Abstract
Porphyromonas gingivalis is one of the major causative organisms of periodontitis and has been shown to be susceptible to toluidine blue-mediated photosensitization in vitro. The aims of the present study were to determine whether this technique could be used to kill the organism in the oral cavities of rats and whether this would result in a reduction in the alveolar bone loss characteristic of periodontitis. The maxillary molars of rats were inoculated with P. gingivalis and exposed to up to 48 J of 630-nm laser light in the presence of toluidine blue. The number of surviving bacteria was then determined, and the periodontal structures were examined for evidence of any damage. When toluidine blue was used together with laser light there was a significant reduction in the number of viable P. gingivalis organisms. No viable bacteria could be detected when 1 mg of toluidine blue per ml was used in conjunction with all light doses used. On histological examination, no adverse effect of photosensitization on the adjacent tissues was observed. In a further group of animals, after time was allowed for the disease to develop in controls, the rats were killed and the level of maxillary molar alveolar bone was assessed. The bone loss in the animals treated with light and toluidine blue was found to be significantly less than that in the control groups. The results of this study show that toluidine blue-mediated lethal photosensitization of P. gingivalis is possible in vivo and that this results in decreased bone loss. These findings suggest that photodynamic therapy may be useful as an alternative approach for the antimicrobial treatment of periodontitis.
Periodontitis is a common disease, with a 5 to 30% prevalence in the adult population (22, 26). It is an inflammatory process of periodontal tissues caused by bacterial infection which results in the destruction of periodontal connective tissue and resorption of alveolar bone. Porphyromonas gingivalis is believed to be one of the major pathogens in the etiology of adult periodontitis (5, 37, 38). Conventional mechanical debridement (i.e., scaling and root planing) can achieve a temporary decrease in the subgingival levels of P. gingivalis together with other pathogens (38). However, organisms cannot be removed from the majority of periodontal pockets by mechanical therapy alone. Antimicrobial chemotherapy may further suppress the periodontal pathogens and increase the benefits obtained by conventional mechanical treatment. Numerous systemic and local antimicrobial chemotherapeutic agents have been evaluated for the treatment of periodontitis with various degrees of success (5, 19, 37, 41). A lack of effectiveness of some of the antibiotics used may be due to development of drug-resistant strains (10, 29). To overcome the problems caused by the emergence of resistance, alternative antimicrobial approaches need to be developed. One potential alternative approach is photodynamic therapy (PDT), which could provide a means of killing microbes in localized, topical infections (21, 40, 43, 45). PDT can be defined as eradication of target cells by reactive oxygen species (ROS) produced by means of a photosensitizing compound and light of an appropriate wavelength (6). Although PDT is more widely known for its application to the treatment of neoplasms, there is also interest in antimicrobial PDT, as a large number of microorganisms (including oral species) have been reported to be killed in vitro by this approach (21, 27, 31, 40, 43, 46, 47). Furthermore, the potencies of some key virulence factors (lipopolysaccharide and proteases) have also been shown to be reduced by photosensitization (18, 32). Due to its localized and noninvasive nature, the side effects associated with many antibiotics (e.g., gastrointestinal disturbance) are unlikely to occur with PDT. Furthermore, development of resistance to PDT would appear to be unlikely since its bactericidal activity is due to singlet oxygen and other reactive species such as hydroxyl radicals which affect a range of cellular targets (1, 4, 7, 14). PDT has been shown to be effective at killing the Escherichia coli and Pseudomonas aeruginosa organisms responsible for infections in animal models (2, 13).
P. gingivalis is very susceptible in vitro to killing by red light when toluidine blue is used as the photosensitizer. Bhatti et al. (3) reported that 100% killing was achieved with 25 μg of toluidine blue per ml in combination with 4.4 J of red light. The aims of this study were to determine whether P. gingivalis could be killed by toluidine blue-mediated photosensitization in vivo in an animal model without damage to the associated periodontal tissues and also to establish whether such treatment would have any effect on the characteristic bone destruction accompanying periodontitis.
MATERIALS AND METHODS
Bacteria.
P. gingivalis W50 was inoculated into fastidious anaerobe broth (BioConnections, Leeds, United Kingdom) and incubated at 37°C in an anaerobic cabinet containing 85% N2, 10% CO2, and 5% H2 (Don Whitley Scientific, Shipley, United Kingdom). Following a 24-h incubation, the bacteria were harvested by centrifugation at 3,000 × g for 15 min. The cells were resuspended in reduced transport fluid (RTF) (42).
Animals.
Male Sprague-Dawley rats (weight, 200 g; Harlan UK Ltd., Bicester, United Kingdom) were used in this study and were housed in accordance with the regulations of the Home Office of the United Kingdom Government.
Photosensitizer and laser.
Toluidine blue solution was prepared by dissolving the powder (Zila Inc, Phoenix, Ariz.) in sterile 0.85% (wt/vol) NaCl. It was then filter sterilized by using 0.22-μm-pore-size membrane filters. The light source used was a diode laser (Diomed, Cambridge, United Kingdom) with a measured output of 0.1 W and a wavelength of 630 nm. The light was distributed by means of a fiber-optic applicator (Medlight SA, Ecublens, Switzerland) with a 0.5-cm cylindrical diffusing tip.
Lethal photosensitization of P. gingivalis in vivo.
