Abstract
The main constraints on the administration of aminoglycosides are the risks of nephrotoxicity and ototoxicity, which can lead to acute, renal, vestibular, and auditory toxicities. In the present study we focused on nephrotoxicity. No reliable predictor of nephrotoxicity has been found to date. We have developed a deterministic model which describes the pharmacokinetic behavior of aminoglycosides (with a two-compartment model), the kinetics of aminoglycoside accumulation in the renal cortex, the effects of aminoglycosides on renal cells, the resulting effects on renal function by tubuloglomerular feedback, and the resulting effects on serum creatinine concentrations. The pharmacokinetic parameter values were estimated by use of the NPEM program. The estimated pharmacodynamic parameter values were obtained after minimization of the least-squares objective function between the measured and the calculated serum creatinine concentrations. A simulation program assessed the influences of the dosage regimens on the occurrence of nephrotoxicity. We have also demonstrated the relevancy of modeling of the circadian rhythm of the renal function. We have shown the ability of the model to fit with 49 observed serum creatinine concentrations for a group of eight patients treated for endocarditis by comparison with 49 calculated serum creatinine concentrations (r2 = 0.988; P < 0.001). We have found that for the same daily dose, the nephrotoxicity observed with a thrice-daily administration schedule appears more rapidly, induces a greater decrease in renal function, and is more prolonged than those that occur with less frequent administration schedules (for example, once-daily administration). Moreover, for once-daily administration, we have demonstrated that the time of day of administration can influence the incidence of aminoglycoside nephrotoxicity. The lowest level of nephrotoxicity was observed when aminoglycosides were administered at 1:30 p.m. Clinical application of this model might make it possible to adjust aminoglycoside dosage regimens by taking into account both the efficacies and toxicities of the drugs.
Aminoglycoside (AG) nephrotoxicity is a well-known occurrence. However, 50 years after the discovery of the first AG (streptomycin), nephrotoxicity is still very difficult to predict and avoid (16).
To date, no model has completely described the pharmacodynamic behavior of AG nephrotoxicity, even though the mechanism of this toxicity has been widely studied (4, 7, 15, 27). AGs are retained in the epithelial cells lining the proximal tubule after glomerular filtration. AGs become attached to the brush-border membrane in their cationic form. The initial points of attachment are probably the acidic phospholipids, especially phosphatidylserine. In this way, aminoglycosides accumulate and cause leakage of intracellular ions (K+, Mg2+, Ca2+), proteins (beta-2-microglobulin, alpha-2-macroglobulin, lysozyme), and enzymes (alanylaminopeptidase, N-acetylglucosaminidase). Thus, the resulting decline in glomerular filtration has a multifactorial origin and involves a combination of tubular and nontubular mechanisms. The most important factor seems to be a tubuloglomerular feedback (19, 24). The kidneys have a strong capacity to compensate for tubular injuries. The importance of regeneration for protection against renal injury is clearly demonstrated by the survival of laboratory rats exposed to repeated administrations of high daily doses of AG (40 mg of gentamicin per kg of body weight per day for at least 42 days). After an initial episode of acute tubular necrosis that occurs within 8 to 10 days and that is associated with marked azotemia, the renal function returns almost to normal, as if the kidney had become refractive (6). Regenerating cells are less differentiated and apparently less susceptible to AG (the level of accumulation of AG is actually reduced in the cortices of animals treated for long periods).
Even though AG pharmacokinetic (PK) behavior in humans has been accurately described (22), the relation between PKs and the conditions of occurrence of AG nephrotoxicity is not clearly established. Many PK studies show that at any given cumulative area under the curve (AUC) for the serum AG concentrations, the risk of nephrotoxicity is lower for once-daily dosing of an AG than for traditional AG dosing (AG administration every 8 or 12 h) (1, 20, 21). This phenomenon is due to the renal uptake of AGs, which is nonlinear and saturable, as shown in studies with animals (10) and humans (1, 19). Studies with megalin indicate that uptake of AGs by tubule cells is saturable (23).
Unlike investigators who have recently reported their findings (20, 21), we believe that AG nephrotoxicity can be represented by a single toxicity effect model that does not depend either on the AUC for the serum AG concentrations or on the administration schedule. We believe that a PK model alone probably cannot explain the clinical or toxicity outcome. We believe that the PK model must include the drug distribution in a potential second compartment and/or a pharmacodynamic (PD) (effect and/or toxicity) model. Also, modeling of AG nephrotoxicity must take into account both the amounts of an AG in the renal cortex, the resulting PD effect represented by leakage from cells into the renal tubule, and the stimulation of the tubuloglomerular feedback that causes the decrease in glomerular filtration.
The objectives of the present study were (i) to develop a deterministic model of AG nephrotoxicity which takes into account both PK and PD variabilities, (ii) to estimate the PK parameter values and, for the first time, the PD parameter values from the deterministic model for a group of patients treated with amikacin over a long period, and (iii) to simulate AG nephrotoxicity and observe the influence of the dosage regimen and the time of administration.
MATERIALS AND METHODS
PK model.
