Abstract
Ciprofloxacin (CPFX) and roxithromycin (RXM) induced apoptosis of activated Jurkat T cells in vitro. CPFX showed concentration-dependent acceleration of apoptosis of activated Jurkat T cells by enhancing the expression of FasL and activities of caspase-3 and -8. RXM accelerated cell death, enhanced expression of FasL and caspase-3 but not caspase-8, and did not show the concentration dependency.
Apoptosis, or programmed cell death, is a regulated physiological process essential for embryogenesis and for maintaining cellular homeostasis. Apoptosis is a constitutive process, but it can be induced or inhibited by various stimuli. Recently, the pathogenesis of numerous diseases, such as cancerous diseases, autoimmune diseases, and inflammatory diseases, have been explained by the alteration of normal regulation of apoptosis (1, 26, 32, 34).
Antibiotics are widely used as bacteriostatic or bactericidal drugs in the therapy of bacterial infections. Besides the respective interactions between antibiotics and bacteria and between the immune system and bacteria, antibiotics also directly interact with the immune system (15, 17, 18). Immunomodulatory effects of antibiotics include alteration of phagocytosis, chemotaxis, endotoxin release, cytokine production, and tumoricidal effects of certain cells. Moreover, some antibiotic agents can affect the life span of cells through inducing or inhibiting apoptosis (4, 6, 18). Among numerous immunomodulatory antibiotics, the immunomodulatory effects of quinolones and macrolides are the major part of the reports in this field (12, 13, 16, 21, 24, 27, 28, 33).
In this study, we wanted to evaluate the effects of ciprofloxacin (CPFX) and roxithromycin (RXM), widely used quinolone and macrolide, on the apoptosis of activated Jurkat T cells. If CPFX or RXM can alter the apoptotic patterns of Jurkat T cells, the immune responses elicited by T cells could be modified and the immunologic effects of CPFX and RXM in clinical practice might be inferred. We analyzed the effect of CPFX or RXM on the viability of activated Jurkat T cells and altered expression of well-known regulators of apoptosis, such as Fas, FasL, Bcl-2, Bax, caspase-3, and caspase-8.
An acute T-cell leukemia cell line, Jurkat (clone E6-1, ATCC TIB-152), was purchased from American Type Culture Collection (Manassas, Va.). Jurkat T cells were maintained in RPMI-1640 (Gibco, Gaithersburg, Md.) containing 10% fetal calf serum (Gibco), 20 mM HEPES, and 1% antibiotic-antimycotic solution (100 U of penicillin, 100 μg of streptomycin, 0.25 μg of amphotericin B; Gibco). The cells were pretreated with various concentrations of CPFX (1, 2.5, 5, and 10 μg/ml; Dong-A Pharm. Co., Ltd., Seoul, Korea) or RXM (1, 10, 20, and 50 μg/ml; Sigma Chemical Co., St. Louis, Mo.) for 2 h at 37°C in 24-well plates (Nunc, Roskilde, Denmark). Cells were then incubated with anti-CD3 antibody for activation (clone HIT3a; 10 ng/ml; PharMingen, San Diego, Calif.).
Viability of Jurkat T cells after treatment was analyzed by MTT (3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) assay (R&D Systems, Inc., Minneapolis, Minn.). A total of 6 × 105 Jurkat T cells per well were incubated at 37°C with anti-CD3 antibody, with or without CPFX or RXM, for the indicated time periods. The absorbance was read at 550 nm with a spectrophotometer, and the percentages of viable cells were calculated.
DNA fragmentation was assessed by an enzyme-linked immunosorbent assay (ELISA) kit (Boehringer Mannheim, Mannheim, Germany). In brief, 3 × 105 Jurkat T cells were labeled with 1 mM 5-bromo-2′-deoxyuridine (BrdU) for 6 h. Anti-CD3 antibody with or without CPFX or RXM was added and incubated for 12 to 72 h. DNA fragmentations were then measured according to the manufacturer's protocols.
