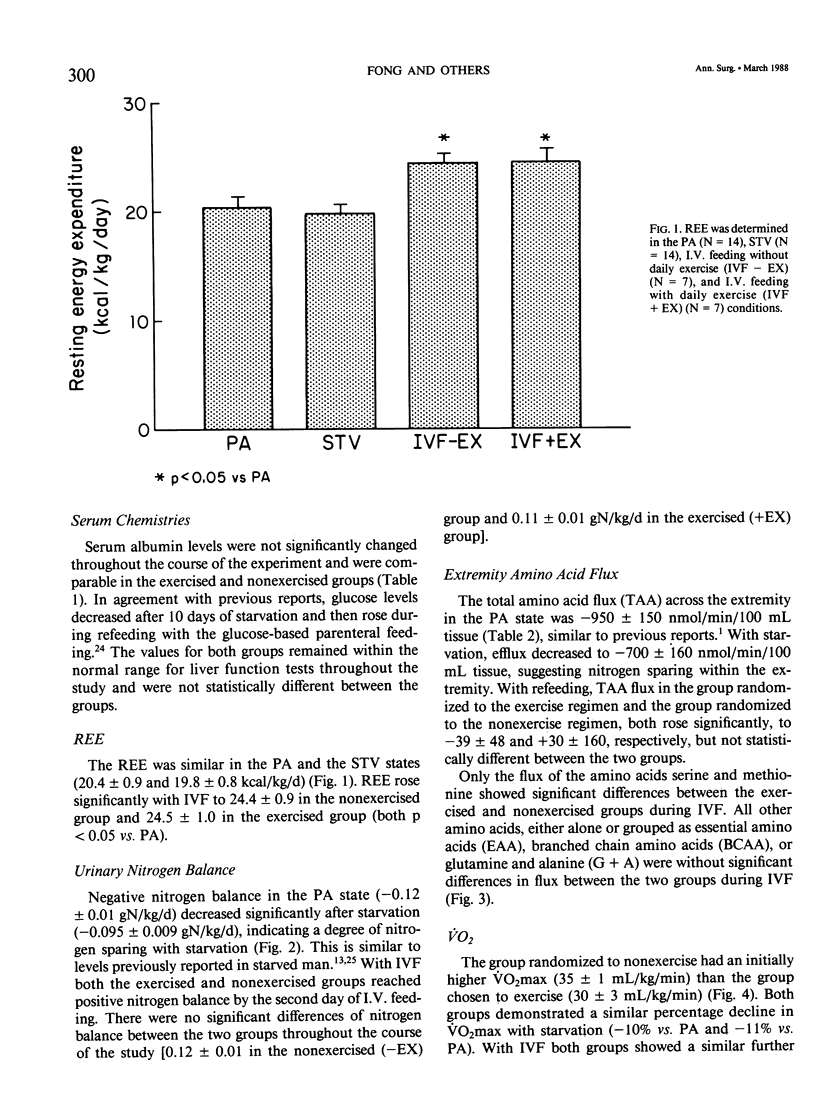
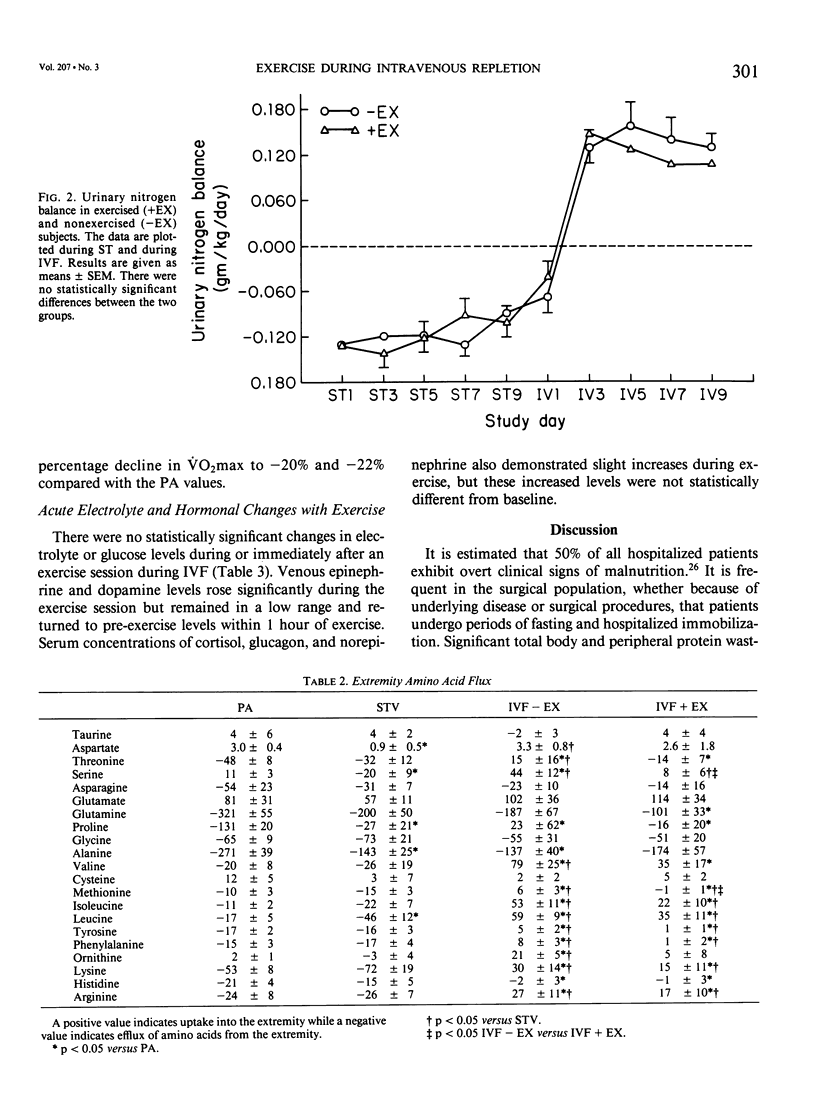
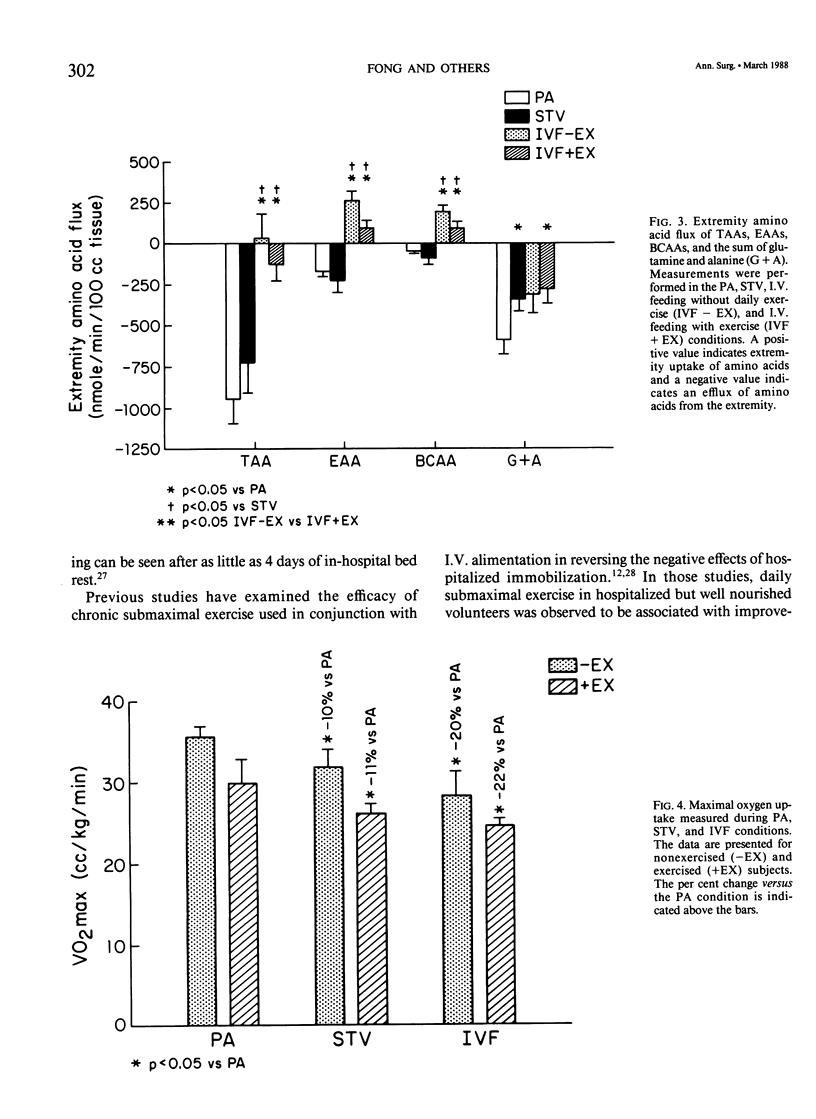
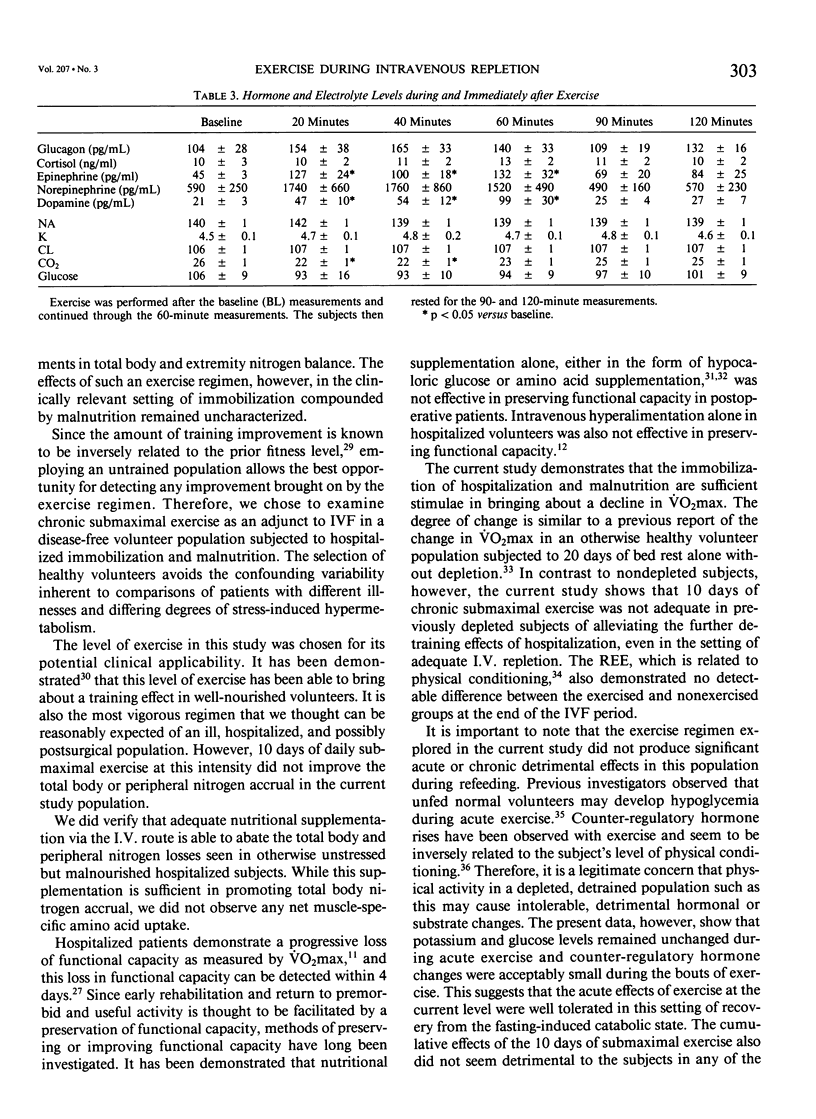
Abstract
The peripheral nitrogen wasting and loss of functional capacity caused by the malnutrition of disease and the immobilization of hospitalization may not be readily reversed by refeeding alone. In order to examine submaximal exercise as an adjunctive anabolic stimulus to intravenous refeeding (IVF) in depleted subjects, 14 volunteers were studied in the postabsorptive (PA) state, after 10 days of total starvation, and again after 10 days of nutritional repletion with I.V. feedings. The subjects were randomized to one group that received IVF alone and one group that performed 1 hour of submaximal (51% of VO2max) stationary bicycle exercise daily during IVF. The exercised group was not significantly different from the nonexercised group in urinary nitrogen balance, resting energy expenditure, extremity amino acid flux, or maximal oxygen consumption. Acute exercise did not induce significant derangements in electrolytes or counter-regulatory hormone concentrations. Ten days of submaximal exercise does not appear to be detrimental in this population recovering from moderate hospitalized malnutrition, but additional anabolic stimulae may be needed for improvements in protein accrual or functional capacity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTRAND P. O., SALTIN B. Maximal oxygen uptake and heart rate in various types of muscular activity. J Appl Physiol. 1961 Nov;16:977–981. doi: 10.1152/jappl.1961.16.6.977. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Albert J. D., Legaspi A., Horowitz G. D., Tracey K. J., Brennan M. F., Lowry S. F. Extremity amino acid metabolism during starvation and intravenous refeeding in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E604–E610. doi: 10.1152/ajpendo.1986.251.5.E604. [DOI] [PubMed] [Google Scholar]
- Bassey E. J., Fentem P. H. Extent of deterioration in physical condition during postoperative bed rest and its reversal by rehabilitation. Br Med J. 1974 Oct 26;4(5938):194–196. doi: 10.1136/bmj.4.5938.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistrian B. R., Blackburn G. L., Vitale J., Cochran D., Naylor J. Prevalence of malnutrition in general medical patients. JAMA. 1976 Apr 12;235(15):1567–1570. [PubMed] [Google Scholar]
- Bruce R. A., Kusumi F., Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973 Apr;85(4):546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Cooper R. R. Alterations during immobilization and regeneration of skeletal muscle in cats. J Bone Joint Surg Am. 1972 Jul;54(5):919–953. [PubMed] [Google Scholar]
- Costill D. L. Carbohydrate nutrition before, during, and after exercise. Fed Proc. 1985 Feb;44(2):364–368. [PubMed] [Google Scholar]
- Dohm G. L., Kasperek G. J., Tapscott E. B., Barakat H. A. Protein metabolism during endurance exercise. Fed Proc. 1985 Feb;44(2):348–352. [PubMed] [Google Scholar]
- Felig P., Cherif A., Minagawa A., Wahren J. Hypoglycemia during prolonged exercise in normal men. N Engl J Med. 1982 Apr 15;306(15):895–900. doi: 10.1056/NEJM198204153061503. [DOI] [PubMed] [Google Scholar]