We chose to use a bicompartmental PK model (8) with intravenous administration. The amount of AG in the serum or central compartment [QS(t); in milligrams] depends on the amount of AG from intravenous administration, the peripheral compartments, and renal tubular reabsorption. QS(t) also depends on the amount of AG in serum which is transferred out of the central compartment to the peripheral compartment or which is eliminated. The AG elimination rate constant can be divided into a principal renal elimination route, which depends linearly on creatinine clearance, and a nonrenal elimination constant (22). QS(t) varies according to the following:
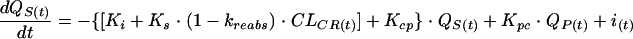 |
(1) |
where C(t) is equal to QS(t)/(V · BW)
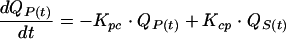 |
(2) |
where t is time (in hours), i(t) (in milligrams per hour) is the infusion rate, Ki (per hour) is the AG nonrenal elimination rate constant, Ks (in milliliters per minute per hour) is the renal elimination constant, kreabs is the tubular reabsorption constant, CLCR(t) (milliliters per minute) is the creatinine clearance, C(t) (in milligrams per liter) is the serum AG concentration, V (in liters per kilogram) is the volume of distribution of AG in the central compartment, BW (in kilograms) is the body weight, Kcp (per hour) is the transfer constant from the central compartment to the peripheral compartment, Kpc (per hour) is the transfer constant from the peripheral compartment to the central compartment, and QP(t) (in milligrams) is the amount in the peripheral compartment.
PD model.
The amount of AG left in the renal cortex [QC(t)] is the difference between the amount of AG that accumulates according to a nonlinear and saturable mechanism (1, 10, 19) such as Michaelis-Menten kinetics (18), as demonstrated in rats (10), and the amount of AG which is eliminated from the renal cortex (10). QC(t) varies as shown below:
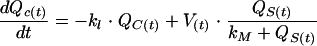 |
(3) |
where V(t) (in milligrams per hour) is the maximum accumulation rate in the renal cortex, QS(t) (in milligrams) is the amount of AG in serum, kM (in milligrams) is the amount of AG in serum for which V(t)/2 is obtained, and k1 (per hour) is the elimination rate constant for AG from the renal cortex.
The AG renal accumulation effect [E(t); in millimolar] is represented by a classic Hill equation (11, 17), which links the amount of AG in the renal cortex to the intracellular components leaking into the renal tubule. In addition, we added a threshold of the amount of AG in the renal cortex under which no effect is observed. E(t) varies as shown below:
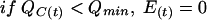 |
(4) |
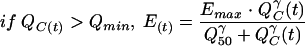 |
(5) |
where Qmin (in milligrams) is the amount of AG in the renal cortex under which no effect is observed, Emax (in millimolar) is the maximum accumulation effect observed, Q50 (in milligrams) is the amount of AG in the renal cortex for which E(t) is equal to Emax/2, and γ (which is dimensionless) is the Hill sigmoidicity parameter.
When the AG treatment lasts more than 1 month, it has been shown in the rat that, despite the occurrence of nephrotoxicity during the first few weeks, renal function returns almost to normal from 6 or 7 weeks after the beginning of treatment (6). The regeneration of tubular cells during AG nephrotoxicity induces a decrease in the level of AG accumulation in the renal cortex (26), i.e., a decrease in V(t), as shown below:
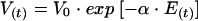 |
(6) |
where V0 (in milligrams per hour) is the maximum accumulation rate at the beginning of the treatment and α (in millimolar−1) is the maximum accumulation rate decrease constant.
The glomerulotubular feedback action can also be represented by a Hill equation (24). This effect is observed on creatinine clearance [CLCR(t); in milliliters per minute] and is associated with a circadian variation, represented here in a sinusoidal form (28), which is an independent factor. CLCR(t) varies during a typical 24-h day as shown below:
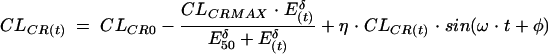 |
(7) |
where CLCR0 (in milliliters per minute) is the creatinine clearance value at the beginning of the treatment, CLCRMAX (in milliliters per minute) is the maximum decrease in creatinine clearance, E50 (in millimolar) is the accumulation effect value for which CLCR(t) is equal to CLCRMAX/2, δ (which is dimensionless) is the Hill sigmoidicity parameter, η is the amplitude of the circadian variation in renal function, ω (which is dimensionless) is the period of the circadian rhythm of the renal function, and ϕ (which is dimensionless) is the value describing the phase relationship of the circadian rhythm.
At this stage, we have introduced a second model without the circadian variation in renal function (model 2), i.e., without the sinusoidal function in equation 7, in order to compare it with model 1, which includes the circadian variation in renal function (equation 7), and to investigate the relevancy of this periodic variation.
The decrease in renal function involves a rise in the serum creatinine level, which depends on the amount produced by muscle daily and the daily renal elimination (13). The amount of creatinine in serum [SCr(t); in milligrams] varies as shown below:
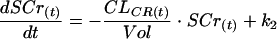 |
(8) |
where CLCR(t) (in milliliters per minute) is the creatinine clearance, Vol (in liters) is the volume of distribution of creatinine, and k2 (in milligrams per hour) is the mean amount of creatinine produced by muscle daily.
Model development.
