Abstract
Background
Using an indirect immunoperoxidase technique, we have studied the distribution of immunoreactive fibers and cell bodies containing neurokinin in the adult human brainstem with no prior history of neurological or psychiatric disease.
Results
Clusters of immunoreactive cell bodies and high densities of neurokinin-immunoreactive fibers were located in the periaqueductal gray, the dorsal motor nucleus of the vagus and in the reticular formation of the medulla, pons and mesencephalon. Moreover, immunoreactive cell bodies were found in the inferior colliculus, the raphe obscurus, the nucleus prepositus hypoglossi, and in the midline of the anterior medulla oblongata. In general, immunoreactive fibers containing neurokinin were observed throughout the whole brainstem. In addition to the nuclei mentioned above, the highest densities of such immunoreactive fibers were located in the spinal trigeminal nucleus, the lateral reticular nucleus, the nucleus of the solitary tract, the superior colliculus, the substantia nigra, the nucleus ambiguus, the gracile nucleus, the cuneate nucleus, the motor hypoglossal nucleus, the medial and superior vestibular nuclei, the nucleus prepositus hypoglossi and the interpeduncular nucleus.
Conclusion
The widespread distribution of immunoreactive structures containing neurokinin in the human brainstem indicates that neurokinin might be involved in several physiological mechanisms, acting as a neurotransmitter and/or neuromodulator.
Background
The mammalian tachykinin peptides include neurokinin A (NKA), neurokinin B (NKB) and substance P (SP) [1]. It is known that these three neuropeptides have a common C-terminal amino acid sequence and that NKA and SP are derived from the preprotachykinin A gene, whereas NKB is derived from the preprotachykinin B gene. It is also known that the biological actions of NKA, NKB and SP are mediated by three receptors, named neurokinin (NK)-1, NK-2 and NK-3 [2]. Thus, the tachykinins have been implicated in several physiological actions such as salivation, the regulation of smooth muscle contraction, depolarization of central neurons, hyperactivity, interaction with dopaminergic A-10 neurons mediating behavioral activation, regulation of blood pressure, and the transmission of the baroreceptor reflex [3-7]. In addition, a loss of tachykinin-containing neurons has been described in neurodegenerative diseases [8], suggesting that the decrease in the amount of tachykinins could be involved in these and other diseases [9,10].
Several inmunocytochemical, " in situ " hybridization and radioimmunoassay studies have demonstrated the distribution of tachykinins in the rat, the cat and the monkey CNS [11-20]. In the human brainstem, the presence of SP has been widely studied [21-27]. Moreover, in humans, the neurokinin B system has been studied in the cerebral cortex, hippocampus and the hypothalamus [28]. However, no data are available in the literature concerning the distribution of neurokinin-immunoreactive structures in the human brainstem. Thus, in the present work we attempted to study in detail the distribution of fibers and cell bodies containing neurokinin in the human brainstem, using an immunoperoxidase technique, and to compare our findings with the distribution of other tachykinins previously described in the human brainstem [23-25,27]. Finally, we report here a widespread distribution of fibers and cell bodies containing neurokinin in the human brainstem.
Results
As shown in Figures 1, 2, 3, neurokinin-like immunoreactive (NK-ir) structures are widely distributed throughout the human brainstem. In general, the distribution of the immunoreactive structures (fibers and cell bodies), as well as the density of such structures, were quite similar in the four brainstems studied. In the three brainstem regions (medulla oblongata, pons and mesencephalon), the highest density of immunoreactive structures was generally observed in their dorsal parts. In addition, the clusters of cell bodies containing NK were almost always observed in the dorsal part of the human brainstem. Finally, in general, the NK-ir cell bodies observed in the human brainstem were large (showing the longest diameter between 20–55 μm).
Figure 1.
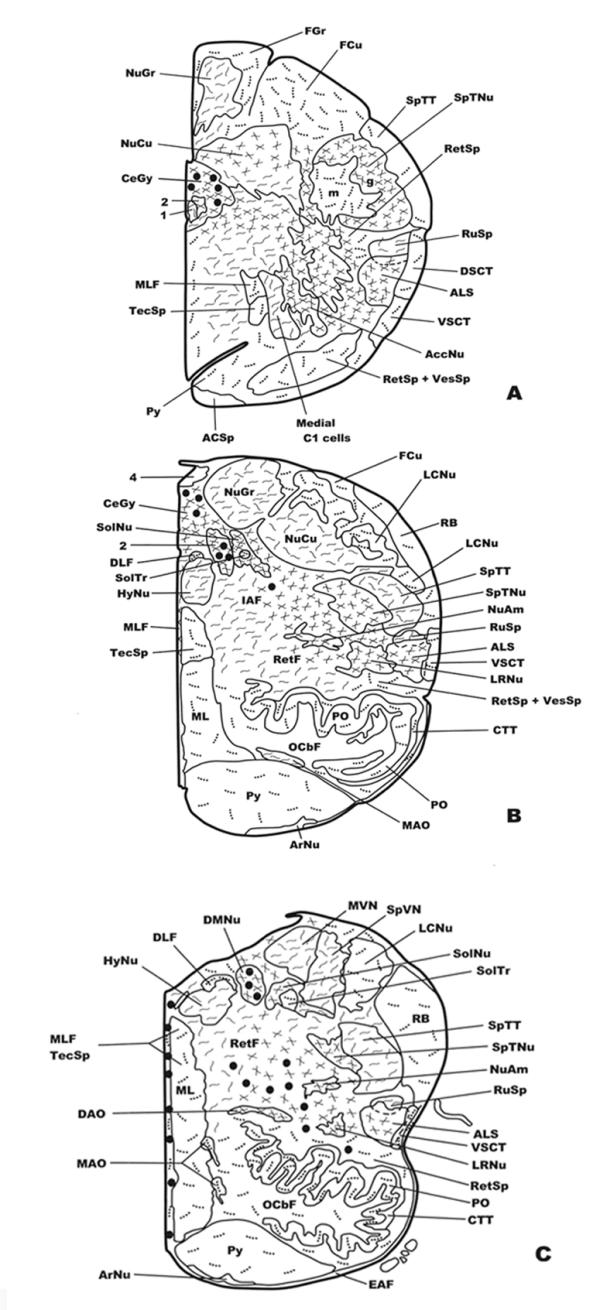
Distribution of NK-ir fibers and cell bodies in frontal planes of the human brainstem from the caudal (Fig. 1A) to the anterior (Fig. 3C) levels. Cell bodies containing neurokinin are represented by closed circles, whereas immunoreactive fibers are represented by dotted lines (single fibers or low density), continuous lines (moderate density) and crossed lines (high density). See list of abbreviations for nomenclature.
Figure 2.