- Gaesser G. A., Rich R. G. Effects of high- and low-intensity exercise training on aerobic capacity and blood lipids. Med Sci Sports Exerc. 1984 Jun;16(3):269–274. [PubMed] [Google Scholar]
- Goldberg A. L. Influence of insulin and contractile activity on muscle size and protein balance. Diabetes. 1979 Jan;28 (Suppl 1):18–24. doi: 10.2337/diab.28.1.s18. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Mechanisms of growth and atrophy of skeletal muscle. Muscle Biol. 1972;1:89–118. [PubMed] [Google Scholar]
- Gollnick P. D. Metabolism of substrates: energy substrate metabolism during exercise and as modified by training. Fed Proc. 1985 Feb;44(2):353–357. [PubMed] [Google Scholar]
- Kapadia C. R., Colpoys M. F., Jiang Z. M., Johnson D. J., Smith R. J., Wilmore D. W. Maintenance of skeletal muscle intracellular glutamine during standard surgical trauma. JPEN J Parenter Enteral Nutr. 1985 Sep-Oct;9(5):583–589. doi: 10.1177/0148607185009005583. [DOI] [PubMed] [Google Scholar]
- Lee P. L. Single-column system for accelerated amino acid analysis of physiological fluids using five lithium buffers. Biochem Med. 1974 Jun;10(2):107–121. doi: 10.1016/0006-2944(74)90013-1. [DOI] [PubMed] [Google Scholar]
- Lowry S. F., Horowitz G. D., Jeevanandam M., Legaspi A., Brennan M. F. Whole-body protein breakdown and 3-methylhistidine excretion during brief fasting, starvation, and intravenous repletion in man. Ann Surg. 1985 Jul;202(1):21–27. doi: 10.1097/00000658-198507000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. A., Lowry S. F., Brennan M. F. Effect of work-induced hypertrophy on skeletal muscle of tumor- and nontumor-bearing rats. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):654–657. doi: 10.1152/jappl.1979.46.4.654. [DOI] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Shephard R. J. Intensity, duration and frequency of exercise as determinants of the response to a training regime. Int Z Angew Physiol. 1968;26(3):272–278. doi: 10.1007/BF00695115. [DOI] [PubMed] [Google Scholar]
- Sigdell J. E. Venous occlusion plethysmography. Part 1: basic principles and applications. Biomed Eng. 1975 Aug;10(8):300–302. [PubMed] [Google Scholar]
- WEIR J. B. DE B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949 Aug;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore J. H., Davis J. A., Norton A. C. An automated system for assessing metabolic and respiratory function during exercise. J Appl Physiol. 1976 Apr;40(4):619–624. doi: 10.1152/jappl.1976.40.4.619. [DOI] [PubMed] [Google Scholar]
- Winder W. W., Hickson R. C., Hagberg J. M., Ehsani A. A., McLane J. A. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):766–771. doi: 10.1152/jappl.1979.46.4.766. [DOI] [PubMed] [Google Scholar]
- Wood C. D. Postoperative exercise capacity following nutritional support with hypotonic glucose. Surg Gynecol Obstet. 1981 Jan;152(1):39–42. [PubMed] [Google Scholar]
- Wood C. D., Shreck R., Tommey R., Towsley K., Guess C. W., Werth R., Pollard M. Relative value of glucose and amino acids in preserving exercise capacity in the postoperative period. Am J Surg. 1985 Mar;149(3):383–386. doi: 10.1016/s0002-9610(85)80113-6. [DOI] [PubMed] [Google Scholar]
- Wood P. D., Terry R. B., Haskell W. L. Metabolism of substrates: diet, lipoprotein metabolism, and exercise. Fed Proc. 1985 Feb;44(2):358–363. [PubMed] [Google Scholar]


