Abstract
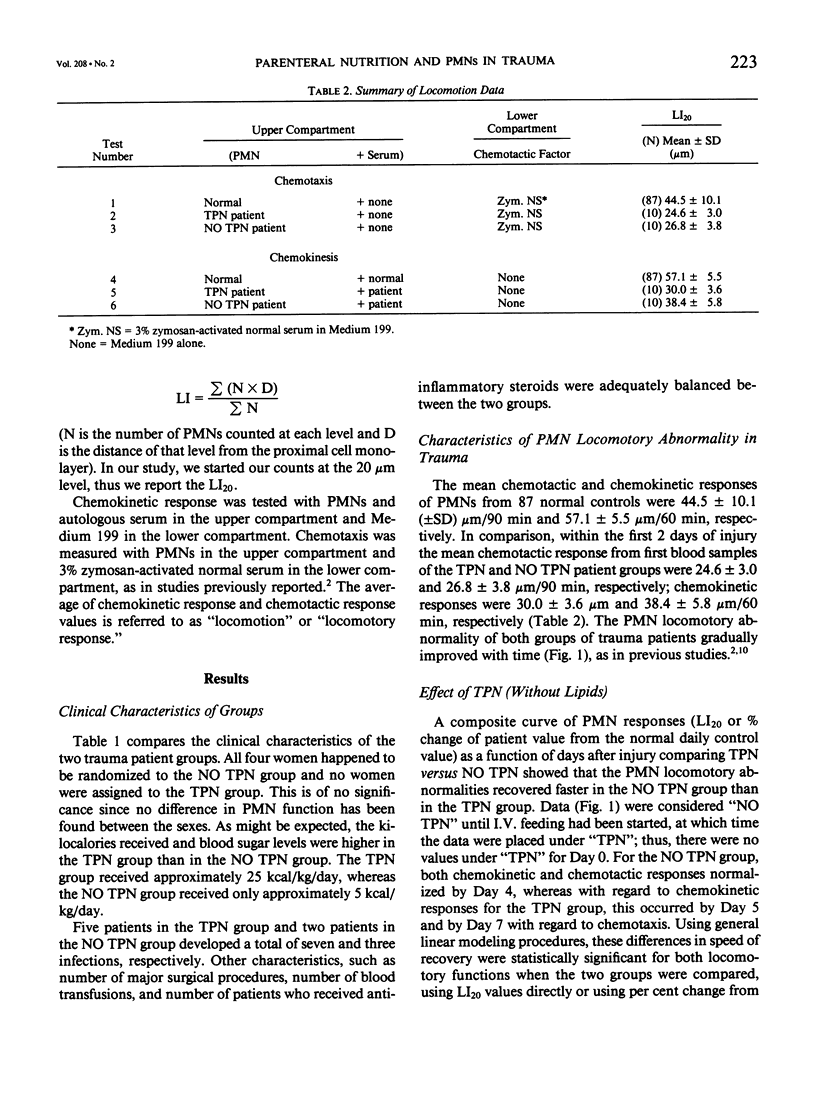
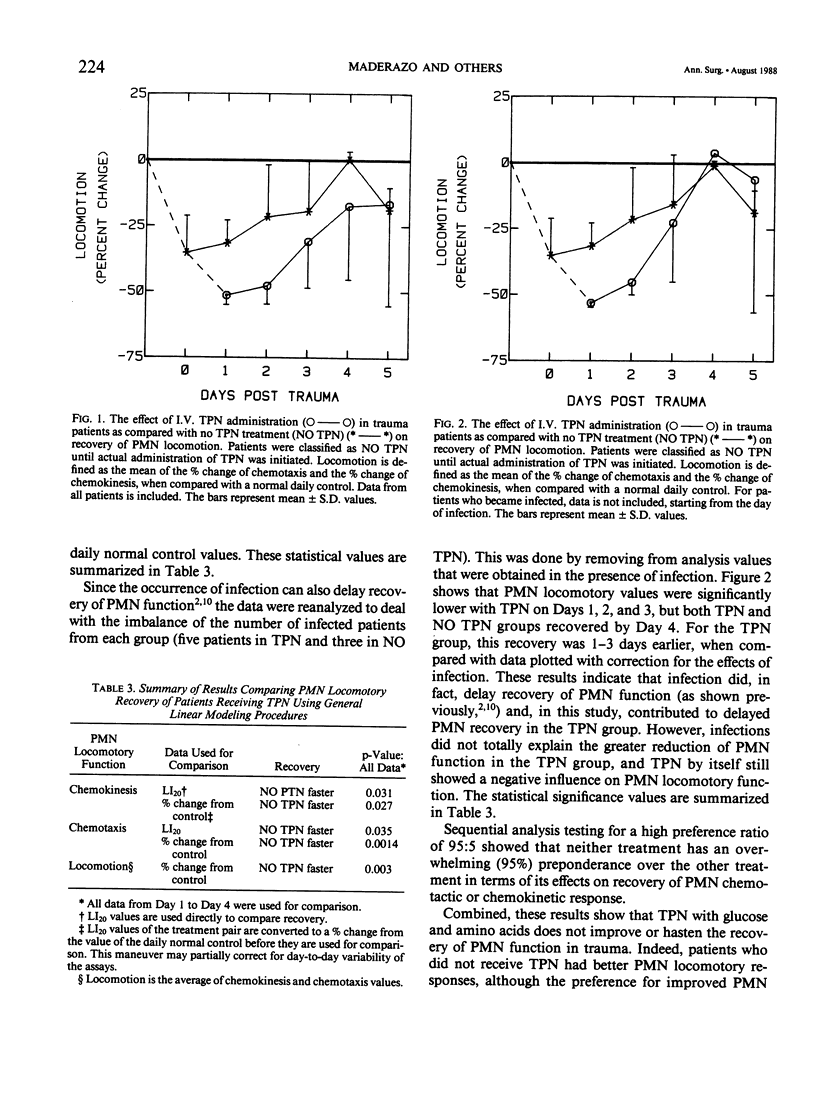
Twenty patients were investigated to determine whether total parenteral nutrition (TPN) influences the recovery of neutrophil (PMN) locomotory dysfunction in blunt trauma. Half were given TPN consisting of amino acids, glucose, electrolytes, and trace minerals, and half were given intravenous (I.V.) fluids consisting of 5% glucose in water or saline, electrolytes, and trace minerals. PMN locomotion was assayed using micropore filters. Analysis of the data by general linear modeling showed that PMN locomotion in TPN patients was significantly slower during the first 3 to 4 days postinjury. By sequential analysis, improved PMN function in the group not given TPN (NO TPN) occurred less than 95% of the time. TPN with amino acids and glucose may worsen and delay the recovery of PMN locomotory responses in blunt trauma, but the preference ratio of NO TPN:TPN for better PMN function was less than 95:5.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W. Immunity, nutrition and trauma: an overview. Acta Chir Scand Suppl. 1985;522:141–150. [PubMed] [Google Scholar]
- Baker S. P., O'Neill B., Haddon W., Jr, Long W. B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974 Mar;14(3):187–196. [PubMed] [Google Scholar]
- Blackburn G. L. Nutritional assessment and support during infection. Am J Clin Nutr. 1977 Sep;30(9):1493–1497. doi: 10.1093/ajcn/30.9.1493. [DOI] [PubMed] [Google Scholar]
- Christou N. V., McLean A. P., Meakins J. L. Host defense in blunt trauma: interrelationships of kinetics of anergy and depressed neutrophil function, nutritional status, and sepsis. J Trauma. 1980 Oct;20(10):833–841. doi: 10.1097/00005373-198010000-00003. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Yawata Y., VanSanten L., Gilberstadt S., Silvis S., Jacob H. S. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N Engl J Med. 1974 Jun 20;290(25):1403–1407. doi: 10.1056/NEJM197406202902504. [DOI] [PubMed] [Google Scholar]
