTB) and three antibiotic-susceptible M. tuberculosis strains (TB) including strain ATCC 27294 swhen either CPZ (A) or TZ (B) was present in the medium at concentrations of 0.01 and 0.1 mg/liter. The control value is the average numbers of CFU for cultures of all six strains to which no phenothiazine was added, inasmuch as the CFU did not differ appreciably between strains.
DISCUSSION
The toxicity associated with chronic use of CPZ is well known (5, 25, 26). In addition, CPZ has been shown to affect the activities of a variety of T lymphocytes in vitro (17, 19, 21), although these inhibitory effects require concentrations of CPZ which are beyond those clinically relevant (i.e., in excess of 1 mg/liter) or fairly close to the highest concentration of CPZ in plasma that might result from its aggressive use (≥0.5 mg/liter). Our previous study (32) and the present study show that an in vitro concentration of 0.1 mg of either CPZ or TZ per liter, which is equivalent to that anticipated to be present in the plasma of a patient treated with either phenothiazine, has no significant toxicity against macrophages or alpha-beta TCR+, gamma-delta TCR+, or CD14+ macrophages.
Concentrations of either CPZ or TZ that are well below their range for toxicity against human macrophages, THP-1 macrophages, or subsets of T lymphocytes in vitro, as shown in this and other studies (32), were shown in the present study to enhance the killing of M. tuberculosis strains that are phagocytosed by either PBMDMs or THP-1 cells. Complete killing is evident by the end of 3 days postinfection at a minimum concentration of 0.1 mg/liter, regardless of the antibiotic susceptibility of the strain. When similar M. tuberculosis strains are exposed to concentrations of CPZ or TZ in vitro, the minimum concentration of either CPZ or TZ required to completely inhibit growth ranges from 8 to over 30 mg/liter (4, 8, 10, 37). The minimum in vitro concentration of either phenothiazine required for complete killing (MBC) in the absence of the macrophage, as shown in our study, ranges from 20 to 50 mg/liter and is similar to that reported previously (4). Because these phenothiazines are concentrated by macrophages (2), cells that have little killing actions of their own (22, 32), it is likely that the concentration of either phenothiazine required to kill M. tuberculosis cells in vitro is achieved because the macrophage is able not only to concentrate the compounds but also to make these compounds available in an active form to the cytoplasmic structure of the macrophage that houses the entrapped phagocytosed bacterium (2). Nevertheless, it is also possible that the lytic action of the macrophage lysosomal apparatus, which is apparently weak, might be sufficiently effective to affect the integrity of the bacterium's cell wall. This weakened cell wall might be more permeable for the penetration of these compounds, which eventually intercalate between the nucleic acid base pairs of the bacterium's DNA, where DNA-dependent functions may be affected (12). Regardless of the cause of killing, in vitro resistance of MDR M. tuberculosis to either CPZ or TZ, as opposed to that of the antibiotic-susceptible strains, is not evident after the MDR M. tuberculosis strain is phagocytosed by macrophages that have previously been loaded with either CPZ or TZ.
The demonstration that clinically relevant concentrations of either CPZ or TZ that are neither toxic in vitro to the macrophage nor inhibitory of T-cell activity in vitro kill phagocytosed mycobacteria supports their use in the management of intracellular M. tuberculosis infections. TZ has significantly fewer serious side effects than CPZ (5), making the use of TZ use as an anti-TB agent far more attractive than the use of CPZ. However, its use is not without some risk, inasmuch as almost twice as many sudden deaths due to cardiac failure take place in patients managed with TZ compared to the number of deaths from such causes in the general population (18). Although TZ may induce episodes of torsade de pointes that result in sudden death, there are only 10 to 15 such events in 10,000 person-years of observation (18). Regardless, careful monitoring of cardiac function during TZ management is strongly recommended.
Admittedly, the use of TZ for the management of an active pulmonary TB infection caused by antibiotic-susceptible M. tuberculosis strains provides no advantage over the use of present therapies. Moreover, because the concentration of TZ needed to kill or even inhibit mycobacterial replication when the bacterium is outside the macrophage is far beyond that which can be achieved in the patient, it cannot be used for the management of a cavitary pulmonary M. tuberculosis infection of moderate to severe status. Nevertheless, even for patients with cavitary infections of moderate status whose origins are associated with a high prevalence of MDR M. tuberculosis, especially when there is a lack or absence of appropriate, effective clinical laboratory support, TZ could serve as an adjunct to conventional therapy during initial management of the patient, at least until the susceptibility data for the causative organism are made available (37). Because the length of such management is anticipated to be weeks, side effects associated with chronic TZ therapy are not anticipated. Normally, such a recommendation would not be made until the effectiveness of TZ therapy was defined subsequent to clinical trials. However, given the severity of MDR M. tuberculosis in parts of the world where effective therapy is not possible, the use of TZ deserves consideration.