Cell surface expression of Fas was assayed by direct immunofluorescence flow cytometry with a saturating concentration of fluorescein isothiocyanate (FITC)-conjugated mouse anti-human APO-1/Fas monoclonal antibody (clone DX2; 10 μl/106 cells; Dako, Glostrup, Denmark) after 18 h of treatment with anti-CD3 antibody with or without CPFX or RXM. Negative control cells were stained with a saturating concentration of irrelevant mouse anti-human APO-1/Fas monoclonal antibody.
Cell surface expression of FasL was assayed by indirect immunofluorescence flow cytometry by using anti-human FasL antibody for primary staining and FITC-conjugated, affinity-purified F(ab′)2 goat anti-human immunoglobulin G (FITC-conjugated anti-mouse immunoglobulin G1; Dako) for secondary staining. Negative control staining was performed by omitting FasL antibody in the primary step. The plates were kept at 4°C until the flow cytometry assay was performed with FACScan and LYSIS II software (Becton Dickinson, San Jose, Calif.). Mean fluorescence intensity was calculated after subtracting the mean fluorescence intensity of the negative control.
Bcl-2 and Bax expressions were measured by a commercial ELISA method (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.). Proteins were extracted from 5 × 105 Jurkat T cells after 18 h of culture with anti-CD3 antibody with or without CPFX or RXM. Concentrations of proteins were measured according to the manufacturer's instructions.
We used caspase-3 and caspase-8 colorimetric assay kits (R&D Systems) for analysis. In brief, 2 × 106 cells were collected, and the cellular pellet was lysed by cell lysis buffer after 18 h of treatment with anti-CD3 antibody with or without CPFX or RXM. Caspase-3 and caspase-8 were measured by colorimetric assay according to the manufacturer's instructions by using a spectrophotometer at 405 nm.
Results are shown as means ± standard errors of the means (SEM) for triplicate or quadruplicate experiments. Statistical analysis was conducted by using analysis of variance with Tukey's method. A P value of <0.05 was judged to be statistically significant.
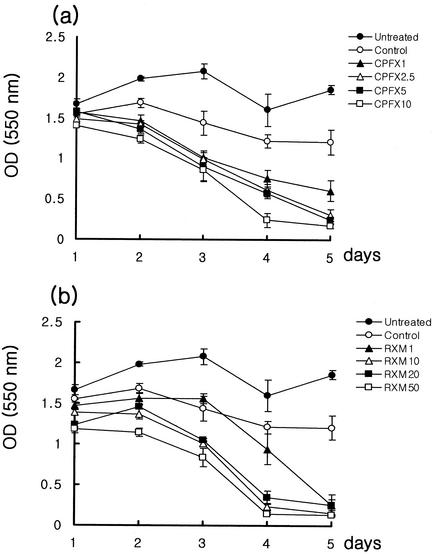
Anti-CD3-activated Jurkat T cells (control) were less viable than untreated cells with time. Addition of CPFX or RXM accelerated the death of activated Jurkat T cells in a concentration-dependent manner, especially CPFX-treated cells (Fig. 1).
FIG. 1.
Effect of CPFX (a) and RXM (b) on the viability of Jurkat T cells. Viability was measured by MTT assay from days 1 to 5 after treatment. The results are presented as means ± SEM of absorbance for triplicate measurements. OD (550 nm), optical density at 550 nm.
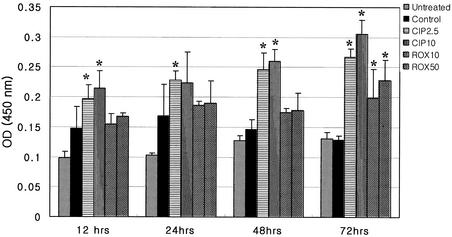
The DNA fragmentation of Jurkat T cells treated with CPFX was greater than that of untreated and control cells. It caused more fragmentation at higher concentrations. But addition of RXM significantly increased fragmentation only at 72 h and did not show concentration dependency (Fig. 2).
