Abstract
Aloe barbadensis Miller (or Aloe vera) has widespread use in health products, and despite numerous reports on the whole plant, little work has been performed on the inner gel, which has been used extensively in these products. This report describes the in vitro susceptibilities of two bacteria to this component.
Global antibiotic resistance by bacteria is becoming an increasing public health concern, and the race to discover new antibacterial agents is on (2). One approach involves the search for new therapeutic agents with novel modes of action from natural resources (4, 11). Plants belonging to the genus Aloe (Liliaceae) have been known for their medicinal properties for many centuries, and Aloe barbadensis Miller (or Aloe vera) is of particular renown (1, 19). In the last decade, aloe vera (AV) has been used extensively in health drinks, topical creams, toiletries, and cosmetics (1), and there are many reported claims of its beneficial properties, encompassing a broad range of conditions (17, 18). These claims remain largely anecdotal, and scientific evidence is often sparse and inconsistent (1, 17). Major sources of confusion arise from preexperimental treatment of the plant, such as storage conditions, the use of fresh or dried components, variations in extraction methods, use of different parts of the plant, growing conditions, and the age of the plants at harvest (12, 13, 21, 23).
The fresh whole leaves of the succulent are fleshy, and removal of the thick outer cuticle reveals a mucilaginous inner gel (1). This is the major component in many reputable commercial products (1, 17), found in a preserved but otherwise untreated form (12, 13, 17). To date, more than 75 active ingredients have been identified from the inner gel (1, 17, 19), each of which may have a range of mechanisms of action, acting synergistically or individually to explain the numerous curative properties. This has led to a trend to isolate and analyze individual ingredients in an attempt to attribute therapeutic potential to each ingredient (20, 22). This has not necessarily shed light on the issue, and in particular, in vivo studies have not been able to distinguish between the antimicrobial effects of the plant and stimulation of the immune system. Whole-leaf components proposed to have direct antibacterial properties include anthraquinones (3) and saponins (17, 19), while polysaccharides have been attributed with indirect bactericidal activity through stimulation of phagocytic leukocytes to destroy bacteria (10, 16).
Since there has been limited research on the inner gel, the aim of this study was to provide evidence for the antibacterial effectiveness of this component prior to extensive experimentation. The ultimate goal would be to attempt to characterize the factors that provide biologic activity. The main microorganism used in this study was Shigella flexneri, a nonmotile rod belonging to the Enterobacteriaceae family. Shigella species are an important cause of gastrointestinal illness, which is manifest by watery diarrhea that progresses to mucoid bloody diarrhea and shigellosis. In the past, antibiotic therapy has been effective (14), but increasing multiple antibiotic resistance, particularly resistance to ampicillin, chloramphenicol, nalidixic acid, and co-trimoxazole, is of major concern (14, 15). There is also increasing evidence that antibiotic therapy can exacerbate the symptoms of gastrointestinal diseases (6, 9), and an alternative treatment would be beneficial. In addition, susceptibility of the gram-positive Streptococcus pyogenes to AV was briefly investigated, as this is another organism which is beginning to show antibiotic resistance (7, 8).
Shigella flexneri (NCTC 9950) and Streptococcus pyogenes Gp A (ATCC 19615) were purchased from the Public Health Laboratory Services (Porton Down, Salisbury, United Kingdom). Stock cultures were subcultured and maintained in tryptic soy broth (TSB; Difco Laboratories Ltd., Detroit, Mich.), at 37°C. Freeze-dried AV was obtained from the United States and consisted of inner gel from plants that were approximately 2 years old at the time of harvest. The powder was reconstituted in phosphate-buffered saline (pH 7.5) at 37°C for 30 min and then sterile filtered through a 0.22-μm-pore-size filter. For comparison, a commercial health drink, Aloe Vera Gel (Forever Living Products, Scottsdale, Ariz.) consisting of undiluted, unfiltered inner-leaf gel, was used as well. This was filtered through sterile Whatman no. 54 filter paper. It was not possible to accurately determine the amount of active material lost by this process. Ampicillin (AMP) and/or nalidixic acid (NAL) (Sigma-Aldrich Ltd., Poole, Dorset, United Kingdom), also used for comparison, were dissolved in phosphate-buffered saline and filtered through a 0.22-μm-pore-size filter. Reagents used in this study were obtained from the following sources: Muller-Hinton broth from Oxoid, Basingstoke, Hampshire, United Kingdom; agar from Unipath Ltd., Bedford, United Kingdom; Alamar blue from Serotec Ltd., Kidlington, United Kingdom; plasticware from Greiner Bio-One Ltd., Stonehouse, United Kingdom, and 96-well tissue culture plates from TPP, Trasadingen, Switzerland; all other reagents were from BDH Ltd., Poole, United Kingdom.