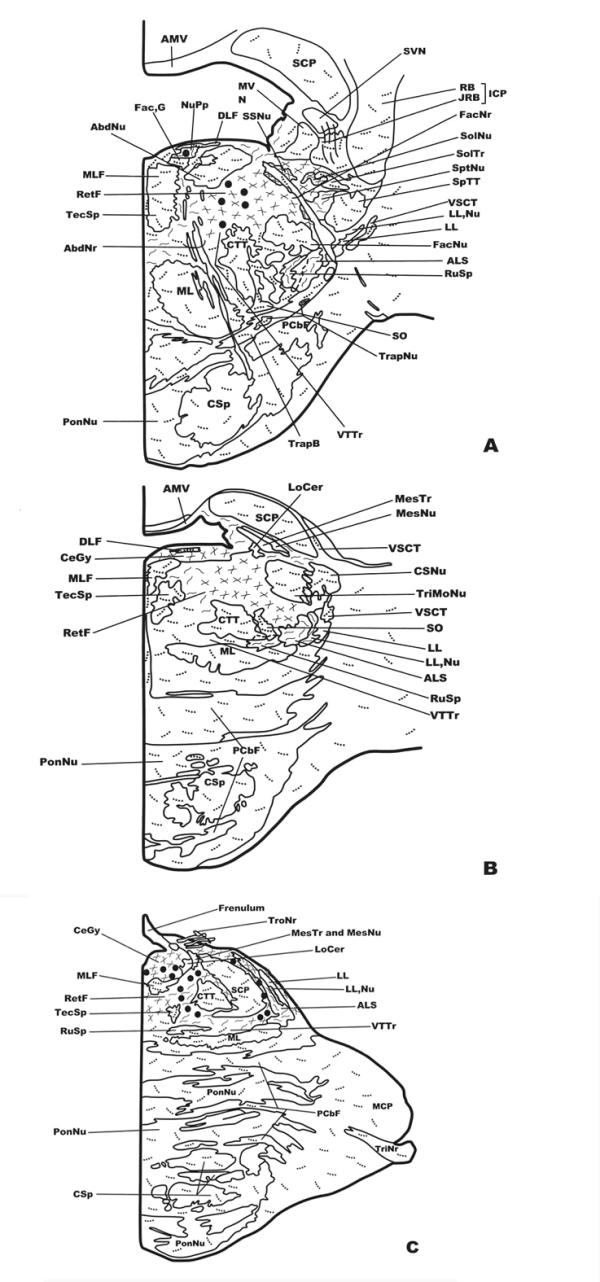
Distribution of NK-ir fibers and cell bodies in frontal planes of the human brainstem from the caudal (Fig. 1A) to the anterior (Fig. 3C) levels. Cell bodies containing neurokinin are represented by closed circles, whereas immunoreactive fibers are represented by dotted lines (single fibers or low density), continuous lines (moderate density) and crossed lines (high density). See list of abbreviations for nomenclature.
Figure 3.
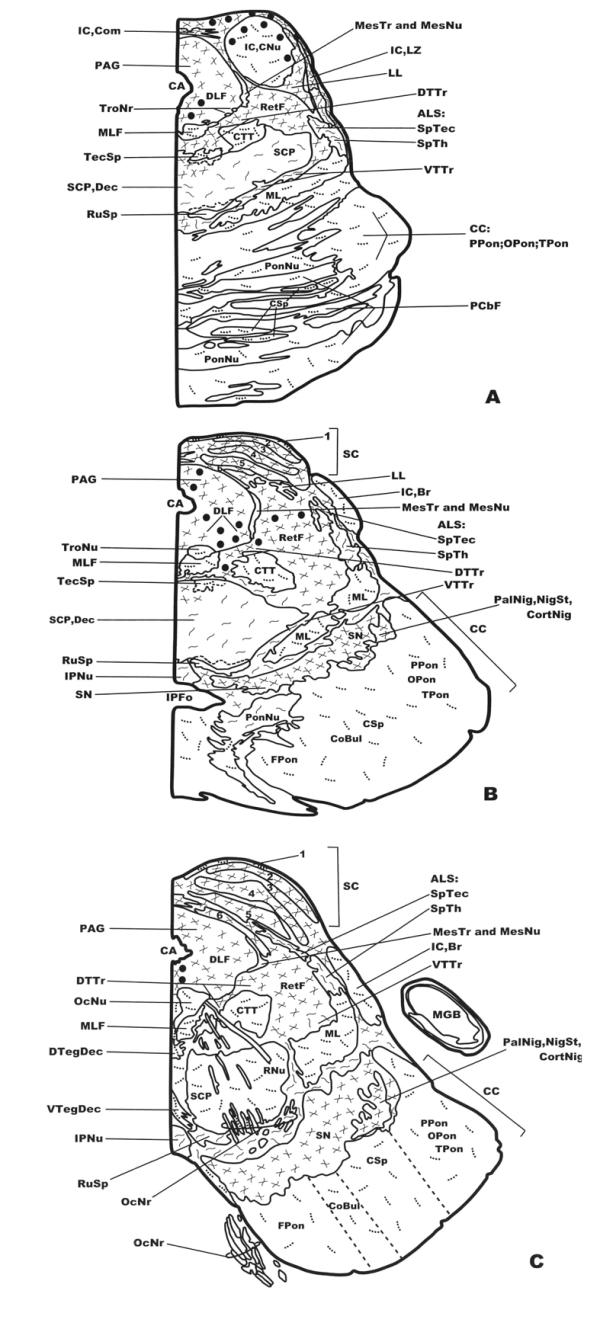
Distribution of NK-ir fibers and cell bodies in frontal planes of the human brainstem from the caudal (Fig. 1A) to the anterior (Fig. 3C) levels. Cell bodies containing neurokinin are represented by closed circles, whereas immunoreactive fibers are represented by dotted lines (single fibers or low density), continuous lines (moderate density) and crossed lines (high density). See list of abbreviations for nomenclature.
Distribution of NK-ir structures in the human medulla oblongata
NK-ir cell bodies
A low density of immunoreactive cell bodies containing NK was observed caudally in the medullary central gray (Figs. 1A; 4A), whereas more rostrally (Fig. 1B) such clusters of NK-ir perikarya showed a moderate density. At the latter level (Fig. 1B), a moderate density was also observed in the dorsal motor nucleus of the vagus and a low density below the nucleus of the solitary tract. More rostrally (Fig. 1C) three populations of immunoreactive cell bodies were observed: the first located along the midline (high density); the second in the dorsal motor nucleus of the vagus (high density)(Fig. 5A,5B); and the third in the reticular formation (nucleus reticularis gigantocellularis included)(moderate density), above the dorsal accessory olivary nucleus and the central tegmental tract (Fig. 6A,6B). In addition, a moderate density of immunoreactive cell bodies containing NK was observed in the raphe obscurus (not shown in Figures).
Figure 4.
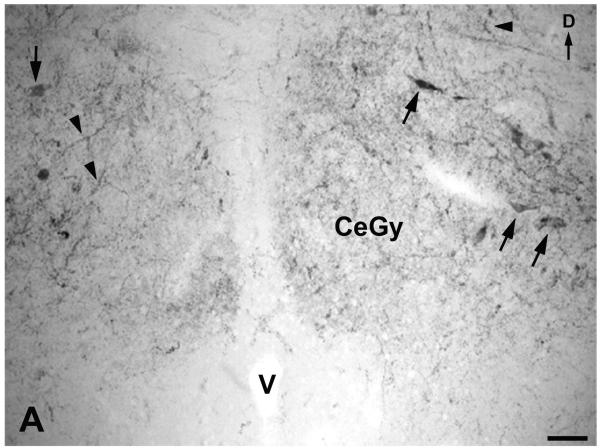
A. Immunoreactive cell bodies (arrows) and fibers (arrowheads) containing NK in the medullary central gray (CeGy). D: dorsal; V: ventricle. Scale bar: 100 μm
Figure 5.