We used the Matlab-Simulink program (Math Works Inc.) for all simulations and estimations. The program makes it possible to select each PK and PD parameter value. The system allows the user to choose as inputs the administration schedule, including the first loading dose, the number of doses, the infusion time, the time of day of the infusion, and the time delay between two infusions. Every simulation began at 12:00 a.m. The glomerular filtration rate shows a repeating pattern of variation over 24 h, i.e., a circadian rhythm (14). This corresponds to an ω value of 2π/24 (equation 7). We chose a sinusoidal adjustment so that the creatinine clearance value was highest at 2:00 p.m. and lowest at 2:00 a.m. (14). This corresponds to a ϕ value of −2π/3 (equation 7). The amplitude of the circadian rhythm was fixed at 10% (η = 0.1) (equation 7) of the creatinine clearance value, which is the most widely used value (14).
Experimental framework.
For the PK and PD parameter estimations, we used data for patients for whom renal toxicity had been observed, as shown by increases in the serum creatinine concentrations (i.e., a decrease in creatinine clearance) with no clinical explanation other than AG administration. The large number of parameters to be estimated led us to select patients for whom a large number of measurements of serum creatinine concentrations were available, i.e., patients treated over a long period. Eight patients who had received amikacin and vancomycin for over 1 month for the treatment of endocarditis were selected. The patients' general characteristics and the treatment conditions are described in Table 1. Amikacin was administered once daily via 30-min infusions. Vancomycin was administered twice daily during the first 2 to 3 days of treatment and then once-daily afterwards via 2-h infusions. Amikacin doses were calculated as a function of the CLCR0, i.e., 15 mg/kg/day when CLCR0 was above 80 ml/min and 7.5 mg/kg/day when CLCR0 was between 30 and 80 ml/min. The doses of amikacin and vancomycin were constant and not adapted during the treatment. Except for vancomycin, no other nephrotoxic drug was administered during the amikacin treatments. The serum creatinine concentrations were measured at different times during treatment and also after treatment had ended in order to observe the overall evolution of the kinetics of the serum creatinine concentrations.
TABLE 1.
General characteristics of eight patients treated for endocarditis with amikacin and vancomycin over a long period and used for estimations
| Characteristic or parameter | Median | Minimum | Maximum |
|---|---|---|---|
| Patient data | |||
| Age (yr) | 51 | 42 | 72 |
| Ht (cm) | 168 | 150 | 178 |
| Wt (kg) | 62 | 48 | 82 |
| Sex ratio | 0.57 | 0.23 | 0.91 |
| CLCR0 (ml/min) | 95 | 45 | 120 |
| Variation of CLCR0 (%) | 74 | 65 | 90 |
| Treatment data for amikacin | |||
| Daily dose (mg) | 451 | 350 | 850 |
| Duration of therapy (days) | 33 | 31 | 38 |
| Peak concn (mg/liter) | 44.3 | 26.6 | 90.1 |
| Trough concn (mg/liter) | 4.5 | 3.6 | 6.5 |
| Treatment data for vancomycin | |||
| Daily dose (mg) | 1,860 | 850 | 2,460 |
| Duration of therapy (days) | 45 | 43 | 48 |
| Peak concn (mg/liter) | 26.3 | 20.3 | 32.6 |
| Trough concn (mg/liter) | 10.4 | 6.5 | 13.4 |
PK and PD parameter values for estimations and simulations.
For the estimations, the Ki parameter value (equation 1) was fixed at 0.006932/h, which corresponds to the Ki value observed for anuric patients (half-life, 100 h) (12). We chose to fix the Ki parameter value in order to put the whole variability of elimination on Ks because nonrenal elimination is very small in comparison with Ks. The kreabs parameter value was fixed at 0.05 (dimensionless) (12); otherwise, the system is not identifiable. Other individual PK parameter values (Ks, V, Kcp, and Kpc) were estimated by use of the NPEM program (25). For the PD parameter values, E50 was fixed at 33.5 mM (24); otherwise, α and Emax are not identifiable. Moreover, V0 was fixed at 1 mg/h in order to obtain Qc(t) values in the range of the data in the literature (10); otherwise, Q50 is not identifiable. Other PD parameters (kM, k1, Qmin, Emax, Q50, γ, CLCRMAX, δ, α, k2, and Vol) were estimated for the eight-patient group by use of the Matlab program with the estimated PK parameter values and the fixed PD parameter values described above (E50 and V0) and by minimization of the least-squares objective function (OF), as shown below:
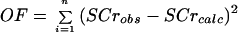 |
(9) |
where OF is the objective function to be minimized, SCrobs (in milligrams per liter) is the measured serum creatinine concentration, and SCrcalc (in milligrams per liter) is the calculated serum creatinine concentration.
To investigate the relevancy of the circadian rhythm of the renal function of model 1 in comparison with model 2 (without a circadian rhythm), we calculated the following errors: err1 = SCrobs − SCrcalc1 for model 1 (where SCrobs is the serum creatinine concentration measured for the eight-patient group treated with amikacin for endocarditis and SCrcalc1 [in milligrams per liter] is the corresponding serum creatinine concentration calculated by use of model 1) and err2 = SCrobs − SCrcalc2 for model 2 (where SCrcalc2 [in milligrams per liter] is the corresponding serum creatinine concentration calculated by use of model 2). Their absolute values are designated |err1| and |err2|, respectively. In order to test the hypothesis that |err2| is greater than |err1|, we applied the Wilcoxon signed-rank test.