The rats received 0.5 ml of a narcotic analgesic (fentanyl [Hypnorm]) and were placed in a supine position. Access to the oral cavity was achieved with a retractor that provided constant opening of the mouth and that held away the cheeks and the tongue. Immediately following the inoculation of 25 μl of a P. gingivalis suspension (1010 CFU/ml in RTF) on the maxillary molar region of each side, the same volume of toluidine blue solution was added. An optical fiber was then immediately placed in the midline of the palate and the area was exposed to 630-nm laser light, after which time samples were collected from the palatal and buccal gingival margins of each side with a sterile endodontic paper point (size 40) in a standardized manner. The paper points were placed in screw-cap vials containing 1 ml of sterile RTF. The samples were transferred to a microbiology laboratory and were processed in less than 3 h.
The effect of photosensitization with toluidine blue at concentrations of 0.01, 0.1, and 1 mg/ml in combination with light doses of 6, 12, 24, and 48 J (with corresponding exposure times of 1, 2, 4, and 8 min) was determined. The effect of toluidine blue alone was tested by application of toluidine blue (at the same concentrations listed above) for the same period of irradiation but in the absence of light. The group that received light only also received sterile 0.85% (wt/vol) NaCl and were exposed to the same light doses mentioned above. The control group did not receive either toluidine blue or light. Each experimental group consisted of six animals.
Samples were vigorously vortexed for 1 min in order to release bacteria from the paper point. Aliquots of serial 10-fold dilutions of the suspension were plated on fastidious anaerobe agar and incubated anaerobically for 10 days, and colonies of P. gingivalis were counted.
Histological evaluation.
Three days after the photosensitization procedure, the animals were killed by carbon dioxide asphyxiation and the maxilla were removed. Specimens were fixed immediately in formic acid-10% formalin. Decalcified specimens were processed and embedded in paraffin. Histological sections were then prepared in the buccopalatal plane and stained with hematoxylin-eosin.
Biodistribution of toluidine blue.
Localization of toluidine blue upon topical application was assessed by digital fluorescence imaging. Twenty-five microliters of toluidine blue (0.01, 0.1, and 1 mg/ml) was applied to the maxillary region. The control group received 0.85% (wt/vol) NaCl in place of toluidine blue. After a 1-min application period, the animals were killed by cervical dislocation. The specimens were removed and were frozen by submerging them in a bath of isopentane that was then placed in liquid nitrogen. The gingival soft tissues were removed with care to avoid traumatizing the tissue. Sections of 10 μm were prepared with a Cryocut E microtome (Reichert Ltd.) and were stored at −70°C. The slides were immediately thawed before digital fluorescence imaging.
A fluorescence microscope (IMT-2; Olympus, Hamburg, Germany), which is an inverted microscope with epifluorescence and phase contrast, was attached to a charge-coupled device camera system (model 1; Wright Instruments Ltd., Cambridge, United Kingdom). Fluorescence was excited with an 8-mW helium-neon laser operating at 632.8 nm, the output of which was directed onto the section with a liquid light guide and dichroic mirrors after removal of the extraneous light with a 10-nm band-pass filter. The fluorescence of toluidine blue was detected in the range of 660 to 700 nm by using a combination of band-pass (Omega Optical Inc.) and long-pass (RG665; Schott) filters. The fluorescence signal was detected with a highly sensitive, cryogenically cooled charge-coupled device camera. Image operations and processing were carried out with an International Business Machines personal computer which generated a digital grey scale that depicted the fluorescence intensity as the number of counts per pixel in arbitrary units. The resolution of the images was 600 to 400 pixels. The mean fluorescence intensity was calculated by the computer after selection of the three regions (e.g., 50 by 50 pixels) of each layer of mucosa. A minimum of three images was taken from each of the three sections of three rats. The sections were subsequently fixed in formalin and stained with hematoxylin-eosin. All specimens were viewed by phase-contrast microscopy to facilitate their orientation.
Effect of photodynamic therapy on alveolar bone loss.
In order to promote colonization of P. gingivalis, the normal oral microflora of the rats was suppressed by administration of antibiotics. The animals received 30 mg of ampicillin per ml in 0.5 ml of sterile water intraperitoneally on a daily basis for 5 days. Following a 2-day interval, the animals were then inoculated orally with P. gingivalis (1010 CFU suspended in 250 μl of 5% low-viscosity carboxymethyl-cellulose) once a day for 5 days.
Following a 2-day interval, the rats received 0.5 ml of fentanyl and were placed in a supine position. Access to the oral cavity was achieved with a retractor, as described above. The mouth was irrigated with toluidine blue solution dissolved in sterile 0.85% (wt/vol) NaCl, with a special emphasis on the maxillary and mandibular molar region. An optical fiber with a 0.5-cm cylindrical tip was placed in the midline of the palate, and the mouth was exposed to 630-nm laser light as described above. Toluidine blue concentrations of 0.01, 0.1, and 1 mg/ml in combination with 48 J of light (exposure time, 8 min) were used. The effect of toluidine blue alone was assessed by application of the dye (by using concentrations identical to those listed above) without irradiation. An additional group of animals received sterile 0.85% (wt/vol) NaCl and was subjected to the same light dose (the light-only group). A control group of animals received neither toluidine blue nor laser light. Each group consisted of six animals.