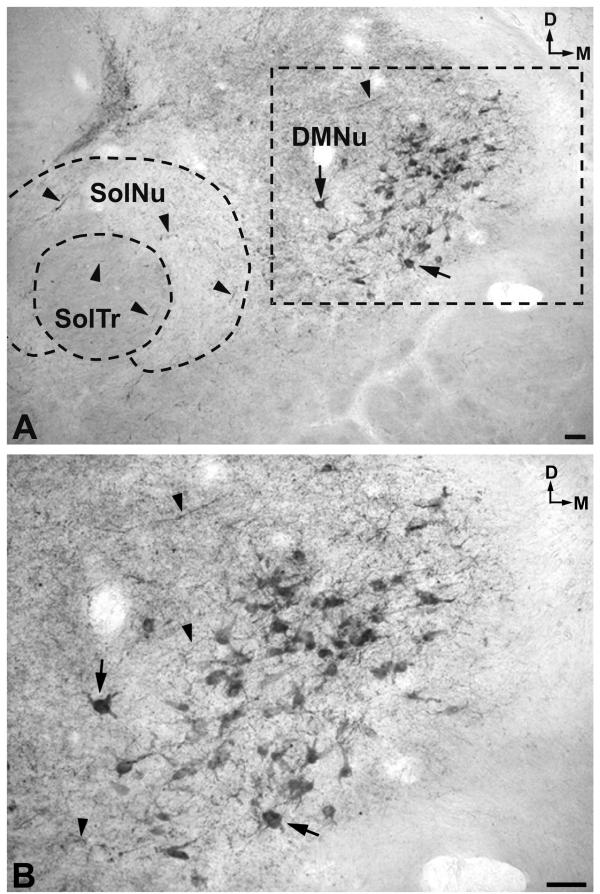
A. Cell bodies (arrows) and fibers (arrowheads) in the dorsal motor nucleus of the vagus (DMNu). Note NK-ir fibers (arrowheads) in both nucleus of the solitary tract (SolNu) and in the solitary tract (SolTr). In addition, NK-ir fibers can be observed around the nucleus of the solitary tract. D: dorsal; M: medial. Scale bar: 100 μm B. High-power image of the area delimited in Figure 5A. D: dorsal; M: medial. Scale bar: 100 μm
Figure 6.
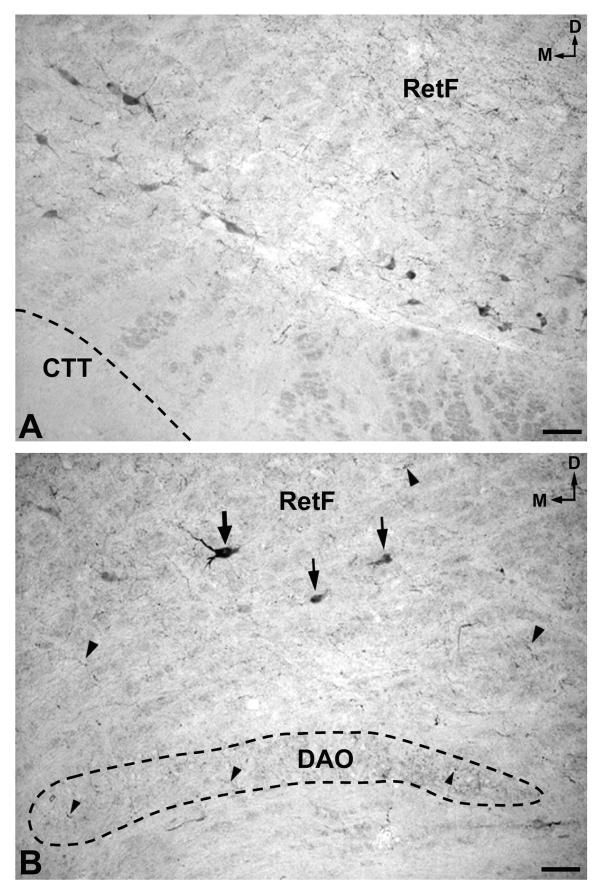
A. Cluster of immunoreactive perikarya located laterally in the bulbar reticular formation (RetF), between the central tegmental tract (CTT) and the lateral reticular nucleus (not shown). D: dorsal; M: medial. Scale bar: 100 μm B. Cell bodies (arrows) and fibers (arrowheads) containing NK in the bulbar reticular formation (RetF). Note immunoreactive fibers (arrowheads) in the dorsal accessory olivary nucleus (DAO). D: dorsal; M: medial. Scale bar: 100 μm
NK-ir fibers
A high density of immunoreactive fibers containing NK was observed in the following regions: in the caudaldorsal part of the cuneate nucleus (Fig. 1A), the medullary central gray (Figs. 1A,1B; 4A), the dorsal motor nucleus of the vagus (Figs. 1A,1B; 5A,5B), the nucleus of the solitary tract (Figs. 1B,1C; 5A), the raphe obscurus, the spinal trigeminal nucleus (gelatinosa part) (Figs. 1A,1B,1C; 7B), the inferior salivatory nucleus, the anterolateral system (Fig. 1A,1B,1C), the nucleus of the accessory nerve (Fig. 1A), the lateral part of the reticular formation (Figs. 1B,1C; 7A), the nucleus ambiguus (Fig. 1B,1C), the lateral reticular nucleus (Fig. 1B,1C), and the dorsal accessory olivary nucleus (Figs. 1C; 6B). A moderate density was found in the gracile nucleus (Fig. 1A,1B), the caudal-ventral part of the cuneate nucleus (Fig. 1A), as well as in the whole nucleus (Fig. 1B), the caudal end of the motor hypoglossal nucleus (Fig. 1A), the rubrospinal tract (Fig. 1A,1B,1C), the medial C1 cells (Fig. 1A), the spinal trigeminal tract (Fig. 1B,1C), the medial part of the reticular formation (nucleus reticularis gigantocellularis included) (Fig. 1B,1C), the motor hypoglossal nucleus (Fig. 1B,1C), the medial accessory olivary nucleus (Fig. 1B), the medial vestibular nucleus (Fig. 1C) and in the spinal (inferior) vestibular nucleus (Fig. 1C).
Figure 7.
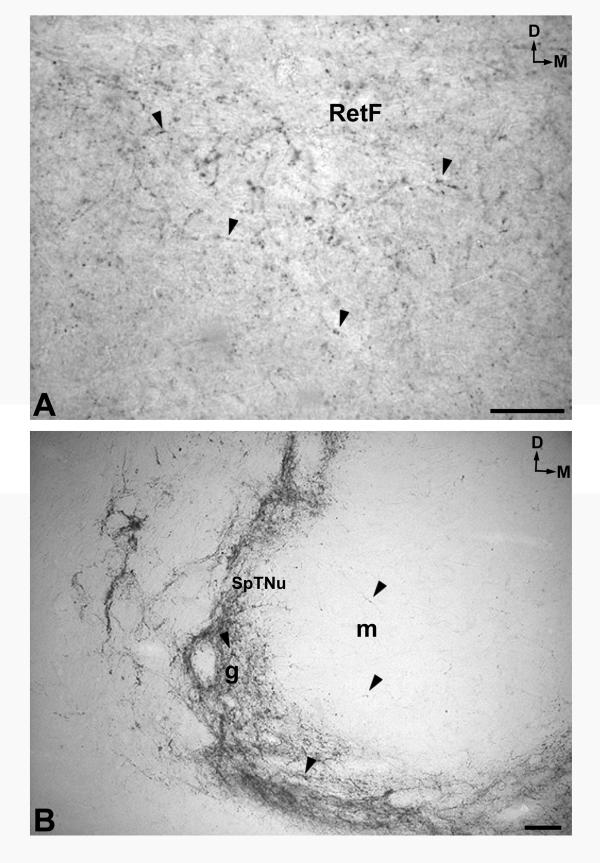
A. Immunoreactive fibers (arrowheads)containing NK in the pontine reticular formation (RetF). D: dorsal; M: medial. Scale bar: 100 μm B. A high density of immunoreactive fibers (arrowheads)in the gelatinosa (g) part of the spinal trigeminal nucleus (SpTNu) and a low density in the magnocellular part (m) of the same nucleus. D: dorsal; M: medial. Scale bar: 100 μm
A low density of immunoreactive fibers containing NK was found in the fasciculus gracilis (Fig. 1A), the fasciculus cuneatus (Fig. 1A,1B), the spinal trigeminal tract (Fig. 1A), the reticulospinal tract (Fig. 1A,1B,1C), the dorsal spinocerebellar tract (Fig. 1A), the vestibulospinal tract (Fig. 1A,1B), the spinal trigeminal nucleus (magnocellular part) (Figs. 1A; 7B), the pyramidal tract (Fig. 1A,1B,1C), the lateral cuneate nucleus (Fig. 1B,1C), the restiform body (Fig. 1B,1C), the medial lemniscus (Fig. 1B,1C), the principal part of the inferior olivary nucleus (Fig. 1B,1C), and in the midline (Fig. 1C), whereas single immunoreactive fibers were observed in the ventral spinocerebellar tract (Fig. 1A,1B,1C), the medial longitudinal fascicle (Fig. 1A,1B,1C), the glossopharyngeal nerve, the tectospinal tract (Fig. 1A,1B,1C), the central tegmental tract(Fig. 1B,1C), the dorsal part of the medial longitudinal fascicle(Fig. 1B,1C), the medial accessory olivary nucleus (Fig. 1C), the solitary tract (Figs. 1C; 5A), and in the glossopharyngeal fibers that join the spinal trigeminal tract.