The zones of inhibition were calculated by agar diffusion. Wells were cut into nutrient agar plates, and 75 μl of the test agent, AV (7 to 450 mg/ml) or antibiotic (2 to 512 μg/ml), was added to each well. An S. flexneri lawn was seeded on the agar for 24 h at 37°C. The diameters of the inhibition zones were measured (Table 1). Concentrations greater than 112 mg of AV per ml, 32 μg of AMP per ml, and 128 μg of NAL per ml showed significant (P < 0.001) growth inhibition compared with untreated control wells. It was not possible to make a comparison with the Aloe Vera Gel due to difficulties encountered with diffusion through the agar. An attempt was made to mix the Aloe Vera Gel with the agar prior to seeding the S. flexneri on the surface to obtain a total viable count, but the agar failed to set, which was attributed to the presence of pectinase in the preparation.
TABLE 1.
Zones of inhibition of AV, Aloe Vera Gel, AMP, and NAF on S. flexneria
| Dilution | Mean zone of inhibition (diam) ± SD (cm) (n = 3)
|
|||
|---|---|---|---|---|
| AV | AVGb | AMP | NAL | |
| 1:2 | 2.8 ± 0.2c | - | 3.0 ± 0.1c | 3.5 ± 0.1c |
| 1:4 | 2.0 ± 0.1c | - | 2.8 ± 0.2c | 3.0 ± 0.1c |
| 1:8 | 1.5 ± 0.1c | - | 1.6 ± 0.2c | 2.8 ± 0.1c |
| 1:16 | 1.3 ± 0.2d | - | - | 2.2 ± 0.2c |
| 1:32 | 0.9 ± 0.1d | - | - | 1.9 ± 0.2c |
| 1:64 | -e | - | - | - |
Wells were cut in nutrient agar, test agent was added to each well and overlaid with a lawn of S. flexneri. The starting concentrations of the test agents were as follows: AV, 450 mg/ml; AVG, 90% (vol/vol); AMP and NAL, 512 μg/ml.
AVG, Aloe Vera Gel (Forever Living Products).
Significant inhibition compared with untreated wells (P < 0.001).
Significant inhibition compared with untreated wells (P < 0.05).
-, no inhibition detected.
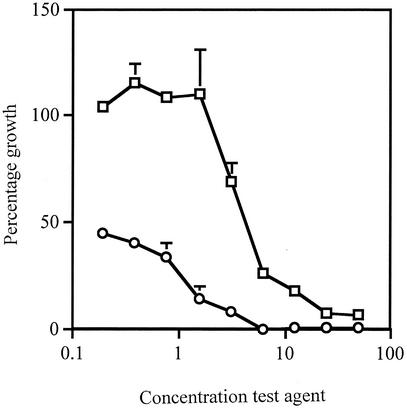
Growth curves were generated as follows. A 96-well microtiter plate containing 100 μl of TSB per well was inoculated with 10 μl of the organism (102 CFU) at 37°C for 30 h (this was done in triplicate). The plate was agitated, and A620 readings were taken at hourly intervals on a Titertek Multiscan MCC/340 plate reader. The number of bacteria per milliliter was calculated from the following equation: (A620 × 2 × 108)/0.2. Growth curves were also set up with 100 μl of test agent: AV (7 to 450 mg/ml), Aloe Vera Gel (5 to 90%), AMP (2 to 512 μg/ml), or NAL (2 to 512 μg/ml). The untreated bacteria reached log phase between 3 to 10 h, with maximum growth after 22 h. All the test agents suppressed bacterial growth for up to 24 h, to various degrees. The 50% inhibition doses were 30 mg of AV per ml, 15% (vol/vol) Aloe Vera Gel, 3 μg of NAL per ml, and 4 μg of AMP per ml. The growth inhibition of AV was further quantified by incubating 100 μl of Muller-Hinton broth with 100 μl of twofold dilutions of AV (in triplicate) and 10 μl of S. flexneri (102 CFU) at 37°C for 6 h. Control wells contained bacteria (positive) or broth (negative) only. Alamar blue (10 μl) was added to each well and incubated at 37°C for a further 2 h. The plate was centrifuged for 10 min at 12,000 × g to pellet the bacteria, and the supernatant was transferred to a fresh microtiter plate. A570 and A600 readings were taken on a SpectraMax 190 spectrometer, and the percentage growth was calculated using the following equation: (mean A570 − A600 of test agent/mean A570 − A600 of the positive control) × 100. Figure 1 shows the dose response of S. flexneri to AV after 8 h. Since the Alamar blue assay incorporates a colorimetric growth indicator to detect metabolic activity, it is useful in cytotoxicity experiments. These assays were designed on a small scale in microtiter plates to reduce the amount of material used; however, the same results were obtained from large-scale experiments using an inoculum size of >5 × 105 CFU/ml. It was important to optimize the assay for microtiter plates, since preliminary follow-up experiments have shown that extraction of the plant material can produce a low yield depending on the solvents used.
