Abstract
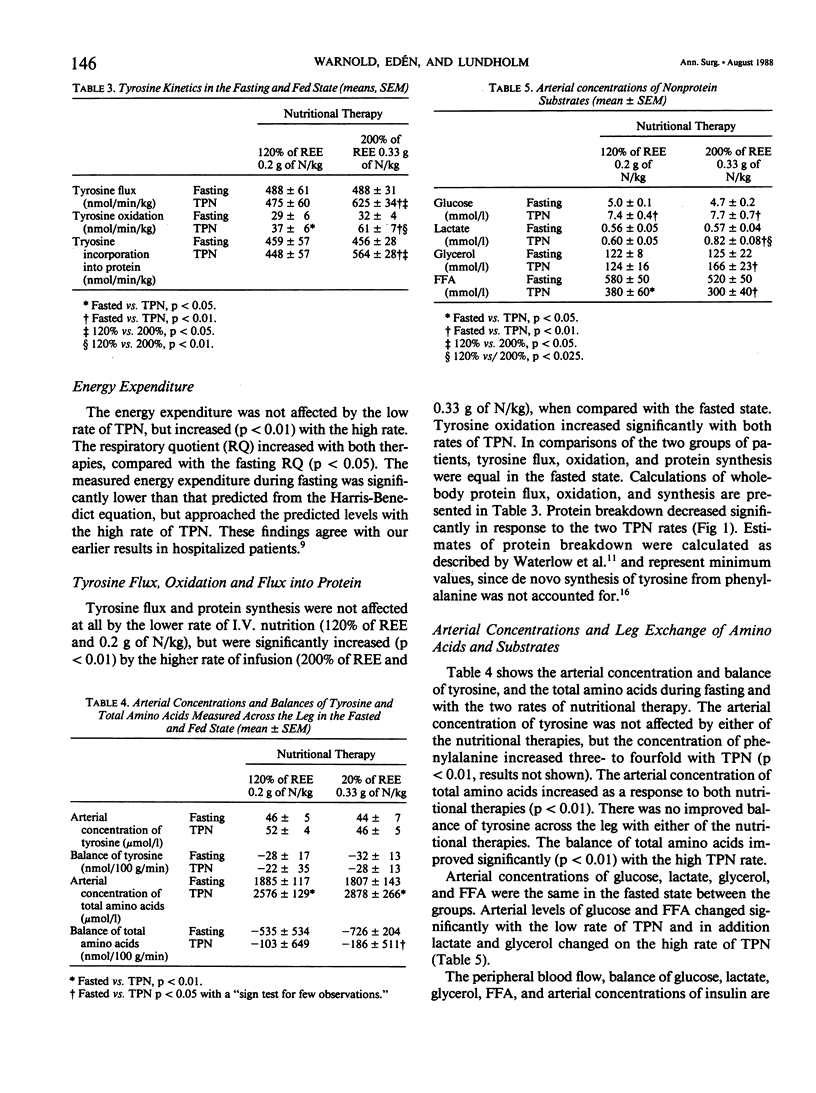
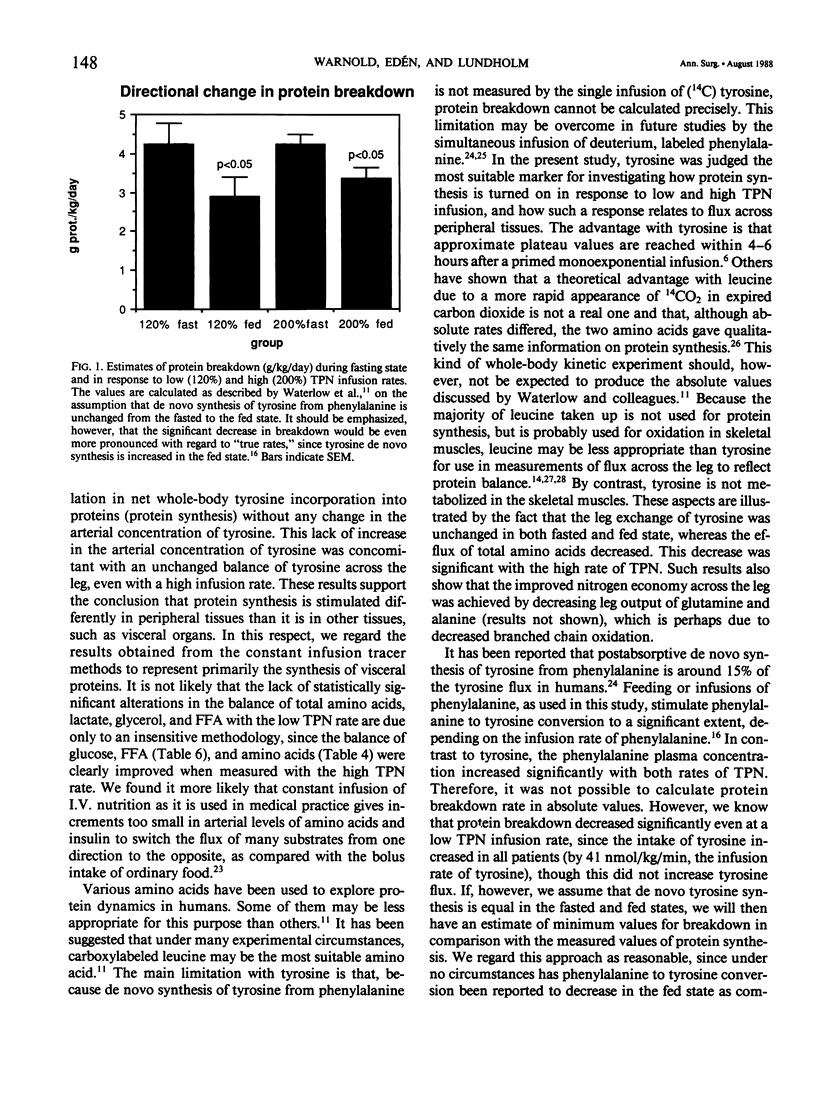
The acute whole-body and peripheral tissue protein response to total parenteral nutrition (TPN) was evaluated before surgery in moderately malnourished patients with stable disease. A primed constant infusion of (U-14C) tyrosine was used in combination with simultaneous measurements of the leg exchange of amino acids, glucose, glycerol, and free fatty acids (FFA). Energy expenditure was measured by indirect calorimetry. Sixteen patients with stable disease and in need of nutritional support were randomized to receive TPN at rates either corresponding to resting requirements (nonprotein calories at 120% of REE with 0.2 g of N/kg/d) or at increased rates (200% of REE with 0.33 g of N/kg/d). Energy expenditure was not affected by the low rate of TPN, but increased with the high rate, with a thermic effect corresponding to 16% of basal levels. Tyrosine flux and incorporation rate into whole-body proteins (protein synthesis) were not altered by the low TPN rate, but increased with the high rate. Estimates of protein breakdown decreased, and tyrosine oxidation increased significantly with both rates of TPN. Protein synthesis was stimulated at the high dose rate only. However, a positive whole-body tyrosine balance (net protein synthesis) measured by the 14C tyrosine technique was associated with a continued negative tyrosine balance across the skeletal muscle compartment in the leg. The results demonstrate that TPN given at rates corresponding to resting needs of 0.2 g of N/kg/day is insufficient to promote protein synthesis in the majority of body proteins. Skeletal muscles may remain in negative protein balance even at high TPN loads. Our results reflect the difficulties of expanding lean body mass through intravenous nutrition in moderately malnourished patients--even those with stable disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J. D., Legaspi A., Horowitz G. D., Tracey K. J., Brennan M. F., Lowry S. F. Extremity amino acid metabolism during starvation and intravenous refeeding in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E604–E610. doi: 10.1152/ajpendo.1986.251.5.E604. [DOI] [PubMed] [Google Scholar]
- Bennegård K., Edén E., Ekman L., Scherstén T., Lundholm K. Metabolic response of whole body and peripheral tissues to enteral nutrition in weight-losing cancer and noncancer patients. Gastroenterology. 1983 Jul;85(1):92–99. [PubMed] [Google Scholar]