Distribution of NK-ir structures in the human pons
NK-ir cell bodies
We observed a moderate density of immunoreactive cell bodies in the caudal (Fig. 2A) and in the rostral (close to the central tegmental tract)(Fig. 2C) reticular formation, between the superior cerebellar peduncle and the anterolateral system, as well as in the tegmental central gray (Fig. 2C). In the nucleus prepositus hypoglossi (Fig. 2A), a low density of immunoreactive cell bodies was found.
NK-ir fibers
In the lateral part (Fig. 2A,2B) and in the medial-most area (Fig. 2C) of the reticular formation, the nucleus of the solitary tract (Fig. 2A), the superior salivatory nucleus (Fig. 2A), and in the tegmental central gray (Fig. 2B,2C) a high density of NK-ir fibers was visualized. A moderate density was observed in the following regions: the rubrospinal tract (Fig. 2A,2B), the anterolateral system (Fig. 2A,2B,2C), the medial part of the reticular formation (Fig. 2A,2B), the medial parabrachial nucleus (close to the superior cerebellar peduncle)(Fig. 2B), the spinal trigeminal nucleus (Fig. 2A), the superior vestibular nucleus, the nucleus prepositus hypoglossi (Fig. 2A), and the nuclei of the lateral lemniscus (Fig. 2B,2C), whereas a low density of immunoreactive fibers containing NK was found in the spinal trigeminal tract(Fig. 2A), the restiform body (Fig. 2A), the medial lemniscus (Fig. 2A,2B,2C), the central tegmental tract (Fig. 2A,2B,2C), the lateral and medial vestibular nuclei (Fig. 2A), the motor facial nucleus (Fig. 2A), the superior olive (Fig. 2A,2B), the corticospinal fibers (Fig. 2A,2B,2C), the pontine nuclei (Fig. 2A,2B,2C), the abducens nucleus (Fig. 2A), the principal trigeminal nucleus (Fig. 2B), the caudal end of the chief sensory nucleus (Fig. 2B), the superior cerebellar peduncle (Fig. 2A,2B,2C) and in the middle cerebellar peduncle (Fig. 2C), Finally, single immunoreactive fibers were visualized in the ventral spinocerebellar tract (Fig. 2A,2B), the medial longitudinal fascicle (Fig. 2A,2B,2C), the tectospinal tract (Fig. 2A,2B,2C), the dorsal part of the medial longitudinal fascicle (Fig. 2A,2B), the solitary tract(Fig. 2A), the internal genu of the facial nerve (Fig. 2A), the facial nerve (Fig. 2A), the trapezoid body (Fig. 2A), the trapezoid nucleus(Fig. 2A), the pontocerebellar fibers (Fig. 2A,2B,2C), the ventral trigeminothalamic tract(Fig. 2A,2B,2C), the juxtarestiform body (Fig. 2A), and the trigeminal nerve (Fig. 2C).
Distribution of NK-ir structures in the human mesencephalon
NK-ir cell bodies
In the mesencephalon two populations of cell bodies containing NK were observed. The first (high density) was located above the periaqueductal gray and the central nucleus of the inferior colliculus and extended into the latter nucleus (Figs. 3A; 8A,8B; 9A,9B). The second was observed in the periaqueductal gray (high density) (Figs. 3B; 10A) and in the reticular formation (low density) (Fig. 3B). In the caudal and rostral regions of the periaqueductal gray a low density of NK-ir perikarya was found in the ventromedial part of the nucleus (Fig. 3A,3C).
Figure 8.
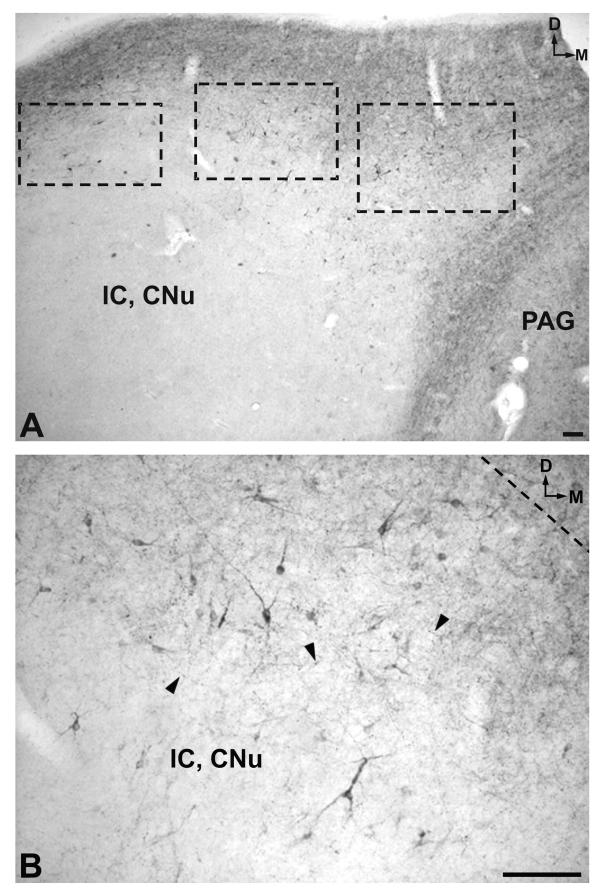
A. Low-magnification image of the periaqueductal gray (PAG) and the central nucleus of the inferior colliculus (IC, CNu) areas. D: dorsal; M: medial. Scale bar: 100 μm B. High magnification of the region delimited in the central rectangle in Figure 8A. Note the immunoreactive cell bodies and fibers (arrowheads). D: dorsal; M: medial. Scale bar: 100 μm
Figure 9.