The parameter values used for the simulations are presented in Table 2. PK parameter values (Ki, Ks, V, Kcp, Kpc, and kreabs) correspond to data from the literature (12). α was defined so that renal function returns almost to normal after 42 days of treatment with amikacin at 1,000 mg per day, which is the delay observed in the rat (6) and which corresponds to an α value of 0.02 mM−1. Other PD parameter values were mainly drawn from past studies: V0 and kM from reference 10; CLCRMAX, E50, and δ from reference 24; and k2 and Vol from reference 13. In the absence of data from the literature, other PD parameter values (k1, Qmin, Emax, Q50, and γ) were those estimated with the Matlab program for the eight-patient group by use of model 1. The body weight was fixed at 70 kg. CLCR0 and the initial serum creatinine concentration (SCr0) were fixed at normal values, i.e., 100 ml/min and 8 mg/liter, respectively.
TABLE 2.
PK and PD parameter values used for simulations and estimated values (median, maximum, minimum) from model 1 (which includes the circadian variation in renal function) for eight patients treated for endocarditis over a long period
| Value | Ki (h−1) (1)a | KS (min/ml/h) (1) | V (liters/kg) (1) | Kcp (h−1) (2) | Kpc (h−1) (2) | kreabs (unitless) (1) | V0 (mg/h) (6) | kM (mg) (3) | k1 (h−1) (3) | Qmin (mg) (4) | Emax (mM) (5) | Q50 (mg) (5) | γ (unitless) (5) | CLCRMAX (ml/min) (7) | E50 (mM) (7) | δ (unitless) (7) | α (mM−1) (6) | k2 (mg/h) (8) | Vol (liters) (8) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simulationb | 0.006932 (12) | 0.00257 (12) | 0.25 (12) | 0.03798 (12) | 0.013 (12) | 0.05 (12) | 1 (10) | 15 (10) | 0.06 | 42.5 | 100 | 50 | 2.5 | 20 (24) | 33.5(24) | 6 (24) | 0.02 (6) | 60 (13) | 5 (13) |
| Estimatedc | |||||||||||||||||||
| Median | 0.006932 | 0.0019 | 0.3024 | 0.0854 | 0.1120 | 0.05 | 1 | 10.2 | 0.06 | 42.5 | 190 | 55.4 | 2.5 | 41 | 33.5 | 5.5 | 0.03 | 64 | 4.8 |
| Minimum | 0.0009 | 0.2708 | 0.0421 | 0.0758 | 8.1 | 0.04 | 37.5 | 30 | 32.5 | 2.4 | 30 | 5 | 0.028 | 52 | 4.3 | ||||
| Maximum | 0.0026 | 0.3842 | 0.1152 | 0.1374 | 13.5 | 0.07 | 50 | 240 | 77.1 | 2.6 | 45 | 6 | 0.045 | 72 | 5.3 | ||||
| |Minimum − maximum|/median (%) | 89 | 38 | 86 | 55 | 53 | 50 | 29 | 110 | 81 | 8 | 37 | 18 | 57 | 31 | 21 |
The numbers in parentheses are those for the equations in Materials and Methods.
Parameter values for all simulations, with reference numbers given in parentheses.
For eight patients treated with amikacin and vancomycin over a long period (>1 month) for endocarditis.
RESULTS
Relevance of model 1 in comparison with that of model 2.
Forty-nine datum points were obtained for the eight patients for use in each of the two models (i.e., 49 paired |err1| and |err2| values), and the Wilcoxon signed-rank test was applied to the differences between |err1| and |err2| and yielded a P value <10−5. As a consequence, we consider model 1, which includes the circadian variation in renal function, to be the more relevant.
Fit of the data with model 1 (which includes the circadian variation in renal function).
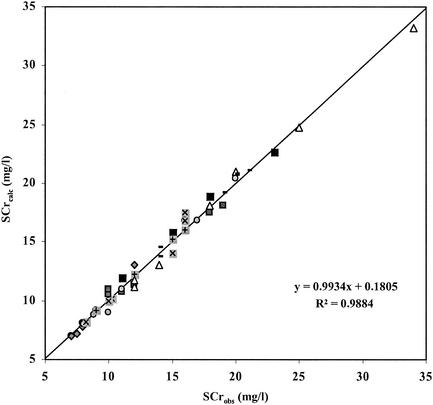
Table 2 shows the medians, minima, and maxima of the PK parameter values (V, Ks, Kcp, and Kpc) estimated with the NPEM program by use of a two-compartment model. Table 2 also provides the medians, minima, and maxima of the PD parameter values (kM, k1, Qmin, Emax, Q50, γ, CLCRMAX, δ, α, k2, and Vol) estimated for the eight patients by minimizing the objective function (equation 9) with the Matlab program. Figure 1 shows the relation between the 49 observed serum creatinine concentrations and the 49 serum creatinine concentrations calculated by use of model 1, as represented by a linear regression with a high and significant correlation coefficient (r2 = 0.9884 with P < 0.001 if the hypothesis that r2 is equal to 0 is put to the test).
FIG. 1.