The animals were killed by carbon dioxide asphyxiation 90 days after the PDT procedure. The maxilla were removed, and alveolar bone loss was measured by morphometric and radiographic methods as described by Klausen et al. (16). In summary, the maxilla were defleshed after they were boiled in water for 5 min under pressure (15 lb/in2). The samples were immersed in 3% hydrogen peroxide overnight, air dried, and stained with 1% methylene blue to delineate the cemento-enamel junction (CEJ). In the morphometric method, the distances from the CEJ to the alveolar bone crest on the buccal and palatal surfaces of the roots of all molars were measured under a dissecting microscope (magnification, ×40) by means of a scale in the eyepiece. In the radiographic method, the maxilla were split along the midline, and standard dental radiographs were taken. The images were then imported into a computer and magnified. The distances from the deepest bony defect to cusp tips on the interproximal sites of all molars were measured. The measurements were performed by two independent examiners, and the means of the two examiners' readings were calculated.
Bone loss was regarded as the distance between CEJ and the alveolar bone crest in the morphometric method and the distance between the deepest bony defect and cusp tips in the radiographic method.
Statistical analysis.
Analysis of variance was performed to determine whether there were significant differences between the different test conditions. If a significant difference was found between the groups as a whole, further analyses were performed to determine where these differences occurred. Bonferroni's correction was applied to allow for the effect of multiple testing.
The Student t test was performed for comparison of the bacterial killing achieved on the palatal and buccal sides of the gingival crevices of animals.
RESULTS
Effect of photosensitization on bacterial viability.
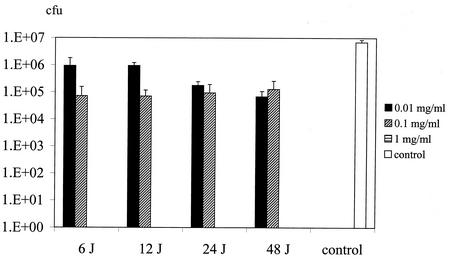
Figure 1 shows the number of P. gingivalis organisms isolated from the gingival margins of treated and control groups (animals that did not receive toluidine blue and that were not irradiated with laser light). When toluidine blue was used in conjunction with light, significant reductions in the viable counts of the organism were achieved with all toluidine blue and light combinations. Use of 1 mg of toluidine blue per ml in combination with all light doses resulted in no detectable viable bacteria (i.e., less than 10 CFU). Reductions of approximately 2 log10 were obtained when 0.1 mg of toluidine blue per ml was used with all light doses (P < 0.001). Even with the lowest concentration of toluidine blue and the lowest light dose used (0.01 mg/ml plus 6 J) there was a 1-log10 reduction in the viable count of the organism (P = 0.004). While 0.01 and 0.1 mg of toluidine blue per ml in the absence of light had no effect on the viability of the bacteria, increasing the concentration to 1 mg/ml caused 1-log10 (P = 0.005) and 2-log10 (P < 0.001) reductions with 4- and 8-min application periods, respectively. Light exposure alone, even with the highest dose tested (48 J), had no effect on the viable count.
FIG. 1.
Effect of toluidine blue-mediated photosensitization on the viability of P. gingivalis on the gingival margins of the maxillary molars of rats. A range of light doses (6 to 48 J) and a range of toluidine blue concentrations (0.01 to 1.0 mg/ml) were used. Control animals were not administered toluidine blue and were not irradiated with laser light. In the case of animals administered 1.0 mg of toluidine blue per ml, no viable bacteria were detected with any of the light doses used.
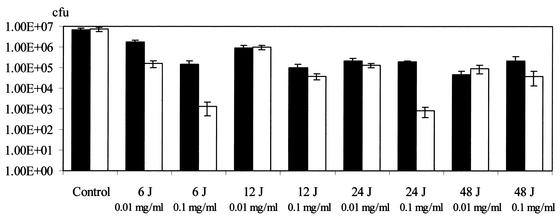
In most of the PDT-treated groups, smaller numbers of bacteria were isolated from the samples taken from the palatal side of the gingival margins than from the samples taken from the buccal side (Fig. 2). The levels of bacterial killing achieved on the palatal and buccal sides were significantly different (P < 0.001).
FIG. 2.
Numbers of viable P. gingivalis organisms from samples taken from the palatal (open bars) and buccal (black bars) gingival margins of maxillary molars after photosensitization. Light energy doses of between 6 and 48 J were used in combination with toluidine blue concentrations ranging from 0.01 to 1.0 mg/ml. In the case of animals administered 1.0 mg of toluidine blue per ml, no viable bacteria were detected in either of the two sites with any of the light doses used.
Histological examination.
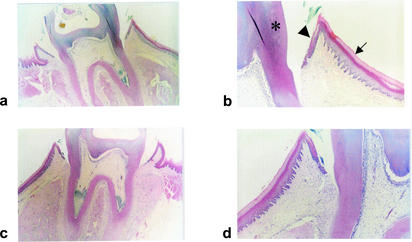
On histological examination, no adverse effects of PDT on the periodontal structures were observed. Even with the highest concentration of toluidine blue and the highest dose of light tested (1 mg of toluidine blue per ml plus 48 J of laser light), no ulcer formation on the epithelium or inflammation of the connective tissue was detected (see Fig. 3). There was no sign of distress in any of the animals.
FIG. 3.
Histological sections of the periodontal structures of rats photosensitized with 1.0 mg of toluidine blue per ml plus 48 J of laser light (a and b) and control animals which were not irradiated and not treated with toluidine blue (c and d). The sections show normal gingival epithelium (arrow) and sulcular epithelium (arrowhead) abutting the teeth (asterisk) (for clarity, the structures are labeled only on panel b). There is no evidence of ulceration or inflammation.
Fluorescence images.