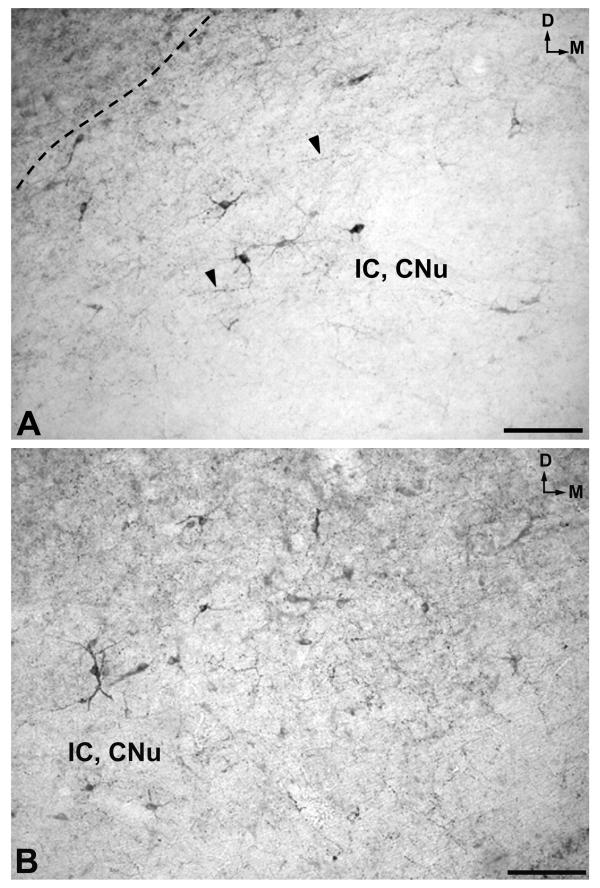
A. High-power image of the region delimited in the left rectangle of Figure 8A. Arrowheads indicate immunoreactive fibers. D: dorsal; IC, CNu: central nucleus of the inferior colliculus; M: medial. Scale bar: 100 μm B. High magnification of the region delimited in the right rectangle of Figure 8A. D: dorsal; IC, CNu: central nucleus of the inferior colliculus; M: medial. Scale bar: 100 μm
Figure 10.
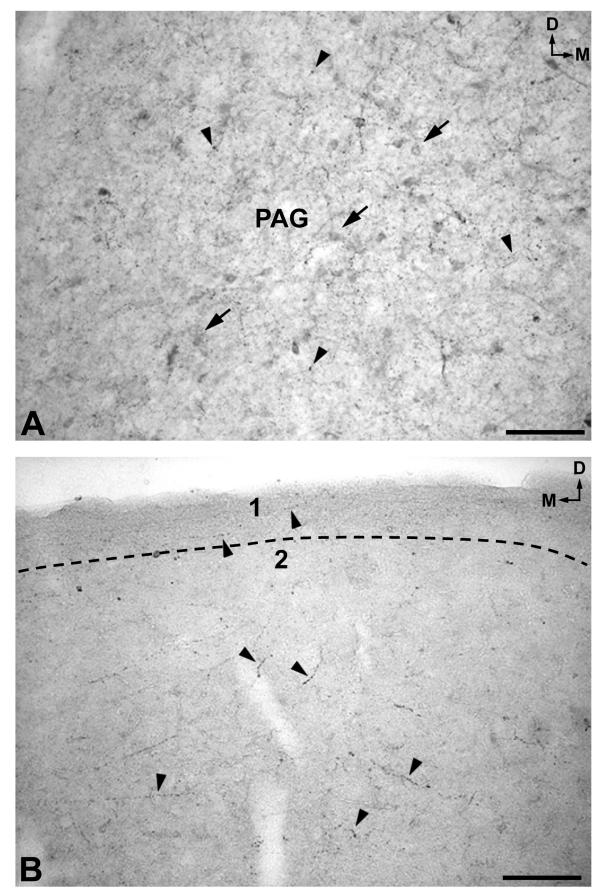
A. NK-ir perikarya (arrows) and fibers (arrowheads) in the periaqueductal gray (PAG). D: dorsal; M: medial. Scale bar: 100 μm B. A low density of immunoreactive fibers is observed in layer 1 of the superior colliculus (SC), whereas a high density is located in layer 2. D: dorsal; M: medial. Scale bar: 100 μm
NK-ir fibers
A high density of NK-ir fibers was found above the periaqueductal gray and the central nucleus of the inferior colliculus (Fig. 3A), the reticular formation (Fig. 3A,3B,3C), the periaqueductal gray (Figs. 3A,3B,3C; 10A), the lateral zone of the inferior colliculus (Fig. 3A), the lateral lemniscus(Fig. 3A), the dorsal trigeminothalamic tract (Fig. 3A,3B,3C), the superior colliculus (layers 2–6)(Figs. 3B,3C; 10B), the substantia nigra(Fig. 3B,3C), and in the pallidonigral, nigrostriatal and corticonigral fibers (Fig. 3B,3C); a moderate density in the spinotectal tract (Fig. 3A,3B,3C), the spinothalamic tract (Fig. 3A,3B,3C), the ventral trigeminothalamic tract(Fig. 3A,3B,3C), the superior cerebellar peduncle (Fig. 3A), the dorsal part of the pontine nuclei (Fig. 3B), the interpeduncular nucleus(Fig. 3B,3C), and the rubrospinal tract (Fig. 3C).
In the central nucleus of the inferior colliculus (Figs. 3A; 8B; 9A,9B), the medial lemniscus (Fig. 3A,3B,3C), the central tegmental tract (Fig. 3A,3B,3C), the corticospinal fibers (Fig. 3A), the pontine nuclei (Fig. 3A), the crus cerebri(Fig. 3A,3B,3C), the red nucleus (Fig. 3C), the superior colliculus (layer l)(Figs. 3B; 10B), the superior cerebellar peduncle (Fig. 3C) a low density of NK-ir fibers was found, whereas single fibers were observed in the medial longitudinal fascicle (Fig. 3A,3B,3C), the tectospinal tract (Fig. 3A,3B), the pontocerebellar fibers (Fig. 3A), the trochlear nerve (Fig. 3B), the oculomotor nucleus(Fig. 3C), the dorsal and ventral tegmental decussations (Fig. 3C), and in the oculomotor nerve(Fig. 3C).
Discussion
This is the first detailed study showing the distribution of NK-ir fibers and cell bodies in the human brainstem without neurological disease. We have to indicate that the intensity and distribution of the immunoreactivity described in our study were found in elderly persons (over age 80). Thus, both intensity and distribution may differ from those observed in other adults. In the medulla oblongata, the pons, and in the mesencephalon, NK-ir structures were observed in almost all the nuclei and tracts of the three brainstem regions studied.
Our results indicate that NK still can be demonstrate 48 h after death. Moreover, in general, we did not find differences in the human brainstems, which were perfused " ex situ " from 24 h to 48 h after death, nor in the density of the immunoreactivity nor in the distribution of the immunoreactive structures. Moreover, no sex differences were found in the distribution of NK-ir structures in the human brainstem.
Distribution of NK in the mammalian brainstem
In a previous study, we described the distribution of NK-ir structures in the cat brainstem [15]. Although the presence of NK-ir fibers is widely distributed in both cat and human brainstems, it seems that the distribution is a little more widespread in humans than in cats. Thus, for example, in the former, we found NK-ir fibers in the superior colliculus, the restiform body, the pyramidal tract, and in the pontine nuclei, in which in the cat no immunoreactive fibers were found [15]. Comparing the distribution of cell bodies containing NK in both cat and human brainstems, it seems that the distribution is widespread in the feline (see [15]). In both species NK-ir perikarya were found in the periaqueductal gray and in the inferior colliculus. However, in the cat cell bodies containing NK were observed in the superior colliculus, the interpeduncular nucleus, the lateral reticular nucleus, and in the nucleus of the solitary tract, as well as in other brainstem localizations, in which we did not observe NK-ir cell bodies in the same brainstem regions of humans. By contrast, in humans, we found NK-ir perikarya located in the dorsal motor nucleus of the vagus, in which in the cat no immunoreactive cell bodies were found [15]. The differences observed in both species concerning the distribution of cell bodies containing NK could be due to technical considerations, since in the cat colchicine was administered in the fourth ventricle in order to accumulate the peptide in cell bodies [15].