Comparison of SCrobs and SCrcalc (n = 49) by use of model 1 (which includes the circadian variation in renal function) for an eight-patient group treated with amikacin and vancomycin for endocarditis for a long period. Different symbols are used for each patient.
Evolution of nephrotoxicity and health conditions of the patients after treatment.
After the treatments had ended, the renal functions of the eight patients almost returned to their initial states. The eight patients recovered from the clinical and bacterial perspectives, indicating the efficacy of the treatment.
Simulations with model 1 (which includes the circadian variation in renal function).
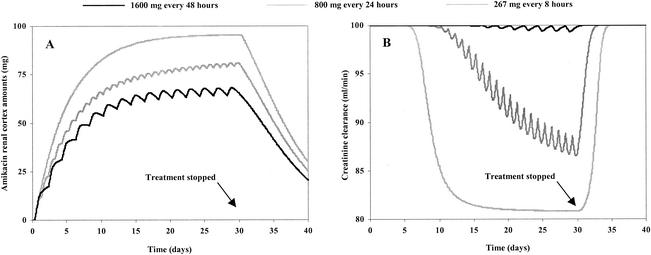
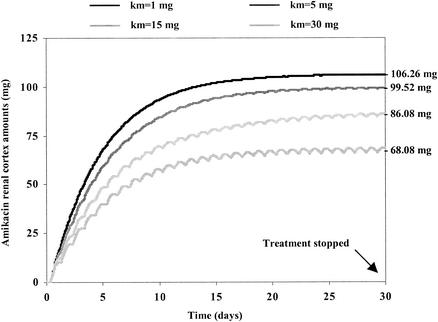
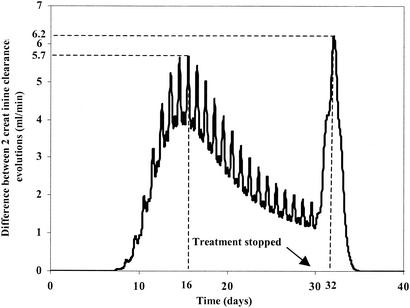
Figure 2 shows the simulated differences between the amounts of amikacin in the renal cortex for three different infusion schedules (which had identical cumulative AUCs for the serum amikacin concentrations) administered over a 30-day period at identical daily doses (800 mg). The doses were 1,600 mg every 48 h, 800 mg once daily, and 267 mg three times daily. The infusion time was fixed at 30 min for all doses administered. Figure 2 shows that the lowest level of accumulation in the renal cortex and, therefore, the lowest decrease in creatinine clearance occurred when amikacin was administered less frequently. Figure 2 shows that with a more frequent dosage regimen, nephrotoxicity (i) appears more rapidly, (ii) induces a greater decrease in renal function, and (iii) is more prolonged. Figure 3 shows the influences of different kM values on the accumulation of AG in the renal cortex with the same dosage regimen, i.e., 1,000 mg of amikacin per day over a 30-day period. Higher levels of accumulation were observed with lower kM values (Qc was equal to 106.26 mg for kM equal to 1 mg, Qc was equal to 99.52 mg for kM equal to 5 mg, Qc was equal to 86.08 mg for kM equal to 15 mg, and Qc was equal to 68.08 mg for kM equal to 30). Figure 4 describes the influence of only the circadian variation in renal function by use of a 30-day amikacin treatment (1,000 mg once daily) and different times of day of administration (1:30 p.m. versus 1:30 a.m.). The lowest level of nephrotoxicity was obtained when amikacin was administrated at 1:30 p.m. The PK and PD parameter values for all simulations are described in Table 2.
FIG. 2.
Evolution of amounts of amikacin in the renal cortex for three different dosage regimens with identical total doses (A) and the resulting effects on mean renal function evolution (B) by use of model 1 (which includes the circadian variation in renal function).
FIG. 3.
Comparison of evolution of the amount of AG in the renal cortex for different possible values of kM for treatment with amikacin at 1,000 mg once daily over a 30 day-period by use of model 1 (which includes a circadian variation in renal function).
FIG. 4.
Difference in creatinine clearance evolution for two dosage regimens by use of model 1 (which includes a circadian variation in renal function), with 1,000 mg of amikacin administered once daily at 1:30 p.m. or 1:30 a.m. over a 30 day-period.
DISCUSSION
Many methods for reducing AG nephrotoxicity have been proposed. Molecular modeling can bring about an intrinsically less toxic AG (19), but these methods are very expensive. Protective approaches such as the administration of polyaspartic acid or deferroxamine can reduce renal injuries caused by an AG. Unfortunately, these approaches could not be translated into clinical applications because of a lack of efficacy and/or intrinsic toxicity (19). In addition, population PK computer programs, used to control serum AG concentrations, are able to provide good predictions of efficacy, as they calculate PD indices such as the AUC/MIC or the maximum concentration in serum/MIC ratio (3). By contrast, the estimated concentrations in the second compartment are not good predictors of nephrotoxicity because they do not take into account nonlinear processes such as the amount of AG taken up in the renal cortex or the tubuloglomerular feedback. Then, a new methodology more suitable for clinical practice was developed. This methodology is based on a PK-PD model. A deterministic model seems to be sufficient to represent AG nephrotoxicity. Our model is complex because it takes into account the renal physiology and describes most precisely the mechanisms involved in AG nephrotoxicity. This approach is very interesting because it gives much information about the PK and PD behaviors of AGs in individual patients.