FIG. 2.
DNA fragmentation of activated Jurkat T cells, assessed by ELISA from 12 to 72 h after CPFX or RXM treatment. The results are presented as means ± SEM of absorbance for quadruplicate measurements. Asterisks indicate a P value of <0.05 compared to that of anti-CD3 antibody-treated Jurkat T cells. OD (450 nm), optical density at 450 nm.
The percentage of Fas-expressing cells was increased in control cells (87.5% ± 3.2%) compared to that in untreated cells (80.5% ± 2.4%). But the addition of CPFX or RXM did not result in any difference in Fas expression compared to that of control cells. The percentage of FasL-expressing cells was also increased in control cells compared to that in untreated cells (21.3% ± 2.1% versus 10.6% ± 3.2%). Addition of CPFX increased FasL-expressing cells to 29.5% ± 1.6% (2.5 μg/ml, P < 0.05) and 34.9% ± 1.1% (10 μg/ml, P < 0.05). Addition of RXM also increased FasL-expressing cells to 26.2% ± 1.8% (10 μg/ml, P < 0.05) and 25.9% ± 1.2% (50 μg/ml, P < 0.05). The expression of FasL was more remarkably increased in the CPFX-treated cells than in RXM-treated cells.
The Bcl-2/Bax ratio decreased with time in untreated Jurkat T cells. There is no significant alteration of the Bcl-2/Bax ratio in control cells or in CPFX- or RXM-treated cells compared to that in untreated cells (data not shown).
Control cells showed enhanced caspase-8 (optical density at 405 nm, 0.54 ± 0.10 versus 0.31 ± 0.09) and caspase-3 (0.86 ± 0.18 versus 0.10 ± 0.06) activities compared to those of untreated cells. Addition of CPFX increased caspase-8 activity at the concentrations of 2.5 μg/ml (0.72 ± 0.11, P < 0.05) and 10 μg/ml (0.83 ± 0.03, P < 0.05), and caspase-3 activity was also increased at the concentrations of 2.5 μg/ml (1.48 ± 0.09, P < 0.05) and 10 μg/ml (1.66 ± 0.12, P < 0.05). Addition of higher concentrations of CPFX tended to induce more caspases than lower concentrations, but there was no statistical difference between lower and higher concentrations (P > 0.05). Addition of RXM significantly increased the activity of only caspase-3 (1.39 ± 0.16, P < 0.05) compared to that of control cells at the concentration of 10 μg/ml, and there were no significant differences between lower and higher concentrations.
Recently, many reports about immunologic modifications, such as alteration of cellular proliferation and cytokines production from various cells, manifested by quinolones (CPFX, trovafloxacin, grepafloxacin) and macrolides (erythromycin, RXM, clarithromycin) have appeared (14, 22, 25, 29). Among immunomodulatory effects exerted by antimicrobials, we wanted to evaluate the effect of CPFX or RXM on apoptosis. In 1995, Aoshiba et al. reported erythromycin-induced apoptosis of human neutrophils, and they concluded that an increase of intracellular cyclic AMP might be the cause of this accelerated apoptosis (2). In 1999, Liu et al. reported doxycycline-induced apoptosis of Jurkat T cells (20). We wanted to analyze the effect of CPFX and RXM on the apoptosis of Jurkat T cells to compare our results with past published data and to find involved pathways of apoptotic processes. We used anti-CD3 antibody to activate Jurkat T cells. Anti-CD3 antibody is known as an activator and inducer of Fas/FasL-mediated apoptosis of T cells (3-5, 10, 20). We used four concentrations of each drug, three of which could be obtained in human blood and one that was beyond the range. The peak serum concentration of CPFX is 0.83 to ∼7.6 μg/ml, and that of RXM is 6 to ∼12 μg/ml according to the dose and route of administration and host factors (6, 12, 16). Addition of CPFX or RXM resulted in faster death than was seen with untreated or control cells, and the pattern of death showed concentration dependency in cells treated with CPFX (Fig. 1).