Our data are in agreement with previous results described in the rat brainstem, using radioimmunoassay techniques [29,30]. Thus, these latter authors found higher levels of NKA and lower levels of NKB in both the midbrain and the medulla oblongata of the rat [30]. In both regions of the human brainstem, we observed a high density of NK-ir structures. Moreover, in the midbrain of the rat those authors described NKA in the substantia nigra, the interpeduncular nucleus and the periaqueductal gray [29], just as we found NK-ir structures in these nuclei of the human brainstem when using immunocytochemical techniques.
Several studies have shown the distribution of NKA (preprotachykinin A mRNA) [12] and NKB (peptide-2; preprotachykinin B mRNA)[14,16,17,20] in the CNS of the rat. The work of Harlan et al. [12] revealed a more widespread distribution of neurons encoding SP and NKA preprotachykinin mRNA in the rat brainstem than the distribution of NK-ir cell bodies observed in the human brainstem. In addition, taking together the results of the other four works [14,16,17,20], it also seems that neurons expressing NKB are more widely distributed in the rat brainstem than those observed containing NK in the human brainstem. However, in the rat, those authors did not find either NKA nor NKB in the dorsal motor nucleus of the vagus and in the nucleus prepositus hypoglossi, in which, in humans, we observed NK-ir structures. Future works must address the issue of whether the discrepancies observed in the distribution of neurokinin in both species are due to technical and/or species differences.
Distribution of tachykinins in the human brainstem
In comparison with previous works in which the distribution of SP was described in the human brainstem [24-27], it seems that in general, taking together the results obtained from the four above-mentioned works, the distribution of NK-ir structures in this region of the human CNS is quite similar. Thus, for example, SP and NK-ir structures have been observed in the same human brainstem areas such as the periaqueductal gray, substantia nigra, parabrachial nucleus, inferior and superior colliculi, reticular formation, nucleus of the solitary tract, red nucleus, interpeduncular nucleus, medial vestibular nucleus, cuneate nucleus, spinal trigeminal nucleus, and the dorsal motor nucleus of the vagus. However, for example, we found NK-ir structures (the antibody used in this study principally recognizes NKA)(see specificity of the antiserum in Methods) in the human brainstem in the lateral cuneate nucleus and in the pyramidal tract, in which no SP-ir structure has been described in humans [24-27]. It is known that both neuropeptides, SP and NKA, are derived from the same precursor. Thus, the differences found in the distribution of both neuroactive substances in the human brainstem could be due to the intraneuronal segregation transport of the peptides and/or the different processing of the precursor [see [31]]. Another possibility could be that the immunoreactivity observed in those nuclei of the human brainstem in which NK-ir structures, but not SP, are found is due to NKB (the antibody used here also recognizes NKB). As is known, this latter tachykinin derives from the pre-protachykinin B gene, whereas SP and NKA derive from another precursor, the pre-protachykinin A gene. Finally, SP-immunoreactive cell bodies have been found in the human brainstem; for example, in the superior colliculus and in the spinal trigeminal nucleus [22,32], in which we did not find NK-ir perikarya.
Possible physiological actions of the NK in the human brainstem
The widespread distribution of NK-ir structures in the human brainstem suggests that the peptide might be involved in several physiological actions, acting as a neurotransmitter and/or neuromodulator. Thus, for example, the presence of NK in the nucleus of the solitary tract, the dorsal motor nucleus of the vagus, the periaqueductal gray, the spinal trigeminal nucleus, the interpeduncular nucleus, the substantia nigra, the parabrachial nucleus, and the superior and inferior colliculi indicates that NK could be involved in a broad range of physiological functions such as in cardiovascular, anti-nociceptive, nociceptive, motor, respiratory, visual and auditory mechanisms. Moreover, a possible interaction between NK and other neuropeptides (e.g., neurotensin and somatostatin) in the human brainstem could be suggested, since in almost all the nuclei, located in the dorsal brainstem of humans, immunoreactive fibers containing NK, neurotensin and somatostatin have been observed [33,34]. This observation is in agreement with the results found in the cat brainstem, in which a clear anatomical relationship between the three above-mentioned neuropeptides has been indicated [15]. Finally, we hope that our study will serve to compare the distribution of NK-ir structures found in normal human brainstems with those observed, in the future, in brainstems showing a given pathology (e.g., Parkinson or Alzheimer disease); since, for example, the loss of SP-containing neurons has been described in the brainstem of people with Parkinson's disease [8].
Conclusions
1. There is a widespread distribution of immunoreactive structures containing neurokinin in the human brainstem.
2. The highest density of fibers and cell bodies containing neurokinin was generally observed in the dorsal part of the human brainstem.
3. The widespread distribution of neurokinin-immunoreactive fibers and cell bodies indicates that neurokinin might be involved in several physiological functions, acting as a neurotransmitter and/or neuromodulator.
Methods
Brainstem material was obtained from four adult human brains with no previous history of neurological or psychiatric disease (two men, 81 and 82 years; two women, 84 and 87 years), who died from renal failure, anaemia, or myocardial infarct. The experimental design, protocols, and procedures of this work have been performed under the guidelines of the Ethics and Legal recommendations of the Spanish and European laws. In addition, this work has been approved by the research commission of the University of Málaga (Spain). Autopsies were carried out within 24–48 h after death. The brains were removed and perfused " ex situ " via the carotid and vertebral arteries. Both arteries were connected to a pressure-driven pump and the brains were perfused at normal mean arterial pressure with 1000 ml of phosphate-buffered saline (PBS) 0.15 M (pH 7.2) followed by 3000 ml of 4% paraformaldehyde in the same buffer. After postfixation (30 days in the latter fixative solution)(4°C), the brains were keept in PBS at 4°C and cryoprotected, at the same temperature, by immersion in increasing sucrose baths (10–30%) until they sank. The brainstems were dissected out and, using a cryostat, 60 μm-thick frontal sections of the mesencephalon, pons and medulla oblongata were cut, collected in PBS, kept at 4°C and processed for immunostaining. In general, six of seven sections were used for immunocytochemistry, and the remaining section was stained for Nissl.
Immunocytochemistry
In order to avoid possible interference by endogenous peroxidase, free-floating sections were treated with distilled water containing NH3 (20%), H2O2 (30%) and NaOH (1%) for 20 min [35]. Then, the sections were washed for 20 min in PBS and preincubated for 30 min in PBS containing 1% normal horse serum and 0.3% Triton X-100. The sections were then washed for 30 min in the last solution containing caseine (0.5%) and were incubated overnight at 4°C in PBS containing Triton X-100 (0.3%) and normal horse serum (1%), as well as anti-neurokinin antiserum, and diluted 1/15000 – 1/30000. The sections were then washed in PBS (30 min) and incubated for 60 min at room temperature with biotinylated antirabbit immunogamma globulin diluted 1/200 in PBS. After a 30 min wash with PBS, the sections were incubated for 1 h with avidin-biotin-peroxidase complex (Vectastain) (diluted 1/100). Finally, after washing the sections in PBS (30 min) and Tris-HCl buffer (pH 7.6)(10 min), the tissue-bound peroxidase was developed by H2O2 using 3, 3' diaminobenzidine as chromogen. The sections were rinsed with PBS and coverslipped with PBS/Glycerol (1/1).