The relevance of taking into consideration the circadian variation in renal function has been demonstrated by fitting the model to the observed data and comparison of that model with a model that does not include the circadian variation (P < 10−5). This shows the importance of taking into account these periodic phenomena in biological models.
The high and significant correlation coefficient (r2 = 0.9884; P < 0.001) (Fig. 1) shows that model 1 (which includes the circadian variation in renal function) is a powerful tool for the representation of renal injuries caused by AG. It might be useful as a predictor of the occurrence of AG nephrotoxicity.
Goldbeter and Claude (9) have shown that the modeling approach can explain the influences of time-patterned drug administration on toxicity in general. AG nephrotoxicity can be influenced by the administration schedule (5), the times of day of administration (2), the parameter kM (equation 3) (10), and inter- and intraindividual variabilities (16). All these aspects are explored below.
For an identical total dose of amikacin, the cumulative AUC for the serum amikacin concentrations remained unchanged for all administration schedules, but the amounts of amikacin in the renal cortex differed (Fig. 2A). As is well known (21), less toxicity is observed when AGs are administered less frequently (Fig. 2B), while the efficacy remains the same (the AUC for the serum AG concentrations and the AUC/MIC ratio remain unchanged). In addition, our model shows that nephrotoxicity (i) appears more rapidly, (ii) induces a greater decrease in renal function, and (iii) is more prolonged when administration schedules are more frequent (Fig. 2B). Accordingly, the differences in the reductions of creatinine clearance between the three dosage regimens for an identical daily dose are maximum on day 30. On that day, the creatinine clearance still represents 99% of the initial creatinine clearance for the dosage regimen of 1,600 mg every 48 h, whereas it represents only 82% of the initial creatinine clearance for the dosage regimen of 267 mg three times daily. Although less frequent administration results in a smaller amount of AG in the renal cortex, the resulting effects on renal function can be very close for the different regimens because of nonlinear relationships. Hence, very similar effects can be observed, and the ability to select a less frequent dosing regimen in order to obtain a lower level of toxicity is not possible when the treatments are prolonged.
Because AG uptake by renal cells is saturable (10, 27), the amount of AG in the renal cortex is also saturable. The more quickly saturated it is (i.e., the lower the kM) (equation 3), the less the amount of AG in the renal cortex is affected by peak concentrations in serum (Fig. 3). However, less renal protection is observed with a lower saturability level (i.e., a lower the kM) (Fig. 3). When the kM is lower, the amount of amikacin in the renal cortex increases. When the kM is higher, the amounts of amikacin in the renal cortex decrease. In fact, 1/kM can be considered an index of the sensibility of using a particular drug for a patient. With a higher kM, the sensibility, the level of accumulation in the renal cortex, and the nephrotoxicity are lower. Patients with high kM values are less sensitive to the nephrotoxic effects of AG.
In accordance with Beauchamp and Labrecque (2), our model shows that AG administration at 1:30 p.m., i.e., during the activity period or food intake, is preferable to administration at 1:30 a.m., i.e., during the rest period, as demonstrated in humans and animals. The mechanisms associated with temporal variations in AG nephrotoxicity are not completely understood, but two hypotheses can be given. First, temporal variations in PK parameters may be of crucial importance, and second, food intake or the activity period may be of crucial importance (2). The relation between food intake and the interactions of AGs with renal tubular cells seems to be linked to the urine pH (2). The interaction is stronger at low pH than at high pH, since these molecules are fully protonated at low pH. It has been shown that the urine pH is higher during the activity period or during the period of food intake and lower during the rest period (2). This could partly explain the lower level of nephrotoxicity during the activity period. Our model confirms that a simple change in the time of administration (at 1:30 p.m. instead of 1:30 a.m.) of an identical dosage causes a decrease in toxicity from day 8. This is quantified by the difference between the two evolutions of the creatinine clearance values (Fig. 4). Administration at 1:30 a.m. induces a higher level of accumulation in the renal cortex and therefore a higher level of nephrotoxicity. The difference between the two creatinine clearance evolutions is positive and becomes maximum at day 16 (5.7 ml/min). After that, the difference in toxicity decreases until the treatment is stopped at day 30. This phenomenon may be explained by the fact that the diminution of creatinine clearance follows a nonlinear process. Two very different amounts of AG in the renal cortex have two very similar effects on creatinine clearance when CLCRMAX, i.e., the maximum diminution of the creatinine clearance (equation 7), is attained. After the end of treatment at day 30, a lower level of reduction of creatinine clearance is still observed when AG is administered at 1:30 p.m. In fact, over time, renal function returns to its initial value for both administration schedules but does so more rapidly with daily administration at 1:30 p.m. After the treatment is stopped, because the amount of AG in the renal cortex is lower when AG is administered at 1:30 p.m. than when it is administered at 1:30 a.m., there is a lower level of decrease in creatinine clearance. The difference between the two creatinine clearance evolutions is maximum at day 32 (6.2 ml/min), i.e., 2 days after the end of the treatment; then, it decreases to zero at day 35, i.e., 5 days after the end of the treatment. It corresponds to the time required for the complete elimination of the AG from the renal cortex. At that time, the creatinine clearance values are equal to those observed before the beginning of treatment because AG nephrotoxicity is reversible, as is well known (19).