We chose two concentrations, one concentration within the range of serum peak level and one at maximal concentration, to maximize the difference in the following experiments. According to the cellular DNA fragmentation ELISA, apoptotic cell death of anti-CD3-activated Jurkat T cells was enhanced by addition of CPFX and increased at higher concentrations. But RXM enhanced apoptosis significantly only at 72 h after stimulation compared to that of control cells. To evaluate the underlying mechanisms of apoptosis, we investigated the expression of some well-known regulators of apoptosis. Fas and FasL, transmembrane receptors on the cell surface, are death regulators that belong to the tumor necrosis factor/nerve growth factor receptor family. Binding of FasL to Fas results in recruitment of a Fas-associated death domain. This complex activates caspases and initiates apoptosis (19, 23, 30, 34). In this study, CPFX and RXM up-regulated expression of FasL in the anti-CD3-activated Jurkat T cells, but up-regulation was more apparent in CPFX-treated Jurkat T cells. These results partially coincided with those of a study of apoptosis in doxycycline-treated Jurkat T cells which revealed more enhanced expression of FasL than Fas by addition of doxycycline (20).
Bcl-2 and Bax, other regulators of apoptosis, belong to the same family but have opposite actions: Bcl-2 inhibits apoptosis, but Bax accelerates apoptosis. In general, Bcl-2 binds to Bax, and the ratio of Bcl-2/Bax determines the fate of the cell (7, 8, 34). We evaluated the change of the Bcl-2/Bax ratio in the CPFX- or RXM-treated Jurkat T cells. Although we expected the additional synergy to accelerate apoptosis by alteration of Bcl-2 and Bax expression, the Bcl-2/Bax ratios were not different between antibiotic-treated and control cells. Therefore, the acceleration of death in CPFX- or RXM-treated Jurkat T cells might not be associated with changes in Bcl-2 and/or Bax activities.
Caspases, cysteine proteases with specificity to cleave after aspartic acid residues in the P1 position of substrate, are well known for the execution of apoptosis. In this study we analyzed caspase-3 and -8 activities. Among more than 13 caspases, caspase-8 is recruited to the Fas/FasL complex through the adaptor protein Fas-associated death domain and can initiate the caspase cascade (1, 11, 34). Caspase-3 is regarded as a key executioner molecule activated by apoptotic stimuli originating either at receptors for exogenous molecules or within cells through the action of drugs, toxins, or radiation (1, 11, 31, 34). The activities of caspase-3 and -8 were increased in the CPFX-treated Jurkat T cells, but only caspase-3 activity was increased in the RXM-treated Jurkat T cells.
Therefore, CPFX could induce apoptosis of activated Jurkat T cells by enhancing the expression of FasL, recruit caspase-8, and finally activate caspase-3 to execute apoptosis. By contrast, RXM increased FasL and caspase-3 activities, which were less than those of CPFX, and did not enhance the activity of caspase-8. Therefore, some differences of mechanisms inducing apoptosis of activated Jurkat T cells between CPFX and RXM could exist. These differences might be caused by biochemical and pharmacological events, such as intracellular accumulation of each drug or changes in intracellular cyclic AMP levels caused by a drug, as Aoshiba et al. noted (2), but presently this is not clear. However, it is certain that RXM could induce apoptosis of activated Jurkat T cells like CPFX, and it could be done by a different way than that of CPFX.