Specificity of the antiserum
Polyclonal anti-neurokinin antibody was raised in rabbits against immunogens prepared by coupling the synthetic NKA peptide (Bachem, Switzerland) to a carrier protein (human serum albumin) with glutaraldehyde. The specificity of the immunostaining was controlled by omission of the neurokinin antibody in the first incubation bath. No immunoreactivity was found. Moreover, control sections were incubated in the primary antiserum previously absorbed by neurokinin A or neurokinin B. Inhibition of staining was obtained after preincubation of the first antiserum with neurokinin A (10-4 and 10-5 M) or with neurokinin B (10-3 M). Moreover, no significant reduction in immunoreactivity was observed on incubating the first antiserum with an excess of SP, eledoisin or kassinin. Thus, the antibody used, in the present work, principally recognizes NKA but shows some cross-reactivity with NKB. For this reason, the term " neurokinin-like immunoreactive " (NK-ir) is used to described the staining results in our material. However, we have to indicate that the immunoreactivity found in this study is mainly due to NKA (see and compare Figs. 6B; 10A; 11A,11B), since the distribution of NKB is much less widespread in the human brainstem, in comparison with the distribution found for NKA(personal observation obtained after using a very specific rabbit anti-NKB antibody from Peninsula). Thus, the anti-NKB antibody (IHC 7357) showed the following crossreactivities: 100% for NKB, 2% for physalaemin, 1% for eledoisin, 0.6% for kassinin, 0.2% for substance P, 0.1% for NKA and 0% for neuropeptide K. Moreover, in the nuclei of the human brainstem in which NKB was observed, the density of the immunoreactivity for NKB was lesser than that found in the same nuclei for NKA (see and compare Figs. 6B; 10A; 11A,11B).
Figure 11.
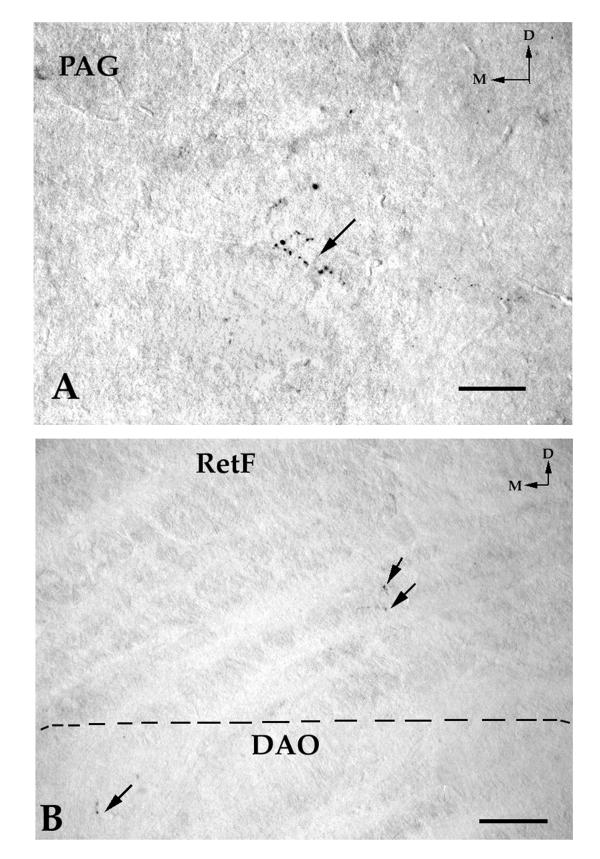
A. Immunoreactive fibers (arrows) containing neurokinin B in the periaqueductal gray (PAG). Compare this Figure with Figure 10A. Note a very low density of immunoreactive fibers containing neurokinin B in the PAG. No immunoreactive cell body containing neurokinin B was found in the PAG. D: dorsal; M: medial. Scale bar: 100 μm B. A few immunoreactive fibers (arrows) containing neurokinin B in the bulbar reticular formation (RetF) and in the dorsal accessory olivary nucleus (DAO). Compare this Figure with Figure 6B. Note a very low density of immunoreactive fibers containing neurokinin B in the RetF and DAO. No immunoreactive cell body containing neurokinin B was observed in the RetF. D: dorsal; M: medial. Scale bar: 100 μm
Mapping
Mapping was carried out according to the atlas of the human brain of Haines [36], and the same atlas was used for the terminology of the brainstem nuclei. In addition, the atlas of Paxinos et al. [37] was consulted.
The number of immunostained cell bodies appearing in each section from each person was counted; a high density of NK-ir perikarya was considered when we found more than 20 cell bodies/section; a moderate density when we found 10–20 cell bodies/section, and a low density when we found fewer than 10 cell bodies/section. The density of the immunopositive fibers was graded under microscopic observation into four categories: high, moderate, low and single. This was accomplished by viewing the sections under bright-field illumination at a constant magnification with reference to photographs of defined series of densities [38].
Finally, photomicrographs were obtained with a CoolSNAP digital camera attached to a Zeiss Axiophot microscope. To improve the visualization of the results, only the brightness and contrast of the images were adjusted without any further manipulation of the photographs.
Abbreviations used in figures
- 1: Caudal end of the motor hypoglossal nucleus
- 2: Caudal end of the dorsal motor nucleus of the vagus
- 4: Area postrema
- AbdNr: Abducens nerve
- AbdNu: Abducens nucleus
- AccNu: The nucleus of the accessory nerve
- ACSp: Anterior corticospinal tract
- ALS: Anterolateral system
- AMV: Anterior medullary velum
- ArNu: Arcuate nucleus
- CA: Cerebral aqueduct
- CC: Crus cerebri
- CeGy: Medullary or tegmental central gray
- CoBul: Corticobulbar fibers
- CortNig: Corticonigral fibers
- CSNu: Principal trigeminal nucleus
- CSp: Corticospinal fibers
- CTT: Central tegmental tract
- D: Dorsal
- DAO: Dorsal accessory olivary nucleus
- DtegDec: Dorsal tegmental decussation
- DLF: Dorsal part of the medial longitudinal fascicle
- DMNu: Dorsal motor nucleus of the vagus
- DSCT: Dorsal spinocerebellar tract
- DTTr: Dorsal trigeminothalamic tract
- EAF: External arcuate fibers
- Fac, G: Facial nerve, internal genu
- FacNr: Facial nerve
- FacNu: Motor facial nucleus
- Fcu: Fasciculus cuneatus
- FGr: Fasciculus gracilis
- Fpon: Frontopontine fibers
- g: Gelatinosa
- HyNu: Motor hypoglossal nucleus
- IAF: Internal arcuate fibers
- IC, Br: Inferior colliculus, brachium
- IC, CNu: Inferior colliculus, central nucleus
- IC, Com: Inferior colliculus, commissure
- IC, LZ: Inferior colliculus, lateral zone
- ICP: Inferior cerebellar peduncle
- IPFo: Interpeduncular fossa
- IPNu: Interpeduncular nucleus
- JRB: Juxtarestiform body
- LCNu: Lateral cuneate nucleus
- LL: Lateral lemniscus
- LL, Nu: Nuclei of the lateral lemniscus (dorsal, ventral)
- LoCer: Locus ceruleus
- LRNu: Lateral reticular nucleus
- M: Medial
- m: Magnocellular
- MAO: Medial accessory olivary nucleus
- MCP: Middle cerebellar peduncle
- MesNu: Mesencephalic trigeminal nucleus
- MGB: Medial geniculate body
- MesTr: Mesencephalic tract of the trigeminal nerve
- ML: Medial lemniscus
- MLF: Medial longitudinal fascicle
- MVN: Medial vestibular nucleus
- NigSt: Nigrostriatal fibers
- NuAm: Nucleus ambiguus
- NuCu: Cuneate nucleus
- NuGr: Gracile nucleus
- NuPp: Nucleus prepositus hypoglossi
- OcbF: Olivocerebellar fibers
- OcNr: Oculomotor nerve
- OcNu: Oculomotor nucleus
- Opon: Occipitopontine fibers
- PAG: Periaqueductal gray
- PalNig: Pallidonigral fibers
- PCbF: Pontocerebellar fibers
- PO: Principal part of the inferior olivary nucleus
- PonNu: Pontine nuclei
- Ppon: Parietopontine fibers
- Py: Pyramidal tract
- RB: Restiform body
- RetF: Reticular formation
- RetSp: Reticulospinal tract
- RNu: Red nucleus
- RuSp: Rubrospinal tract
- SC: Superior colliculus
- SCP: Superior cerebellar peduncle
- SCP, Dec: Superior cerebellar peduncle, decussation
- SN: Substantia nigra
- SO: Superior olive
- SolNu: Nucleus of the solitary tract
- SolTr: Solitary tract
- SpTec: Spinotectal tract
- SpTh: Spinothalamic tract
- SpTNu: Spinal trigeminal nucleus
- SpTT: Spinal trigeminal tract
- SpVN: Spinal (inferior) vestibular nucleus
- SSNu: Superior salivatory nucleus
- SVN: Superior vestibular nucleus
- TecSP: Tectospinal tract
- Tpon: Temporopontine fibers
- TrapB: Trapezoid body
- TrapNu: Trapezoid nucleus
- TriMoNu: Trigeminal motor nucleus (caudal part)
- TriNr: Trigeminal nerve
- TroNr: Trochlear nerve
- V: Ventricle
- VesSp: Vestibulospinal tract
- VSCT: Ventral spinocerebellar tract
- VTegDec: Ventral tegmental decussations
- VTTr: Ventral trigeminothalamic tract
Authors' contribution
RC carried out the cartography, conceived the study and participated in its design and coordination. FM and MB carried out the immunocytochemical technique and the cartography. VS, PS and ER initials carried out the autopsies and the perfusion of the brains, as well as they obtained the brain sections. ZDC, JAN and PM initials carried out the immunocytochemical technique, the cartography and drafted the manuscript. GT obtained the first antibody used in this study. SGB initials drafted the manuscript.