Acknowledgments
We thank the members of Cost Action B16 for valuable comments and advice during the course of this study and the Institute of Hygiene and Tropical Medicine and its Scientific Council for support beyond that provided by grant POCTI-37579/FCB/2001, provided by the Fundação para a Ciência e Tecnologia of Portugal.
REFERENCES
- 1.Amaral, L., and J. E. Kristiansen. 2000. Phenothiazines: an alternative to conventional therapy for the initial management of suspected multi-drug resistant tuberculosis. Int. J. Antimicrob. Agents 14:173-176. [DOI] [PubMed] [Google Scholar]
- 2.Amaral, L., and J. E. Kristiansen. 2001. Phenothiazines: potential management of Creutzfeldt-Jacob disease and its variants. Int. J. Antimicrob. Agents 18:411-417. [DOI] [PubMed] [Google Scholar]
- 3.Amaral, L., J. E. Kristiansen, and V. Lorian. 1990. Synergistic effect of chlorpromazine on the activity of some antibiotics. J. Antimicrob. Chemother. 30:556-558. [DOI] [PubMed] [Google Scholar]
- 4.Amaral, L., J. E. Kristiansen, L. S. Abebe, and W. Millet. 1996. Inhibition of the respiration of multi-drug resistant clinical isolates of Mycobacterium tuberculosis by thioridazine: potential use for initial therapy of freshly diagnosed tuberculosis. J. Antimicrob. Chemother. 38:1049-1053. [DOI] [PubMed] [Google Scholar]
- 5.Amaral, L., J. E. Kristiansen, M. Viveiros, and J. Atouguia. 2001. Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as an anti-tuberculosis therapy. J. Antimicrob. Chemother. 47:505-511. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society, the Centers for Disease Control, and the Infectious Disease Society of America. 1993. Control of tuberculosis in the United States. Joint statement of the American Thoracic Society, the Centers for Disease Control, and the Infectious Disease Society of America. Respir. Care 38:929-939. [PubMed] [Google Scholar]
- 7.Bates, J. H., and W. W. Stead. 1993. The history of tuberculosis as a global epidemic. Med. Clin. N. Am. 77:1205-1217. [DOI] [PubMed] [Google Scholar]
- 8.Bettencourt-Viveiros, M., S. Bosne-David, and L. Amaral. 2000. Comparative in vitro activity of phenothiazines against multidrug-resistant Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 16:69-71. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier, P., P. Gaillot, R. Jacob, J. Gaudechon, and P. Buisson. 1952. Recherches sur les dimthylaminopropyl N-phenothiazines. C. R. Acad. Sci. 235:59-60. [Google Scholar]
- 10.Crowle, A. J., G. S. Douvas, and M. H. May. 1992. Chlorpromazine: a drug potentially useful for treating mycobacterial infections. Exp. Chemother. 38:410-419. [DOI] [PubMed] [Google Scholar]
- 11.David, S., D. Ordway, M. J. Arroz, J. Costa, and R. Delgado. 2001. Activity against Mycobacterium tuberculosis with concomitant induction of cellular immune responses by tetra-macrocycle with acetate pendant arms. Res. Microbiol. 152:569-576. [DOI] [PubMed] [Google Scholar]
- 12.de Mol, N. J., R. M. Posthuma, and G. R. Mohn. 1983. Induction of repairable DNA damage in Escherichia coli and interaction with DNA in vitro by the radical cation of chlorpromazine. Chem. Biol. Interact. 47:223-237. [DOI] [PubMed] [Google Scholar]
- 13.Dievler, A. 1997. Fighting tuberculosis in the 1990s: how effective is planning in policy making? J. Public Health Policy 18:167-187. [PubMed] [Google Scholar]
- 14.Elferink, J. G. R. 1979. Chlorpromazine inhibits phagocytosis and exocytosis in rabbit polymorphonuclear leukocytes. Biochem. Pharmacol. 28:965-968. [DOI] [PubMed] [Google Scholar]
- 15.Fisher, R. A., and E. Teller. 1959. Clinical experience with ataractic therapy in tuberculous psychiatric patients. Dis. Chest 34:134-139. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara, P. I. 2002. A decade of successful tuberculosis control in New York City—the role of DOT vs. DOTS. Kekkaku 77:29-35. [PubMed] [Google Scholar]
- 17.Ghezzi, P. 1991. Effects of chlorpromazine on polymorphonucleocyte-mediated activities in vivo and in vitro. Immunology 72:138-143. [PMC free article] [PubMed] [Google Scholar]
- 18.Glassman, A. H., and J. T. Bigger. 2001. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am. J. Psychiatry 158:1174-1182. [DOI] [PubMed] [Google Scholar]