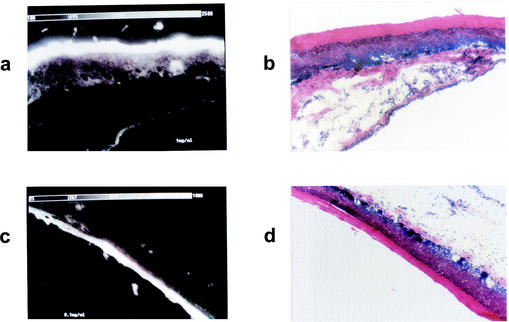
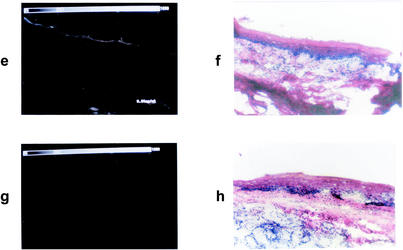
The fluorescence intensity of topically applied toluidine blue on the periodontal structures can be seen in Fig. 4. Fluorescence microscopy images showed that toluidine blue had penetrated throughout the epithelium, with the highest intensity being on the keratinized layer. The intensity of the fluorescence in the tissues correlated with the concentration of toluidine blue in the solutions applied.
FIG. 4.
Fluorescence microscopy images showing the biodistribution of toluidine blue administered topically to the rat gingiva. (a, c, e, and g) Fluorescence images after application of toluidine blue at 1.0, 0.1, and 0.01 mg/ml and no toluidine blue, respectively. (b, d, f, and h) Corresponding sections stained with hematoxylin-eosin. Scale for each panel, 880 by 550 μm.
Alveolar bone loss.
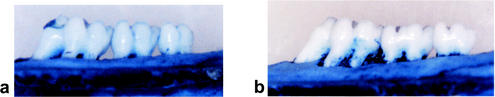
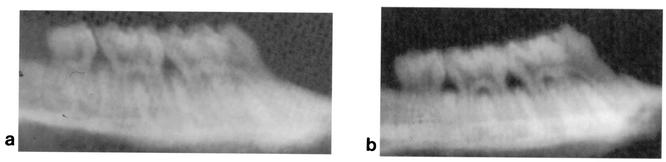
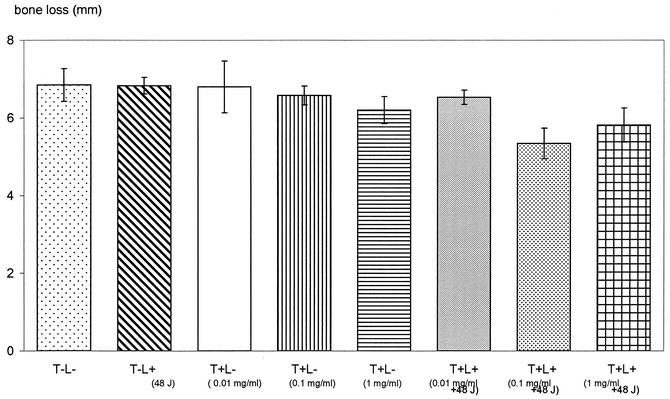
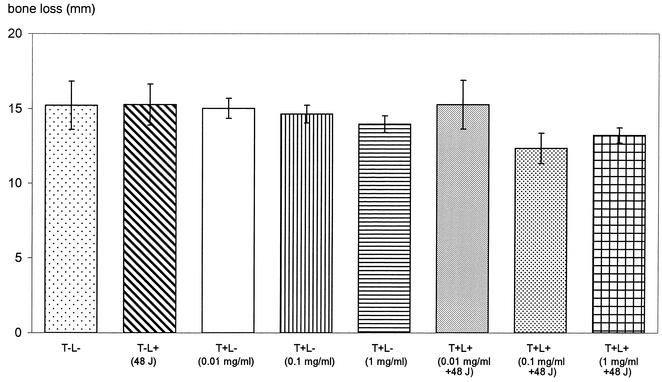
Figures 5 and 6 show photographs representing the alveolar bone levels of the maxillary molars evaluated by morphometric and radiographic methods, respectively. There was no statistically significant reduction in bone loss when 0.01 mg of toluidine blue per ml was used in conjunction with 48 J of light. The results obtained from morphometric measurements (Fig. 7) showed that when 0.1 and 1 mg of toluidine blue per ml was used together with 48 J of laser light, there were significant reductions in bone loss (P = 0.01 and P = 0.04, respectively) compared to that for the control group. When either toluidine blue (at all concentrations studied) or light was used alone, there was no significant effect on bone resorption. The results obtained from radiographic measurements correlated with those obtained from morphometric measurements, except that 1 mg of toluidine blue per ml in the absence of light also resulted in significant reductions in bone loss (P = 0.04). There were statistically significant differences in bone loss between the control group and animals treated with 0.1 mg of toluidine blue per ml (P = 0.002) and 1 mg of toluidine blue per ml (P = 0.01) when the toluidine blue-treated animals were irradiated with 48 J of laser light (Fig. 8).
FIG. 5.
Representative photographs showing the alveolar bone supporting the maxillary molars of rats administered toluidine blue (0.1 mg/ml) and irradiated with 48 J of laser light (a) and control animals which were not irradiated and not treated with toluidine blue (b).
FIG. 6.
Representative radiographs showing the alveolar bone of the maxillary molars of rats administered toluidine blue (0.1 mg/ml) and irradiated with 48 J of laser light (a) and control animals which were not irradiated and not treated with toluidine blue (b).
FIG. 7.