The deterministic aspect of this nonlinear model is shown in particular in Fig. 4, which shows the periodic oscillations (period of 24 h) corresponding to the administration schedules. Hence, the nonlinear deterministic model (but not a stochastic model) is forced by periodic oscillations.
Some of the PD parameters estimated for the eight patients treated with amikacin for endocarditis for a long period show higher degrees of variability than others (Table 2). The great variability observed during the time of AG nephrotoxicity (16) is partly due to interindividual variability in the level of accumulation in the renal cortex (equation 3), especially for kM (53%) and k1 (50%) (Table 2). Another source of interindividual variability is the resulting effect of the accumulation of the AG in the renal cortex, i.e., the leaking of AG from the intracellular components into the renal tubule, as shown by the parameters Emax (110%) and Q50 (81%). Some other PD parameters showed slight interindividual variabilities, such as CLCRMAX (37%) and δ (18%), which are tubuloglomerular feedback parameters. These results may explain why some patients are more sensitive than others to AG nephrotoxicity. In fact, a high level of interindividual variability is observed for parameters linked to the AG itself. By contrast, small interindividual variability is observed for the physiological process that induces a decrease in renal function. We point out the fact that the PD parameters described in Table 2 are those estimated for the concurrent administration of amikacin and vancomycin, the latter of which is considered an additional risk factor for nephrotoxicity. As a consequence, the values of the PD parameters estimated here apply only to the group of patients evaluated in the present study. The model also takes into account intraindividual variability, i.e., changes in parameter values during a treatment. For example, intraindividual variability can be observed in some clinical situations when the infection itself induces a decrease in renal function. When the infection is treated, the renal function is restored. In addition, experimental studies show that the level of accumulation of gentamicin is actually reduced in the cortices of animals treated for long periods by a mechanism that involves a decrease in the rate of accumulation of AG in the renal cortex, i.e., a decrease in V(t) (equation 6) (26).
One of the most interesting aspects of this deterministic model is that it makes it possible, for the first time, estimation of some PD parameter values with the help of serum creatinine concentrations. However, in the clinical routine, the use of serum creatinine concentrations as a marker of renal function is not 100% reliable. In fact, serum creatinine concentrations increase only when renal mechanisms fail to compensate for all the toxicity. In short, the serum creatinine concentration is a belated marker of renal function. The use of an early marker of renal injuries like enzymuria (alanylaminopeptidase) as a complement to the serum creatinine concentrations is necessary to better estimate the PD parameter values, chiefly, those with great interindividual variability.
The deterministic model presented in this report is a powerful tool that can be used to simulate and control AG nephrotoxicity. Its use brings rationality to the empirical observations made over the past 50 years. However, we must point out the extreme limitation of this study: it only applies to eight humans. Moreover, the model takes into account only nephrotoxicity, not efficacy or other toxicities. As a consequence, these results should be confirmed by the joint use of an efficacy model (such as the model of Zhi [29] or other models) and the toxicity model described in this report. The population PK computer programs cannot be used to achieve an overall optimum therapy. The aim of any therapy is to obtain the greatest efficacy without exceeding toxicity limits. In future, it might not be idle to consider incorporation of this model into the population PK programs, which would enable them to achieve this type of optimization.
REFERENCES
- 1.Barclay, M. L., C. M. J. Kirkpatrick, and E. J. Begg. 1999. Once-daily aminoglycoside therapy. Is it less toxic than multiple daily doses and how should it be monitored? Clin. Pharmacokinet. 36:89-98. [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp, D., and G. Labrecque. 2001. Aminoglycoside nephrotoxicity: do time and frequency of administration matter? Curr. Opin. Crit. Care 7:401-408. [DOI] [PubMed] [Google Scholar]
- 3.Corvaisier, S., P. H. Maire, M. Y. Bouvier d'Yvoire, X. Barbaut, N. Bleyzac, and R. W. Jelliffe. 1998. Comparison between antimicrobial pharmacodynamic indices and bacterial killing as described by using the Zhi model. Antimicrob. Agents Chemother. 42:1731-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Broe, M. E., G. J. Paulus, G. Verpooten, F. Roels, N. Buyssens, R. Wedeen, and P. M. Tulkens. 1984. Early effects of gentamicin, tobramycin and amikacin on the human kidney. Kidney Int. 25:643-652. [DOI] [PubMed] [Google Scholar]
- 5.De Broe, M. E., and G. A. Verpooten. 1991. Influence of dosage schedules on renal accumulation of amikacin and tobramycin in man. J. Antimicrob. Chemother. 27(Suppl. C):41-47. [DOI] [PubMed] [Google Scholar]
- 6.Elliott, W. C., D. C. Houghton, D. N. Gilbert, J. Baines-Hunter, and W. M. Bennett. 1982. Gentamicin nephrotoxicity. I. Degree and permanence of acquired insensitivity. J. Lab. Clin. Med. 100:501-512. [PubMed] [Google Scholar]
- 7.Evan, A. P., and F. C. Luft. 1982. Gentamicin-induced glomerular injury, p. 67-78. In Néphrotoxicité, ototoxicité médicamenteuses. Editions INSERM (Institut National de la Santé et de la Recherche Médical), Paris, France.