FIG. 1.
Effects of AV and AMP on growth of S. flexneri. S. flexneri was incubated at 37°C for 8 h in Muller-Hinton broth with AV (□) or AMP (○). The test agent concentrations on the x axis are shown in milligrams per milliliter for AV and in micrograms per milliliter for AMP. Alamar blue was used to ascertain the percentage of growth of S. flexneri. The results are expressed as the means ± standard deviations of three readings.
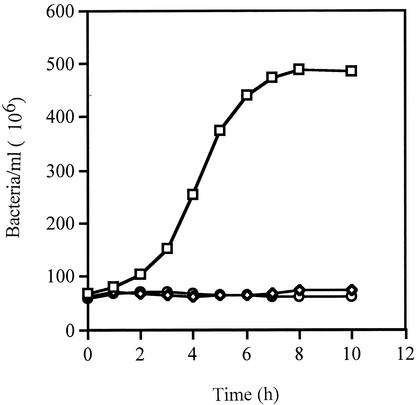
To compare the effect of AV against a gram-positive bacterium, growth curves were set up with S. flexneri and Streptococcus pyogenes. Effective growth inhibition, up to 24 h, was achieved with concentrations of more than 100 mg of AV per ml for S. flexneri and 25 mg of AV per ml for Streptococcus pyogenes (Fig. 2). The reduction in the amount of AV needed to suppress the growth of the gram-positive bacterium was attributed to the structural differences between the two organisms. As far as we are aware, this is the first report of the susceptibility of both these organisms to AV.
FIG. 2.
Effects of AV and AMP on growth of Streptococcus pyogenes. Streptococcus pyogenes was incubated at 37°C in TSB alone (□) or with AV (25 mg/ml) (⋄) or AMP (25 μg/ml) (○). The number of bacteria were calculated from A620 readings. The results are expressed as the mean A620s ± standard deviations of three readings.
In view of the complexities of examining the pharmacology of this plant, simple assays, which can be easily replicated to test multiple fractions are essential to establish antimicrobial activity. The assays described in this report enable easy multiparameter comparison and allow a range of bacterial species to be examined. This preliminary study established the susceptibilities of S. flexneri and Streptococcus pyogenes to the inner gel of A. barbadensis Miller or A. vera. The next stage will involve solvent and aqueous extraction of the inner gel to isolate and identify molecules for further research. Although the activities of the AV and Aloe Vera Gel appear to be low in comparison to those of NAL and AMP, individual components may have greater activity. Glycosides of anthraquinones and dihydroxyanthraquinones (3, 22), acemannan (5), and saponins (1, 17) are active antibacterial components found in the whole plant and known to be constituents of the inner gel. Therefore, further research on these and other molecules will provide further evidence of the therapeutic potential of the inner gel. The direct effect of the inner gel on bacteria which are found in accessible areas of the body enables future development of antimicrobial products, which can protect the mucosa.
Acknowledgments
We thank Roger Poore (Aloe Vera of America Inc., Dallas, Tex.), Aidan O'Hare (Forever Living Products, Dallas, Tex.), Charles Smith (Forever Living Products, Shotts, United Kingdom), Alexander Gray (Department of Pharmaceutical Sciences, University of Strathclyde), and Graham Park (Lamellar Therapeutics Ltd., Glasgow, United Kingdom) for invaluable advice and assistance.
REFERENCES
- 1.Atherton, P. 1998. Aloe vera: magic or medicine? Nurs. Stand. 41:49-54. [DOI] [PubMed] [Google Scholar]
- 2.Bax, R., N. Mullan, and J. Verhoef. 2000. The millenium bugs-the need for and development of new antibacterials. Int. J. Antimicrob. Agents 16:51-59. [DOI] [PubMed] [Google Scholar]
- 3.Boateng, J. S. 2000. Analysis of commercial samples of aloe. Ph.D. thesis. University of Strathclyde, Glasgow, United Kingdom.