Resorption of alveolar bone of the maxillary molars assessed by morphometric measurement. The bone loss was regarded as the distance between the CEJ and the alveolar bone crest. Animals in the test group were treated with various concentrations of toluidine blue and irradiated with 48 J of laser light (T+L+). Control groups consisted of rats which were not irradiated with laser light and which were not administered toluidine blue (T−L−), rats which were not irradiated but which were administered toluidine blue (T+L−), and rats which were not administered toluidine blue but which were irradiated with 48 J of laser light (T−L+).
FIG. 8.
Resorption of alveolar bone of the maxillary molars assessed by radiographic measurement. The bone loss was regarded as the distance between the deepest bony defect and cusp tips. Animals in the test group were treated with various concentrations of toluidine blue and irradiated with 48 J of laser light (T+L+). Control groups consisted of rats which were not irradiated with laser light and which were not administered toluidine blue (T−L−), rats which were not irradiated but which were administered toluidine blue (T+L−)and rats which were not administered toluidine blue but which were irradiated with 48 J of laser light (T−L+).
DISCUSSION
The results of this study have shown that it is possible to kill P. gingivalis in the gingival crevice of rats by using topically applied toluidine blue and red light from a diode laser without causing any detectable damage to the adjacent tissues. Furthermore, the treatment also resulted in a significant decrease in the characteristic bone loss that accompanies periodontitis.
Studies of the susceptibility of P. gingivalis to lethal photosensitization in vitro have reported that the organism can be killed with low concentrations of toluidine blue and low light energy doses. For example, Bhatti et al. (3) found that 100% killing of P. gingivalis organisms could be achieved with 25 μg of toluidine blue per ml and 4.4 J of red light. However, a number of investigations have demonstrated that the killing obtained may be adversely affected by environmental factors such as the presence of saliva and serum and pHs other than neutral (3, 17). It was likely, therefore, that in the rat model used in this study, the presence of saliva and gingival crevicular fluid would interfere with lethal photosensitization of the target organism. The results obtained confirmed this supposition, as the bacterial killing resulting from the lowest light dose and photosensitizer concentration used were considerably less than those found in vitro. Nevertheless, substantial killing was obtained with higher photosensitizer concentrations, and the number of P. gingivalis organisms was reduced to undetectable levels in some cases.
Interestingly, there was little evidence of a light energy dose-related effect on the bacterial killing obtained. However, at the lower light energy doses used (6 and 12 J), the extent of bacterial killing was found to increase markedly with increasing photosensitizer concentration, and this was not attributable to any light-independent toxicity of the toluidine blue itself. These results imply that even at the lowest light energy dose used (6 J), enough photons were being supplied to activate all of the photosensitizer molecules present in the gingival crevice (resulting from the application of 0.01 mg of toluidine blue per ml to the site) to release sufficient ROS to enable killing of most (approximately 90%) of the bacteria present. What appeared to be limiting the killing obtained with this light energy dose was the concentration of the photosensitizer at the site or, more strictly, its ROS-generating capacity. The latter is affected not only by the number of photosensitizer molecules but also by the extent of their aggregation, their proximity to the target organism, and the presence of ROS-quenching molecules (e.g., many proteins and lipids) in their immediate environment (3, 20). As the concentration of toluidine blue increased, the yield of ROS (from the same light dose) must also have increased, and this resulted in correspondingly greater killing of the P. gingivalis bacteria until, at a concentration of 1.0 mg/ml, no viable bacteria were detectable. Interestingly, in six of the eight study groups, a decrease in the killing of P. gingivalis organisms implanted on the buccal side compared to the level of killing of organisms on the palatal side was noted. This is most likely attributable to the higher light energy dose received by the palatal regions, as they were closer to the light source and were not shielded by intervening tissues. In this study, the cylindrical fiber optic was placed in the midpoint of the palate, and the distance from the fiber to each alveolar process was approximately 0.5 cm. The distance between the sampling sites on the palatal and buccal regions was approximately 0.3 cm. The diffusion of red light in the tissues decreases in intensity logarithmically every 3 mm, so that the buccal regions would have received a considerably lower light dose than the palatal regions. Laser systems which can provide uniform illumination of the oral cavity are being developed, and this mode of light delivery is likely to be able to achieve better killing at all oral sites. Hence, it may be possible to achieve a one-stage full-mouth disinfection, and this has been reported to result in improved outcomes compared to those of standard treatment strategies in the treatment of adult periodontitis (35).
Prior to the clinical use of this approach to killing of P. gingivalis, it is important to ensure that damage to host tissues does not occur. Histological examination of the periodontal tissues of the rats following PDT showed no adverse effects, in that no ulcer formation on the epithelium or inflammation in the connective tissue was detected even with the highest light doses and toluidine blue concentrations used. Furthermore, there was no evidence of distress in any of the animals. Topically administered toluidine blue at a concentration of 1% has been used for the early diagnosis of precancerous and cancerous lesions in the mouth (44), with no reactions or side effects following its application. Toluidine blue also appears to be a promising photosensitizer for use in antimicrobial PDT. In a previous study, toluidine blue has been shown to be an effective photosensitizer of Helicobacter mustelae implanted on ferret gastric mucosa following its activation by light from a copper vapor dye laser. A 90% reduction in the viable counts of bacteria sensitized with 0.75 mg of toluidine blue per kg of body weight was seen after irradiation with 200 J of red light/cm2 (25). In a clinical study, toluidine blue has been used as a photosensitizer to kill bacteria on implants prior to insertion and was found to be effective in decreasing peri-implantitis (12).
