Abstract
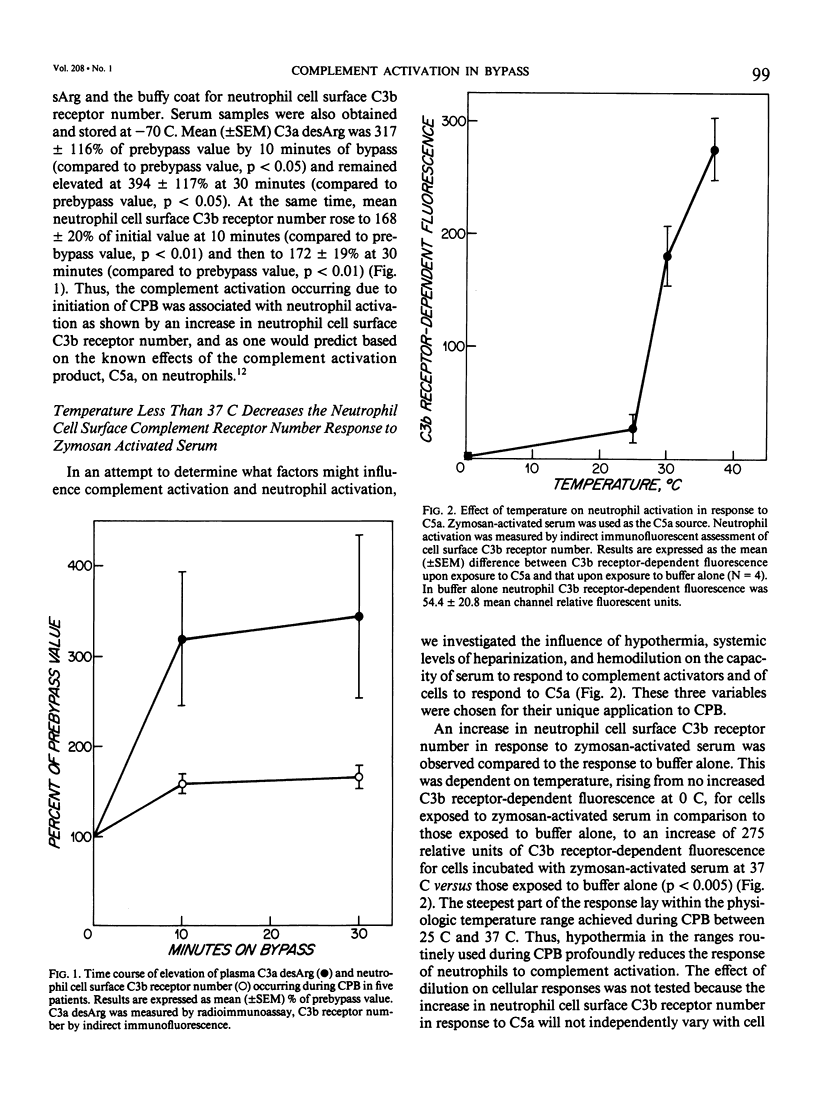
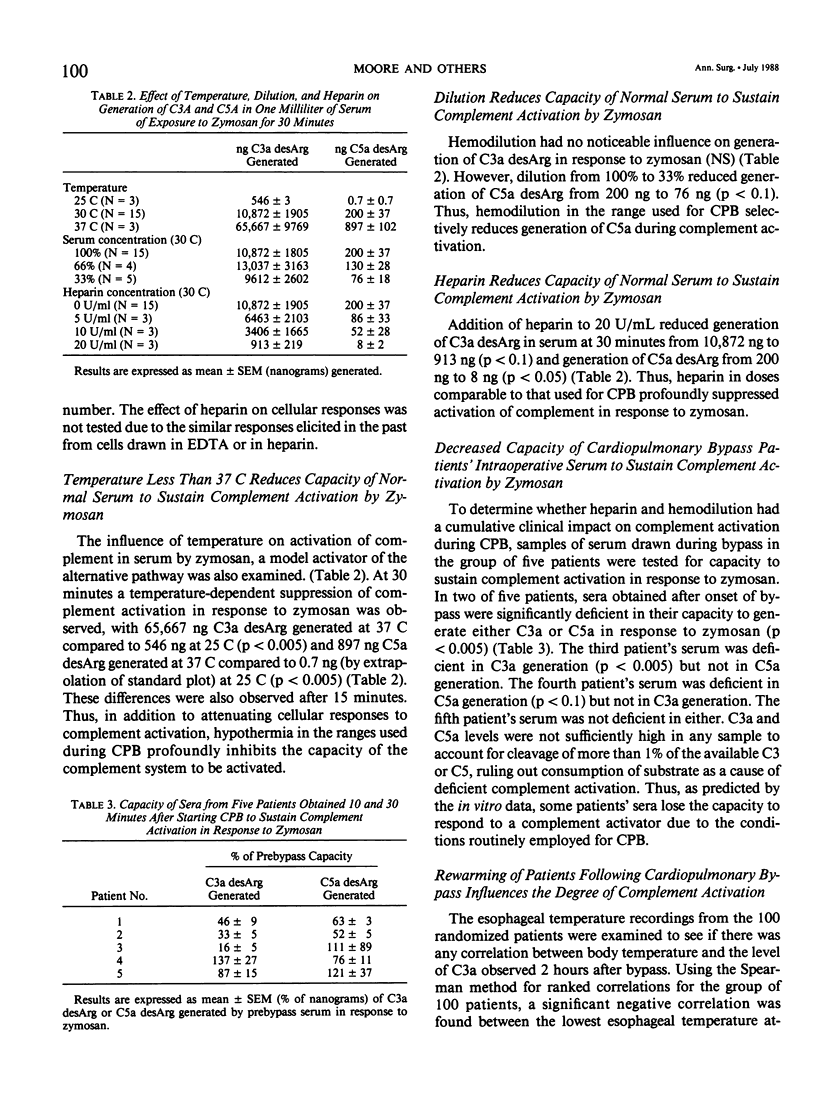
Complement activation was examined prospectively in 100 cardiopulmonary bypass (CPB) patients. Plasma C3a desArg (C3a) increased (cannulation: 234 +/- 33 ng/mL; 20 minutes on CPB: 622 +/- 51; 2 hours after CPB: 1143 +/- 109, p less than 0.0001). C3a at 2 hours was higher in the 13 patients requiring mechanical ventilation for longer than 1 day (1023 +/- 274) than in the 67 without respiratory complication (568 +/- 45, p less than 0.004). Five more patients were studied for neutrophil activation to confirm that a biologic effect of complement activation occurs during CPB; in these five patients C3a increased to 317% of baseline after 10 minutes on CPB with a corresponding rise in neutrophil cell surface receptors for the complement opsonin C3b (as measured by indirect immunofluorescence) to 168% (p less than 0.05). Both increases were sustained at 30 minutes. Temperature, dilution, and heparin were studied as variables relevant to CPB. Exposure of normal neutrophils to C5a in vitro caused an increase in C3b receptors which was dependent on temperature (0 specific fluorescence at 0 C, 30 at 25 C, 180 at 30 C, and 275 at 37 C). Generation of C3a and C5a in normal serum by zymosan was also temperature-dependent (ng/mL C5a generated: 0.7 at 25 C, 200 at 30 C, and 897 at 37 C; ng/mL C3a generated: 546 at 25 C, 10,872 at 30 C, and 65,667 at 37 C). Serum dilution to 33% decreased ng/mL C5a generated in the same system from 200 to 76 with no effect on C3a. Addition of heparin to 20 U/mL decreased ng/mL C3a generated from 10,872 to 913 and C5a from 200 to 8. Thus, hypothermia, dilution, and heparin protect CPB patients from complement activation by reducing both generation of C3a/C5a and the subsequent cellular response of neutrophil activation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavarocchi N. C., Pluth J. R., Schaff H. V., Orszulak T. A., Homburger H. A., Solis E., Kaye M. P., Clancy M. S., Kolff J., Deeb G. M. Complement activation during cardiopulmonary bypass. Comparison of bubble and membrane oxygenators. J Thorac Cardiovasc Surg. 1986 Feb;91(2):252–258. [PubMed] [Google Scholar]
- Cavarocchi N. C., Schaff H. V., Orszulak T. A., Homburger H. A., Schnell W. A., Jr, Pluth J. R. Evidence for complement activation by protamine-heparin interaction after cardiopulmonary bypass. Surgery. 1985 Sep;98(3):525–531. [PubMed] [Google Scholar]