We used a Jurkat T-cell line in this experiment. Jurkat T cells have some differences from normal peripheral T cells. Jurkat T cells can secrete interleukin-2 when its antigen receptor is cross-linked with anti-receptor monoclonal antibodies. Also, one of the Jurkat cell line's most interesting features is that it stops growing when its antigen receptor is cross-linked (9). Although more evidence should be discovered by additional studies about immunomodulatory effects of antimicrobials on human T cells, it might be possible that CPFX or RXM induced alteration of apoptosis of a T-cell line in humans and can alter the immune response to stimuli. If these observed findings could take place in T cells in the human body, we can expect that these immunomodulatory antibiotics lessen the host tissue damage, primarily by killing the invading microorganisms and additionally by inducing apoptosis of activated T cells in infectious diseases.
In conclusion, our preliminary experiments revealed that CPFX and RXM have the potential to accelerate apoptosis of activated Jurkat T cells in vitro. This acceleration of apoptosis is conducted by inducing the expression of FasL, by interaction of more Fas and FasL, and finally by activating caspases. Further investigations about detailed and precise regulatory mechanisms in the cells, effects on the other cells (for example, normal peripheral blood cells), and clinical relevance are warranted.
REFERENCES
- 1.Andreoli, T. E. 1999. The apoptosis syndrome. Am. J. Med. 107:488-506.10569304 [Google Scholar]
- 2.Aoshiba, K., A. Nagai, and K. Konno. 1995. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob. Agents Chemother. 39:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner, T., R. J. Mogil, D. LaFace, N. J. Yoo, A. Mahboubi, and F. Echeverri. 1995. Cell-autonomous Fas(CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441-444. [DOI] [PubMed] [Google Scholar]
- 4.Dearstyne, E. A., and N. I. Kervliet. 2002. Mechanism of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced decrease in anti-CD3-activated CD4+ T cells: the role of apoptosis, Fas, and TNF. Toxicology 170:139-151. [DOI] [PubMed] [Google Scholar]
- 5.Dhein, J., H. Walczak, C. Bäumler, K. M. Debatin, and P. H. Krammer. 1995. Autocrine T-cell suicide mediated by APO-1(Fas/CD95). Nature 373:438-441. [DOI] [PubMed] [Google Scholar]
- 6.Herold, C., M. Ocker, M. Ganslmayer, H. Gerauer, E. G. Hahn, and D. Schuppan. 2002. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br. J. Cancer 86:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh, N., Y. Tsujimoto, and S. Nagata. 1993. Effect of bcl-2 on Fas-mediated cell death. J. Immunol. 151:621-627. [PubMed] [Google Scholar]
- 8.Iwai, K., T. Miyawaki, T. Takizawa, A. Konno, K. Ohta, A. Yachie, H. Seki, and N. Taniguchi. 1994. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood 84:1201-1208. [PubMed] [Google Scholar]
- 9.Janeway, C. A., P. Travers, M. Walport, and J. D. Capra (ed.). 1999. Immunobiology, 4th ed. Elsevier Science Ltd./Garland Publishing, New York, N.Y.