- Bennegård K., Lindmark L., Edén E., Svaninger G., Lundholm K. Flux of amino acids across the leg in weight-losing cancer patients. Cancer Res. 1984 Jan;44(1):386–393. [PubMed] [Google Scholar]
- Bruce A., Andersson M., Arvidsson B., Isaksson B. Body composition. Prediction of normal body potassium, body water and body fat in adults on the basis of body height, body weight and age. Scand J Clin Lab Invest. 1980 Sep;40(5):461–473. doi: 10.3109/00365518009101869. [DOI] [PubMed] [Google Scholar]
- Clarke J. T., Bier D. M. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of L-[ring-2H5]phenylalanine and L-[1-13C] tyrosine in the postabsorptive state. Metabolism. 1982 Oct;31(10):999–1005. doi: 10.1016/0026-0495(82)90142-1. [DOI] [PubMed] [Google Scholar]
- Desai S. P., Moldawer L. L., Bistrian B. R., Blackburn G. L. Amino acid and protein metabolism in hospitalized patients as measured by L-[U-14C]tyrosine and L-[1-14C]leucine. Clin Sci (Lond) 1983 Nov;65(5):499–505. doi: 10.1042/cs0650499. [DOI] [PubMed] [Google Scholar]
- Edén E., Ekman L., Bennegård K., Lindmark L., Lundholm K. Whole-body tyrosine flux in relation to energy expenditure in weight-losing cancer patients. Metabolism. 1984 Nov;33(11):1020–1027. doi: 10.1016/0026-0495(84)90231-2. [DOI] [PubMed] [Google Scholar]
- Haider M., Haider S. Q. Assessment of protein-calorie malnutrition. Clin Chem. 1984 Aug;30(8):1286–1299. [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- James W. P., Garlick P. J., Sender P. M., Waterlow J. C. Studies of amino acid and protein metabolism in normal man with L-[U-14C]tyrosine. Clin Sci Mol Med. 1976 Jun;50(6):525–532. doi: 10.1042/cs0500525. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy K. N. Protein nutrition in clinical practice. Br Med Bull. 1981 Jan;37(1):11–17. doi: 10.1093/oxfordjournals.bmb.a071669. [DOI] [PubMed] [Google Scholar]
- Jeevanandam M., Lowry S. F., Horowitz G. D., Legaspi A., Brennan M. F. Influence of increasing dietary intake on whole body protein kinetics in normal man. Clin Nutr. 1986 Feb;5(1):41–48. doi: 10.1016/0261-5614(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Johnson D. J., Jiang Z. M., Colpoys M., Kapadia C. R., Smith R. J., Wilmore D. W. Branched chain amino acid uptake and muscle free amino acid concentrations predict postoperative muscle nitrogen balance. Ann Surg. 1986 Nov;204(5):513–523. doi: 10.1097/00000658-198611000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann W. D., Heinrich H. C. Impaired phenylalanine-tyrosine conversion in patients with iron-deficiency anemia studied by a L-(2H5)phenylalanine-loading test. Am J Clin Nutr. 1986 Oct;44(4):468–474. doi: 10.1093/ajcn/44.4.468. [DOI] [PubMed] [Google Scholar]