Other photosensitizers used for PDT, on the other hand, have been reported to cause tissue damage. Meyer et al. (24) observed gingival ulceration in rabbits following systemic administration of 5 mg of disulfonated phthalocyanine per kg and exposure to 20 J of light with a wavelength of 675 nm. With higher doses of laser light, muscle necrosis (small ulcers with 50 J and large ulcers with 100 J) and necrotizing sialometaplasia in salivary glands (with 100 J) were observed. Nevertheless, the healing was uneventful in all sites. By using the same regimen, the walls of rabbit carotid arteries showed full-thickness cell death, although collagen and elastin were shown to be unaffected and the mechanical properties of the vessels remained intact (11). Treatment with a hematoporphyrin derivative at 20 mg/kg in combination with light at 90 or 180 J/cm2 was found to result in vesicle formation, which disturbed the stratification of the epithelium and which caused edema, cellular infiltration, and a reduction in the number of vessels, but muscle fibers remained intact (33). In contrast to disulfonated phthalocyanine and the hematoporphyrin derivative, therefore, toluidine blue appears to exhibit a greater selectivity for bacteria than for mammalian tissue.
The studies of the biodistribution of topically applied toluidine blue on the gingival structures demonstrated that the photosensitizer penetrated throughout the epithelium. However, even when it was administered systemically in ferrets, the surface of the gastric epithelium was found to be the brightest under fluorescence (25). Peng et al. (34) reported that a strong fluorescence of a methylene blue derivative occurred in the keratinized epithelium of the epidermis but that almost no fluorescence was found in the fibrous connective tissue at 1 to 48 h postinjection of 10 mg/kg into rats. Following oral administration of aminolevulinic acid in patients, maximum fluorescence was found in the oral epithelium. The photodynamic effect, following subsequent illumination, was also limited to the epithelium (8). On the other hand, intravenous application of meta-tetrahydroxyphenylchlorin resulted in slightly higher levels in the connective tissue (9). Fluorescence imaging studies showed that the penetration of toluidine blue into the periodontal tissues was related to the concentration of the photosensitizer used. The most intense fluorescence throughout the epithelial layer was observed when the highest concentration was used. However, the fluorescence intensity in the connective tissue was very low. This could be due to the thick keratin layer (of the rat gingiva), which may form a barrier against the penetration of water-soluble photosensitizers. An increased penetration in human periodontal structures may be expected because of the nonkeratinized nature of the sulcular epithelium. Penetration through the epithelium and connective tissues may be important, as the periodontopathogens may infiltrate through the epithelial barrier into the connective tissue (23). Elimination of bacteria that have migrated into the tissues may therefore be possible by PDT.
In addition to gingival and connective tissue inflammation, periodontitis is characterized by alveolar bone resorption. The loss of bone supporting the teeth may lead to tooth mobility and, eventually, tooth loss. The results obtained from this study have shown that toluidine blue-mediated PDT can significantly reduce the level of alveolar bone loss in animals with periodontitis even after only a single application. Indeed, the 1.75-mm reduction (determined radiographically) in bone loss found in this study compares extremely favorably with that obtained during the evaluation of other therapeutic strategies. For example, Rajapakse et al. (36) reported that in their rat model of P. gingivalis-induced bone loss, immunization with a proteinase-adhesin complex resulted in a statistically significant decrease in alveolar bone loss of approximately 0.8 mm, which is approximately half that achieved by PDT. Furthermore, in the study of Katz et al. (15), immunization of rats with a recombinant hemagglutinin B of P. gingivalis achieved a reduction in bone loss approximately nine times less than that resulting from PDT in the present study.
There is considerable interest in the use of locally applied antimicrobial agents in the treatment of periodontitis (39). A major advantage of this approach over the systemic administration of such agents is that it minimizes disruption of the normal microflora at other body sites, so helping to avoid opportunistic infections at these sites. However, one problem with this approach is the difficulty in maintaining therapeutic levels of the agent for a sufficient period of time due to elution of the agent by gingival crevicular fluid (30). To overcome this problem, the insertion of fibers, strips, and resorbable cellulose that slowly release agents into the periodontal pocket has been used (28, 39). The use of PDT, however, is not beset by such problems, as the photosensitizer needs to be retained in the periodontal pocket for only a short time; this may be minutes or seconds, depending on the power of the laser light delivery system used. For example, in this study we have shown that 1 mg of toluidine blue per ml was sufficient to reduce the number of viable P. gingivalis organisms to below detectable levels by using a light exposure period of only 60 s.
This study has shown that toluidine blue-mediated lethal photosensitization of an important periodontopathogen, P. gingivalis, can be achieved in the gingival crevices of rats without damaging the adjacent periodontal tissues and also that the characteristic bone destruction accompanying periodontitis can be significantly reduced by this approach. PDT has also been used to kill E. coli and P. aeruginosa in mice without causing damage to host tissues (2, 13). The encouraging results of this preliminary study suggest that further investigations of this novel approach to antimicrobial therapy are worth undertaking. Further animal studies are required to determine whether repeated applications of PDT leads to a greater reduction in bone loss and to establish the optimum treatment parameters before proceeding to clinical trials.
Acknowledgments
We thank the Charles Wolfson Charitable Trust for funding this research.
We thank A. Mosse for advice on fiber optics and J. Woodhams for advice on animal experimentation.