- 8.Gibaldi, M., and D. Perrier. 1991. Drug and pharmaceutical sciences: pharmacokinetics, vol. 15, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 9.Goldbeter, A., and D. Claude. 2002. Time-patterned drug administration: insights from a modelling approach. Chronobiol. Int. 19:157-175. [DOI] [PubMed] [Google Scholar]
- 10.Guiliano, R. A., G. A. Verpooten, L. Verbist, R. P. Wedeen, and M. E. De Broe. 1986. In vivo uptake kinetics of aminoglycosides in the kidney cortex of rats. J. Pharmacol. Exp. Ther. 236:470-475. [PubMed] [Google Scholar]
- 11.Hill, A. V. 1910. The possible effects of the aggregation of hemoglobin on its dissociation curve. J. Physiol. 40:iv-vii.
- 12.Hurst, A. K., K. T. Iseri, M. A. Gill, J. K. Nogushi, T. M. Gilman, and R. W. Jelliffe. 1987. Comparison of four methods for predicting serum gentamicin concentrations in surgical patients with perforated or gangrenous appendicitis. Clin. Pharm. 6:234-238. [PubMed] [Google Scholar]
- 13.Jelliffe, R. W., and S. Jelliffe. 1972. A computer program for estimation of creatinine clearance from unstable serum creatinine levels. Math. Biosci. 14:17-24. [Google Scholar]
- 14.Koopman, M. G., G. C. Koomen, R. T. Krediet, E. A. de Moor, F. G. Hoek, and L. Arisz. 1989. Circadian rhythm of glomerular filtration rate in normal individuals. Clin. Sci. 77:105-111. [DOI] [PubMed] [Google Scholar]
- 15.Kosek, J. C., R. I. Mazze, and J. Cousins. 1974. Nephrotoxicity of gentamicin. Lab. Investig. 30:48-57. [PubMed] [Google Scholar]
- 16.Maire, P. H., S. Corvaisier, F. Rougier, M. Bouvier D'Yvoire, D. Claude, X. Barbaut, G. Carret, F. Jehl, M. Ducher, and R. W. Jelliffe. 2000. Pharmacocinétique/pharmacodynamie clinique des antibiotiques, p. 715-731. In Précis de bactériologie clinique. Editions ESKA, Paris, France.
- 17.Maurin, M., F. Rougier, and P. Maire. 2000. Note de calcul sur les lois de Hill: aspects probabiliste déterministe et épistémologique. Internal report LTE2026. Institut National de la Recherche sur les Transports et leur Sécurité, Lyon, France.
- 18.Michaelis, L., and M. L. Menten. 1913. Die Kinetik der Invertinwirkung. Biochem. Z. 49:333-369. [Google Scholar]
- 19.Mingeot-Leclerq, M. P., and P. M. Tulkens. 1999. Aminoglycosides: nephrotoxicity. Antimicrob. Agents Chemother. 43:1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murry, K. R., P. S. McKinnon, B. Mitrzyk, and M. J. Rybak. 1999. Pharmacodynamic characterization of nephrotoxicity associated with once-daily aminoglycoside. Pharmacotherapy 19:1252-1260. [DOI] [PubMed] [Google Scholar]
- 21.Rybak, M. J., B. J. Abate, S. L. Kang, M. J. Ruffing, S. A. Lerner, and G. L. Drusano. 1999. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob. Agents Chemother. 43:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schentag, J. J. 1977. Renal clearance and tissue accumulation of gentamicin. Clin. Pharmacol. Ther. 22:364-370. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz, C., J. Hilpert, C. Jacobsen, C. Boensch, E. I. Christensen, F. C. Luft, and T. E. Willnow. 2002. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 277:618-622. [DOI] [PubMed] [Google Scholar]
- 24.Schnermann, J., D. A. Häberle, J. M. Davis, and K. Thurau. 1992. Tubuloglomerular feedback control of renal vascular resistance, p. 1675-1705. In Renal physiology. Oxford University Press, Oxford, United Kingdom.
- 25.Schumitzky, A. 1991. Nonparametric EM algorithms for estimating prior distributions. Appl. Math. Comput. 45:143-157. [Google Scholar]
- 26.Sundin, D. P., C. Meyer, R. Dahl, A. Geerdes, R. Sandoval, and B. A. Molitoris. 1997. Cellular mechanism of aminoglycoside tolerance in long term gentamicin treatment. Am. J. Physiol. 41:C1309-C1318. [DOI] [PubMed] [Google Scholar]
- 27.Tulkens, P. M. 1989. Nephrotoxicity of aminoglycosides. Toxicol. Lett. 46:107-123. [DOI] [PubMed] [Google Scholar]
- 28.Ware, J. H., and R. E. Bowden. 1977. Circadian rhythm analysis when output is collected at intervals. Biometrics 33:566-571. [PubMed] [Google Scholar]
- 29.Zhi, J., C. H. Nightingale, and R. Quintilini. 1986. A pharmacodynamic model for the activity of antibiotics against microorganisms under nonsaturable conditions. J. Pharm. Sci. 75:1063-1067. [DOI] [PubMed] [Google Scholar]