- Changelian P. S., Jack R. M., Collins L. A., Fearon D. T. PMA induces the ligand-independent internalization of CR1 on human neutrophils. J Immunol. 1985 Mar;134(3):1851–1858. [PubMed] [Google Scholar]
- Chenoweth D. E., Cooper S. W., Hugli T. E., Stewart R. W., Blackstone E. H., Kirklin J. W. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981 Feb 26;304(9):497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donabedian H., Gallin J. I. Deactivation of human neutrophil chemotaxis by chemoattractants: effect on receptors for the chemotactic factor f-Met-Leu-Phe. J Immunol. 1981 Sep;127(3):839–844. [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Hakim R. M., Lowrie E. G. Effect of dialyzer reuse on leukopenia, hypoxemia and total hemolytic complement system. Trans Am Soc Artif Intern Organs. 1980;26:159–164. [PubMed] [Google Scholar]
- Hammerschmidt D. E., Stroncek D. F., Bowers T. K., Lammi-Keefe C. J., Kurth D. M., Ozalins A., Nicoloff D. M., Lillehei R. C., Craddock P. R., Jacob H. S. Complement activation and neutropenia occurring during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1981 Mar;81(3):370–377. [PubMed] [Google Scholar]
- Khuri S. F., Marston W. A., Josa M., Braunwald N. S., Cavanaugh A. C., Hunt H., Barsamian E. M. Observations on 100 patients with continuous intraoperative monitoring of intramyocardial pH. The adverse effects of ventricular fibrillation and reperfusion. J Thorac Cardiovasc Surg. 1985 Feb;89(2):170–182. [PubMed] [Google Scholar]
- Kirklin J. K., Chenoweth D. E., Naftel D. C., Blackstone E. H., Kirklin J. W., Bitran D. D., Curd J. G., Reves J. G., Samuelson P. N. Effects of protamine administration after cardiopulmonary bypass on complement, blood elements, and the hemodynamic state. Ann Thorac Surg. 1986 Feb;41(2):193–199. doi: 10.1016/s0003-4975(10)62668-9. [DOI] [PubMed] [Google Scholar]
- Kirklin J. K., Westaby S., Blackstone E. H., Kirklin J. W., Chenoweth D. E., Pacifico A. D. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983 Dec;86(6):845–857. [PubMed] [Google Scholar]
- Lee J., Hakim R. M., Fearon D. T. Increased expression of the C3b receptor by neutrophils and complement activation during haemodialysis. Clin Exp Immunol. 1984 Apr;56(1):205–214. [PMC free article] [PubMed] [Google Scholar]
- Moore F. D., Jr, Davis C., Rodrick M., Mannick J. A., Fearon D. T. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N Engl J Med. 1986 Apr 10;314(15):948–953. doi: 10.1056/NEJM198604103141503. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]