REFERENCES
- 1.Bagchi, B., and B. Sreeradha. 1989. Role of dye molecules remaining outside the cell during photodynamic inactivation of Escherichia coli in the presence of acriflavine. Photochem. Photobiol. 29:403-405. [DOI] [PubMed] [Google Scholar]
- 2.Berthiaume, F. S., R. Reiken, M. Toner, R. G. Tompkins, and M. L. Yarmush. 1994. Antibody-targeted photolysis of bacteria in vivo. Bio/Technology 12:703-705. [DOI] [PubMed] [Google Scholar]
- 3.Bhatti, M., A. MacRobert, S. Meghji, B. Henderson, and M. Wilson. 1997. Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem. Photobiol. 65:1026-1031. [DOI] [PubMed] [Google Scholar]
- 4.Bhatti, M., A. MacRobert, S. Meghji, B. Henderson, and M. Wilson. 1998. A study of the uptake of toluidine blue O by Porphyromonas gingivalis and the mechanism of lethal photosensitisation. Photochem. Photobiol. 68:370-376. [PubMed] [Google Scholar]
- 5.Chaves, E. S., M. K. Jeffcoat, C. C. Ryerson, and B. Snyder. 2000. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J. Clin. Periodontol. 27:897-903. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty, T. J., C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan, and Q. Peng. 1998. Photodynamic therapy. J. Natl. Cancer Inst. 90:889-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenberg, B., E. Gross, Y. Nitzan, and Z. Malik. 1993. Electric depolarization of photosensitized cells: lipids vs. protein alterations. Biochim. Biophys. Acta 1151:257-264. [DOI] [PubMed] [Google Scholar]
- 8.Fan, K. F., C. Hopper, P. M. Speight, G. Buonaccorsi, A. J. MacRobert, and S. G. Bown. 1996. Photodynamic therapy using 5-aminolaevulinic acid for premalignant lesions of the oral cavity. Cancer 78:1374-1383. [DOI] [PubMed] [Google Scholar]
- 9.Fan, K. F., C. Hopper, P. Speight, G. Buonaccorsi, and S. C. Bown. 1997. Photodynamic therapy using mTHPC for malignant disease in the oral cavity. Int. J. Cancer 73:25-32. [DOI] [PubMed] [Google Scholar]
- 10.Feres, M., A. D. Haffajee, C. Goncalves, K. A. Allard, S. Som, C. Smith, J. M. Goodson, and S. S. Socransky. 1999. Systemic doxycycline administration in the treatment of periodontal infections. II. Effect on antibiotic resistance of subgingival species. J. Clin. Periodontol. 26:784-792. [DOI] [PubMed] [Google Scholar]
- 11.Grant, W. E., G. Buonaccorsi, P. Speight, A. J. MacRobert, C. Hopper, and S. C. Bown. 1995. The effect of photodynamic therapy on the mechanical integrity of normal rabbit carotid arteries. Laryngoscope 105:867-871. [DOI] [PubMed] [Google Scholar]
- 12.Haas, R., M. Baron, O. Dortbudak, and G. Watzek. 2000. Lethal photosensitisation, autologous bone and e-PTFE membrane for the treatment of peri-implatitis: preliminary results. Int. J. Oral. Maxillofac. Implants 15:374-382. [PubMed] [Google Scholar]
- 13.Hamblin, M. R., D. A. O'Donnell, N. Murthy, C. H. Contag, and T. Hasan. 2002. Rapid control of wound infections by targeted photodynamic therapy monitored by in vivo bioluminescence imaging. Photochem. Photobiol. 75:51-57. [DOI] [PubMed] [Google Scholar]
- 14.Ito, T., and K. Kobayashi. 1977. In vivo evidence for the photodynamic membrane damage as a determining step of the inactivation of yeast cells sensitized by toluidine blue. Photochem. Photobiol. 25:399-401. [DOI] [PubMed] [Google Scholar]
- 15.Katz, J., K. P. Black, and S. M. Michalek. 1999. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun. 67:4352-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klausen, B., R. T. Evans, and C. Sfintescu. 1989. Two complementary methods of assessing periodontal bone level in rats. Scand. J. Dent. Res. 97:494-499. [DOI] [PubMed] [Google Scholar]
- 17.Komerik, N., and M. Wilson. 2002. Factors influencing the susceptibility of gram-negative bacteria to toluidine blue-mediated lethal photosensitisation. J. Appl. Microbiol. 92:618-623. [DOI] [PubMed] [Google Scholar]
- 18.Komerik, N., M. Wilson, and S. Poole. 2000. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem. Photobiol. 72:676-680. [DOI] [PubMed] [Google Scholar]
- 19.Loesche, W. J., S. A. Syed, E. C. Morrison, G. A. Kerry, T. Higgins, and J. Stoll. 1984. Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks. J. Periodontol. 55:325-335. [DOI] [PubMed] [Google Scholar]
- 20.MacRobert, A. J., S. G. Bown, and D. Phillips. 1989. What are the ideal photoproperties for a sensitizer? Ciba Found. Symp. 146:4-12. [DOI] [PubMed] [Google Scholar]
- 21.Malik, Z., J. Hanania, and Y. Nitzan. 1990. Bactericidal effects of photoactivated porphyrins—an alternative approach to antimicrobial drugs. J. Photochem. Photobiol. 5:281-293. [DOI] [PubMed] [Google Scholar]
- 22.Manson, J. D., and B. M. Eley. 1995. Outline of periodontics, 3rd ed. Wright, Oxford, United Kingdom.