- Elwyn D. H., Bryan-Brown C. W., Shoemaker W. C. Nutritional aspects of body water dislocations in postoperative and depleted patients. Ann Surg. 1975 Jul;182(1):76–85. doi: 10.1097/00000658-197507000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N., Parker P., Greene H. L., Ghishan F. K. Intravenous lipid emulsions and human neutrophil function. J Pediatr. 1981 Dec;99(6):913–916. doi: 10.1016/s0022-3476(81)80019-4. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Grisham M. B., Jefferson M. M., Melton D. F., Thomas E. L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem. 1984 Aug 25;259(16):10404–10413. [PubMed] [Google Scholar]
- Heymsfield S. B., Bethel R. A., Ansley J. D., Nixon D. W., Rudman D. Enteral hyperalimentation: an alternative to central venous hyperalimentation. Ann Intern Med. 1979 Jan;90(1):63–71. doi: 10.7326/0003-4819-90-1-63. [DOI] [PubMed] [Google Scholar]
- Maderazo E. G., Albano S. D., Woronick C. L., Drezner A. D., Quercia R. Polymorphonuclear leukocyte migration abnormalities and their significance in seriously traumatized patients. Ann Surg. 1983 Dec;198(6):736–742. doi: 10.1097/00000658-198312000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderazo E. G., Woronick C. L., Albano S. D., Breaux S. P., Pock R. M. Inappropriate activation, deactivation, and probable autooxidative damage as a mechanism of neutrophil locomotory defect in trauma. J Infect Dis. 1986 Sep;154(3):471–477. doi: 10.1093/infdis/154.3.471. [DOI] [PubMed] [Google Scholar]
- Maderazo E. G., Woronick C. L. Micropore filter assay of human granulocyte locomotion: problems and solutions. Clin Immunol Immunopathol. 1978 Oct;11(2):196–211. doi: 10.1016/0090-1229(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Moore F. D. Energy and the maintenance of the body cell mass. JPEN J Parenter Enteral Nutr. 1980 May-Jun;4(3):228–260. doi: 10.1177/014860718000400302. [DOI] [PubMed] [Google Scholar]
- Moore F. D. The problem of trauma. Acta Chir Scand Suppl. 1985;522:13–23. [PubMed] [Google Scholar]
- Nordenström J., Jarstrand C., Wiernik A. Decreased chemotactic and random migration of leukocytes during Intralipid infusion. Am J Clin Nutr. 1979 Dec;32(12):2416–2422. doi: 10.1093/ajcn/32.12.2416. [DOI] [PubMed] [Google Scholar]
- Schopfer K., Douglas S. D. Neutrophil function in children with kwashiorkor. J Lab Clin Med. 1976 Sep;88(3):450–461. [PubMed] [Google Scholar]