- 4.Bombardelli, E. 2001. Approaches to the quality characteristics of medicinal plant derivatives. Eur. Phytojournal 1:30-33. [Google Scholar]
- 5.Davis, R. H., J. J. D. Donato, G. M. Hartman, and R. C. Haas. 1994. Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J. Am. Podiatric Med. Assoc. 84:77-81. [DOI] [PubMed] [Google Scholar]
- 6.Dofferhoff, A. S. M., M. T. Esselink, and H. G. de Vries-Hospers. 1993. The release of endotoxin from antibiotic-treated Escherichia coli and the production of tumour necrosis factor by human monocytes. J. Antimicrob. Chemother. 31:373-384. [DOI] [PubMed] [Google Scholar]
- 7.Gur, D., M. Ozalp, B. Sumerkan, A. Kaygusuz, K. Toreci, I. Koksal, U. Over, and G. Soyletir. 2002. Relevance of antimicrobial resistance in Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis and Streptococcus pyogenes: results of a multicentre study in Turkey. Int. J. Antimicrob. Agents 19:207-211. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh, P. R., L. J. Teng, L. N. Lee, P. C. Yang, S. W. Ho, H. C. Lue, and K. T. Luh. 2002. Increased prevalence of erythromycin resistance in streptococci: substantial upsurge in erythromycin-resistant M phenotype in Streptococcus pyogenes (1979-1998) but not in Streptococcus pneumoniae (1985-1999) in Taiwan. Microb. Drug Resist. 8:27-33. [DOI] [PubMed] [Google Scholar]
- 9.Hurley, J. C. 1992. Antibiotic induced release of endotoxin: a reappraisal. Clin. Infect. Dis. 15:840-854. [DOI] [PubMed] [Google Scholar]
- 10.Lawless, J., and J. Allan. 2000. The chemical composition of Aloe vera, p. 161-171. In Aloe vera natural wonder cure. Thorsons, Publishing Ltd., London, United Kingdom.
- 11.Marr, C., and S. Bent. 1999. An evidence based review of the 10 most commonly used herbs. West. J. Med. 171:168-171. [PMC free article] [PubMed] [Google Scholar]
- 12.McAnalley, B. H. April 1988. Process for preparation of aloe products, produced thereby and composition thereof. U.S. patent 4,735,935.
- 13.McAnnalley, B. H. April 1990. Process for preparation of aloe products, produced thereby and composition thereof. U.S. patent 4,917,890.
- 14.Navia, M. M., L. Capitano, J. Ruiz, M. Vargas, H. Urassa, D. Schellemberg, J. Gascon, and J. Vila. 1999. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J. Clin. Microbiol. 37:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prats, G., B. Mirelis, T. Llovet, C. Munoz, E. Miro, and F. Navarro. 2000. Antibiotic resistance trends in enteropathogenic bacteria isolated in 1985-1987 and 1995-1998 in Barcelona. Antimicrob. Agents Chemother. 44:1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pugh, N., S. A. Ross, M. A. Elsohly, and D. S. Pasco. 2001. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J. Agric. Food Chem. 49:1030-1034. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds, T., and A. C. Dweck. 1999. Aloe vera leaf gel: a review update. J. Ethnopharmacol. 68:3-37. [DOI] [PubMed] [Google Scholar]
- 18.Shelton, R. M. 1991. Aloe vera: its chemical and therapeutic properties. Int. J. Dermatol. 30:679-683. [DOI] [PubMed] [Google Scholar]
- 19.Urch, D. 1999. Aloe vera the plant, p. 8-17. In Aloe vera nature's gift. Blackdown Publications, Bristol, United Kingdom.
- 20.Vazquez, B., G. Avila, D. Segura, and B. Escalante. 1996. Anti-inflammatory activity of extracts from Aloe vera gel. J. Ethnopharmacol. 55:69-75. [DOI] [PubMed] [Google Scholar]
- 21.Vogler, B. K., and E. Ernst. 1999. Aloe vera: a systemic review of its clinical effectiveness. Br. J. Gen. Pract. 447:823-828. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, H. H., J. G. Chung, C. C. Ho, C. T. Wu, and S. H. Chang. 1998. Aloe-emodin effects on arylamine N-acetyl transferase activity in the bacteria Helicobacter pylori. Planta Med. 64:176-178. [DOI] [PubMed] [Google Scholar]
- 23.Yarron, A. 1993. Characterisation of Aloe vera gel composition and autodegradation, and stabilisation of the natural fresh gel. Phytother. Res. 7:S11-S13. [Google Scholar]