- 23.Meyer, D. H., K. P. Mintz, and P. M. Fives-Taylor. 1997. Models of invasion of enteric and periodontal pathogens into epithelial cells: a comparative analysis. Crit. Rev. Oral Biol. Med. 8:389-409. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, M., P. Speight, and S. G. Bown. 1991. A study of the effects of photodynamic therapy on the tissues of the rabbit jaw. Br. J. Cancer 64:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millson, C. E., M. Wilson, A. J. MacRobert, and S. G. Bown. 1996. Ex-vivo treatment of gastric Helicobacter infection by photodynamic therapy. J. Photochem. Photobiol. B Biol. 32:59-65. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki, H., T. Pilot, M. H. Leclercq, and D. E. Barmes. 1991. Profiles of periodontal conditions in adults measured by CPITN. Int. Dent. J. 41:74-80. [PubMed] [Google Scholar]
- 27.Mohr, H., B. Bachmann, A. Klein-Struckmeier, and B. Lambrecht. 1997. Virus inactivation of blood products by phenothiazine dyes and light. Photochem. Photobiol. 65:441-445. [DOI] [PubMed] [Google Scholar]
- 28.Norkiewicz, D. S., L. G. Breault, S. T. Wonderlich, and K. H. Malone. 2001. The use of chemotherapeutic agents in localized periodontal pockets. Mil. Med. 166:940-946. [PubMed] [Google Scholar]
- 29.Olsvik, B., and F. C. Tenover. 1993. Tetracycline resistance in periodontal pathogens. Clin. Infect. Dis. 16(Suppl. 4):S310-S313. [DOI] [PubMed] [Google Scholar]
- 30.Oosterwaal, P. J., F. H. Mikx, and H. H. Renggli. 1990. Clearance of a topically applied fluorescein gel from periodontal pockets. J. Clin. Periodontol. 17:613-615. [PubMed] [Google Scholar]
- 31.Paardekooper, M., A. W. De Bruijne, J. V. Steveninck, and P. J. Van den Broek. 1995. Intracellular damage in yeast cells caused by photodynamic treatment with toluidine blue. Photochem. Photobiol. 61:84-89. [DOI] [PubMed] [Google Scholar]
- 32.Packer, S., M. Bhatti, T. Burns, and M. Wilson. 2000. Inactivation of proteolytic enzymes from Porphyromonas gingivalis using light-activated agents. Las. Med. Sci. 15:24-30. [DOI] [PubMed] [Google Scholar]
- 33.Pe, M. B., K. Sano, and T. Inokuchi. 1993. Effects of photodynamic therapy in the normal mouse tongue. J. Oral Maxillofac. Surg. 51:1129-1134. [DOI] [PubMed] [Google Scholar]
- 34.Peng, Q., S. B. Brown, J. Moan, J. M. Nesland, M. Wainwright, J. Griffths, B. Dixon, J. Cruse-Sawyer, and D. Vernon. 1993. Biodistribution of a methylene blue derivative in tumour and normal tissues of rats. J. Photochem. Photobiol. B Biol. 20:63-71. [DOI] [PubMed] [Google Scholar]
- 35.Quirynen, M., C. Mongardini, M. de Soete, M. Pauwels, W. Coucke. J. van Eldere, and D. van Steenberghe. 2000. The role of chlorhexidine in the one-stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations. J. Clin. Periodontol. 27:578-589. [DOI] [PubMed] [Google Scholar]
- 36.Rajapakse, P. S., N. M. O'Brien-Simpson, N. Slakeski, B. Hoffmann, and E. C. Reynolds. 2002. Immunization with the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infect. Immun. 70:2480-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renvert, S., G. Dahlen, and M. Wikstrom. 1996. Treatment of periodontal disease based on microbiological diagnosis. Relation between microbiological and clinical parameters during 5 years. J. Periodontol. 67:562-571. [DOI] [PubMed] [Google Scholar]
- 38.Sbordone, L., I. Ramaglia, E. Gulletta, and V. Iacono. 1990. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J. Periodontol. 61:579-584. [DOI] [PubMed] [Google Scholar]
- 39.Slots, J., and M. J. Jorgensen. 2002. Effective, safe, practical and affordable periodontal antimicrobial therapy: where are we going, and are we there yet? Periodontol. 2000 28:298-312. [DOI] [PubMed] [Google Scholar]
- 40.Spikes, J. D., and G. Jori. 1987. Photodynamic therapy of tumours and other diseases using porphyrins. Las. Med. Sci. 2:3-15. [Google Scholar]
- 41.Stabholz, A., A. A. Nicholas, G. J. Zimmerman, and U. M. Wikesjo. 1998. Clinical and antimicrobial effects of a single episode of subgingival irrigation with tetracycline HCl or chlorhexidine in deep periodontal pockets. J. Clin. Periodontol. 25:794-800. [DOI] [PubMed] [Google Scholar]
- 42.Syed, S. A., and W. J. Loesche. 1972. Survival of human dental plaque flora in various transport media. Appl. Microbiol. 24:638-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 44.Warnakulasuriya, K., and N. W. Johnson. 1996. Sensitivity and specificity of orascan toluidine blue mouthrinse in the detection of oral cancer and precancer. J. Oral Pathol. Med. 25:97-103. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, M. 1993. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease: a review. J. Appl. Bacteriol. 75:299-306. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, M., J. Dobson, and W. Harvey. 1992. Sensitization of oral bacteria to killing by low-power laser radiation. Curr. Microbiol. 25:77-81. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, M., and C. Yianni. 1995. Killing of methicillin-resistant Staphylococcus aureus by low-power laser light. J. Med. Microbiol. 42:62-66. [DOI] [PubMed] [Google Scholar]