- Lindmark L., Bennegård K., Edén E., Ekman L., Scherstén T., Svaninger G., Lundholm K. Resting energy expenditure in malnourished patients with and without cancer. Gastroenterology. 1984 Aug;87(2):402–408. [PubMed] [Google Scholar]
- Lindmark L., Bennegård K., Edén E., Svaninger G., Ternell M., Lundholm K. Thermic effect and substrate oxidation in response to intravenous nutrition in cancer patients who lose weight. Ann Surg. 1986 Dec;204(6):628–636. doi: 10.1097/00000658-198612000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm K., Bennegård K., Zachrisson H., Lundgren F., Edén E., Möller-Loswick A. C. Transport kinetics of amino acids across the resting human leg. J Clin Invest. 1987 Sep;80(3):763–771. doi: 10.1172/JCI113132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldawer L. L., Kawamura I., Bistrian B. R., Blackburn G. L. The contribution of phenylalanine to tyrosine metabolism in vivo. Studies in the post-absorptive and phenylalanine-loaded rat. Biochem J. 1983 Mar 15;210(3):811–817. doi: 10.1042/bj2100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacht C. A., Schutz Y., Vernet O., Christin L., Jéquier E. Continuous versus single bolus enteral nutrition: comparison of energy metabolism in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E524–E529. doi: 10.1152/ajpendo.1986.251.5.E524. [DOI] [PubMed] [Google Scholar]
- O'Keefe S. J., Moldawer L. L., Young V. R., Blackburn G. L. The influence of intravenous nutrition on protein dynamics following surgery. Metabolism. 1981 Dec;30(12):1150–1158. doi: 10.1016/0026-0495(81)90034-2. [DOI] [PubMed] [Google Scholar]
- Pocock S. J., Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975 Mar;31(1):103–115. [PubMed] [Google Scholar]
- Steffee W. P., Goldsmith R. S., Pencharz P. B., Scrimshaw N. S., Young V. R. Dietary protein intake and dynamic aspects of whole body nitrogen metabolism in adult humans. Metabolism. 1976 Mar;25(3):281–297. doi: 10.1016/0026-0495(76)90086-x. [DOI] [PubMed] [Google Scholar]
- Streat S. J., Beddoe A. H., Hill G. L. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987 Mar;27(3):262–266. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- Symreng T. Arm anthropometry in a large reference population and in surgical patients. Clin Nutr. 1982 Dec;1(3):211–219. doi: 10.1016/0261-5614(82)90015-2. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]