- 10.Ju, S. T., D. J. Panka, H. Cui, R. Ettinger, M. El-Khatib, D. H. Sherr, D. H., B. Z. Stanger, and A. Marshak-Rothstein. 1995. Fas(CD95)/FasL interaction required for programmed cell death after T-cell activation. Nature 373:444-448. [DOI] [PubMed] [Google Scholar]
- 11.Juo, P., C. J. Kuno, J. Yuan, and J. Blenis. 1998. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr. Biol. 8:1001-1008. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki, S., H. Takizawa, T. Ohtoshi, N. Takeuchi, T. Kohyama, H. Nakamura, T. Kasama, K. Kobayashi, K. Nakahara, Y. Morita, and K. Yamamoto. 1998. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob. Agents Chemother. 42:1499-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan, A., T. R. Slifer, and J. S. Remington. 1998. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob. Agents Chemother. 42:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch, C. C., D. J. Esteban, A. C. Chin, M. E. Olson, R. R. Read, H. Ceri, D. W. Morck, and A. G. Buret. 2000. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J. Antimicrob. Chemother. 46:19-26. [DOI] [PubMed] [Google Scholar]
- 15.Labro, M. T. 1997. The prohost effect of antimicrobial agents as a predictor of clinical outcome. J. Chemother. 9(Suppl. 1):100-108. [PubMed] [Google Scholar]
- 16.Labro, M. T. 1998. Immunologic effects of macrolides. Curr. Opin. Infect. Dis. 11:681-688. [DOI] [PubMed] [Google Scholar]
- 17.Labro, M. T. 1998. Antibacterial agents-phagocytes: new concepts for old in immunomodulation. Int. J. Antimicrob. Agents 10:11-21. [DOI] [PubMed] [Google Scholar]
- 18.Labro, M. T. 2000. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy tales”? Clin. Microbiol. Rev. 13:615-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liles, W. C., P. A. Kienner, J. A. Ledbetter, A. Aruffo, and S. J. Klebanoff. 1996. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J. Exp. Med. 184:429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J., C. A. Kuszynski, and B. T. Baxter. 1999. Doxycycline induced Fas/Fas ligand-mediated apoptosis in Jurkat T lymphocytes. Biochem. Biophys. Res. Commun. 260:562-567. [DOI] [PubMed] [Google Scholar]
- 21.Morikawa, K., F. Oseko, S. Morikawa, and K. Iwamoto. 1994. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob. Agents Chemother. 38:2643-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morikawa, K., H. Watabe, M. Araake, and S. Morikawa. 1996. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 40:1366-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy, K., S. B. Haudek, M. Thompson, and B. P. Giroir. 1998. Molecular biology of septic shock. New Horiz. 6:181A-193A. [PubMed] [Google Scholar]
- 24.Ono, Y., Y. Ohmoto, K. Ono, Y. Sakada, and K. Murata. 2000. Effect of grepafloxacin on cytokine production in vivo. J. Antimicrob. Chemother. 46:91-94. [DOI] [PubMed] [Google Scholar]
- 25.Purswani, M., S. Eckert, H. Arora, R. Johann-Liang, and G. J. Noel. 2000. The effect of three broad-spectrum antimicrobials on mononuclear cell responses to encapsulated bacteria: evidence for down-regulation of cytokine mRNA transcription by trovafloxacin. J. Antimicrob. Chemother. 46:921-929. [DOI] [PubMed] [Google Scholar]
- 26.Raff, M. 1998. Cell suicide for beginners. Nature 396:119-122. [DOI] [PubMed] [Google Scholar]
- 27.Riesbeck, K., and A. Forsgren. 1994. Limited effects of temifloxacin compared with ciprofloxacin on T-lymphocyte function. Antimicrob. Agents Chemother. 38:879-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sassa, K., Y. Muzushima, and M. Kobayashi. 1999. Differential modulatory effects of clarithromycin on the production of cytokines by a tumor. Antimicrob. Agents Chemother. 43:2787-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz, M. J., P. Speelman, S. Zaat, S. J. J. van Deventer, and T. van der Poll. 1998. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob. Agents Chemother. 42:1605-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, A., and B. B. Aggarwal. 1998. Death domain receptors and their role in cell demise. J. Interferon Cytokine Res. 18:439-450. [DOI] [PubMed] [Google Scholar]
- 31.Sun, X. M., M. MacFarlane, J. Zhuang, B. B. Wolf, D. R. Green, and G. M. Cohen. 1999. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem. 274:5053-5060. [DOI] [PubMed] [Google Scholar]
- 32.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 33.Vazifeh, D., A. Bryskier, and M. T. Labro. 2000. Effect of proinflammatory cytokines on the interplay between roxithromycin, HMR 3647, or HMR 3004 and human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 44:511-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodle, E. S., and H. R. Kulkarni. 1998. Programmed cell death. Transplantation 65:681-691. [DOI] [PubMed] [Google Scholar]