- 19.Grabski, R., J. Dewit, J. De Braekeleer, M. Malicka-Blaskiewicz, P. De Baetselier, and H. Verschueren. 2001. Inhibition of T-cell invasion across cultured fibroblast monolayers by phenothiazine-related calmodulin inhibitors: impairment of lymphocyte motility by trifluoperazine and chlorpromazine, and alteration of the monolayer bypimozide. Biochem. Pharmacol. 61:1313-1317. [DOI] [PubMed] [Google Scholar]
- 20.Guth, P. S., and M. A. Spirtes. 1964. The phenothiazine tranquilizers: biochemical and biophysical actions. Int. Rev. Neurobiol. 7:231-278. [DOI] [PubMed] [Google Scholar]
- 21.Hieronymus, T., P. Grotsch, N. Blank, M. Grunke, D. Capraru, T. Geiler, S. Winkler, J. R. Kalden, and H. M. Lorenz. 2000. Chlorpromazine induces apoptosis in activated human lymphoblasts: a mechanism supporting the induction of drug-induced lupus erythematosus. Arthritis Rheum. 43:1994-2004. [DOI] [PubMed] [Google Scholar]
- 22.Hoidal, J. R., D. Schmeling, and P. K. Peterson. 1981. Phagocytosis and bacterial killing and metabolism by purified lung phagocytes. J. Infect. Dis. 144:61-71. [DOI] [PubMed] [Google Scholar]
- 23.Hu, O. Y., and S. H. Curry. 1989. Stability, human blood distribution and rat tissue localization of promazine and desmonomethylpromazine. Biopharm. Drug Dispos. 10:537-548. [DOI] [PubMed] [Google Scholar]
- 24.Kaminska, M. 1967. Role of chlorpromazine in the treatment of pulmonary tuberculosis in psychiatric patients. Folia Med. Cracov 9:115-143. [PubMed] [Google Scholar]
- 25.Kaplowitz, N., T. Y. Aw, F. R. Simon, and A. Stolz. 1986. Drug-induced hepatotoxicity. Ann. Intern. Med. 104:826-839. [DOI] [PubMed] [Google Scholar]
- 26.Kodovanti, U. P., V. G. Lockard, and H. M. Mehendale. 1990. In vivo toxicity and pulmonary effects of promazine and chlorpromazine in rats. J. Biochem. Toxicol. 5:245-251. [DOI] [PubMed] [Google Scholar]
- 27.Kristiansen, J. E., and L. Amaral. 1997. The potential management of resistant infections with non-antibiotics. J. Antimicrob. Chemother. 40:319-327. [DOI] [PubMed] [Google Scholar]
- 28.Kristiansen, J. E., and B. Vergmann. 1986. The antibacterial effect of selected phenothiazines and thioxanthenes on slow growing mycobacteria. APMIS 94:393-398. [DOI] [PubMed] [Google Scholar]
- 29.Levaditi, R., H. Chaigneau-Erhard, and J. Henry-Eveno. 1951. L′antihistaminique 3277RP (Phenergan) agit-il curativement dans la tuberculose experimentale de la souris? C. R. Soc. Biol. 145:1454-1456. [PubMed] [Google Scholar]
- 30.Maccagnani, G. 1959. Pharmacotherapy in tubercular schizophrenics, p. 145-147. In N. E. Cline (ed.), Psycopharmacology frontiers. Little, Brown & Co., Boston, Mass.
- 31.Molnar, J., I. Beladi, and I. Foldes. 1977. Studies on the anti-tubercular action of some phenothiazine deriviatives in vitro. Zentbl. Bakteriol. Parasitenkd. Infektkrankh. Hyg. Abt. 1 Orig. 239:521-526. [PubMed] [Google Scholar]
- 32.Ordway, D., M. Viveiros, C. Leandro, M. J. Arroz, and L. Amaral. 2002. Intracellular activity of clinical concentrations of phenothiazines against phagocytosed Staphylococcus aureus. Int. J. Antimicrob. Agents 20:34-43. [DOI] [PubMed] [Google Scholar]
- 33.Portugal, I., L. Brum, M. Viveiros, J. Moniz Pereira, and H. David. 1999. Outbreak of multiple-drug-resistant tuberculosis in Lisbon: detection by restriction fragment length polymorphism analysis. Int. J. Tuberc. Lung Dis. 3:207-213. [PubMed] [Google Scholar]
- 34.Rusch-Gerdes, S. 1999. Epidemiology of resistant tuberculosis in Europe. Infection 27(Suppl. 2):17-18. [DOI] [PubMed] [Google Scholar]
- 35.Schraufnagel, D. E. 1999. Tuberculosis treatment for the beginning of the next century. Int. J. Tuberc. Lung Dis. 3:651-662. [PubMed] [Google Scholar]
- 36.Viveiros, M., I. Portugal, R. Bettencourt, T. C. Victor, A. M. Jordaan, C. Leandro, D. Ordway, and L. Amaral. 2002. Isoniazid-induced transient high level resistance of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2804-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viveiros, M., and L. Amaral. 2001. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 17:225-228. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. 1998. Anti-tuberculosis drug resistance in the world. Report 1. The W.H.O./IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. World Health Organization, Geneva, Switzerland.