Acknowledgments
Acknowledgements
The authors wish to thank N. Skinner for a revision of the English text. This work has been supported by the D.G.I.C.Y.T. (PB99/0160) and the Ministerio de Ciencia y Tecnología (BFI2001-1905), Spain.
Contributor Information
Rafael Coveñas, Email: covenas@gugu.usal.es.
Francisco Martin, Email: fran@ioba.med.uva.es.
Magdalena Belda, Email: belda@gugu.usal.es.
Victor Smith, Email: smith@uma.es.
Pablo Salinas, Email: salinas@uma.es.
Eva Rivada, Email: rivada@uma.es.
Zaida Diaz-Cabiale, Email: zaida@uma.es.
Jose Angel Narvaez, Email: bueno@uma.es.
Pilar Marcos, Email: mpmarcos@correo.med-ab.uclm.es.
Gerard Tramu, Email: g.tramu@neurocyto.u-bordeaux.fr.
Salvador Gonzalez-Baron, Email: sgonzalez@uma.es.
References
- Maggio JE. Tachykinins. Annu Rev Neurosci. 1988;11:13–28. doi: 10.1146/annurev.ne.11.030188.000305. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Krause JE, Mantyh PW, Couture R, Bannon MJ. Diversity in mammalian tachykinin peptidergic neurons; multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990;4:1606–1615. [PubMed] [Google Scholar]
- Burns LH, Kelley AE. Neurokinin-alpha injected into the ventral tegmental area elicits a dopamine-dependent behavioral activation in the rat. Pharmacol Biochem Behav. 1988;31:255–263. doi: 10.1016/0091-3057(88)90343-7. [DOI] [PubMed] [Google Scholar]
- Erspamer V. The tachykinin peptide family. Trends Neurosci. 1981;4:267–269. [Google Scholar]
- Nagashima A, Takano K, Tateishi Y, Matsuoka T, Hamaoka T, Kamiya H-O. Central pressor actions of neurokinin B: increases in neurokinin B contents in discrete nuclei in spontaneously hypertensive rats. Brain Res. 1989;499:198–203. doi: 10.1016/0006-8993(89)91154-2. [DOI] [PubMed] [Google Scholar]
- Nagashima A, Takano K, Tateishi Y, Matsuoka T, Hamaoka T, Kamiya H-O. Cardiovascular roles of tachykinin peptides in the nucleus tractus solitarii of rats. Brain Res. 1989;487:392–396. doi: 10.1016/0006-8993(89)90848-2. [DOI] [PubMed] [Google Scholar]
- Paris JM, Mitsushio H, Lorens SA. Intra-raphe neurokinin-induced hyperactivity: effects of 5,7-dihydroxytryptamine lesions. Brain Res. 1989;476:183–188. doi: 10.1016/0006-8993(89)91556-4. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PRC, Blessing WW, Geffen LB. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson's disease. Ann Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Mauborgue A, Javoy-Agid F, Legrand JC, Agid Y, Cesselin F. Decrease of substance P immunoreactivity in the substantia nigra and pallidum of Parkinsonian brains. Brain Res. 1983;268:167–170. doi: 10.1016/0006-8993(83)90403-1. [DOI] [PubMed] [Google Scholar]
- Pearson J, Brandeis L, Cuello AC. Depletion of substance P-containing axons in substantia gelatinosa of patients with diminished pain sensitivity. Nature. 1982;295:61–63. doi: 10.1038/295061a0. [DOI] [PubMed] [Google Scholar]
- Arai H, Emson PC. Regional distribution of neuropeptide K and other tachykinins (neurokinin A, neurokinin B and substance P) in rat central nervous system. Brain Res. 1986;399:240–249. doi: 10.1016/0006-8993(86)91514-3. [DOI] [PubMed] [Google Scholar]
- Harlan RE, García MM, Krause JE. Cellular localization of substance P- and neurokinin A-encoding preprotachykinin mRNA in the female rat brain. J Comp Neurol. 1989;287:179–212. doi: 10.1002/cne.902870204. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A, Hökfelt T, Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat-I. Cell bodies and nerve terminals. Neuroscience. 1978;3:861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hibridization. Neuroscience. 1992;51:317–345. doi: 10.1016/0306-4522(92)90318-v. [DOI] [PubMed] [Google Scholar]
- Marcos P, Coveñas R, de León M, Narváez JA, Tramu G, Aguirre JA, González-Barón S. Neurokinin A-like immunoreactivity in the cat brainstem. Neuropeptides. 1993;25:105–114. doi: 10.1016/0143-4179(93)90089-s. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunocytochemistry and in situ hybridization. J Comp Neurol. 1992;317:341–356. doi: 10.1002/cne.903170403. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Maderdrut JL, 0'Harte F, Conlon JM. Localization of neurokinin B in the central nervous system of the rat. Peptides. 1992;13:815–829. doi: 10.1016/0196-9781(92)90192-6. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ, Eskay RL. Distribution of immunoreactive substance P neurons in the hypothalamus and pituitary of the rhesus monkey. J Comp Neurol. 1984;224:51–59. doi: 10.1002/cne.902240105. [DOI] [PubMed] [Google Scholar]
- Velasco A, de León M, Coveñas R, Marcos P, Narváez JA, Tramu G, Aguirre JA, González-Barón S. Distribution of neurokinin A in the cat diencephalon: an immunocytochemical study. Brain Res Bull. 1993;31:279–285. doi: 10.1016/0361-9230(93)90218-z. [DOI] [PubMed] [Google Scholar]
- Warden MK, Young WS., III Distribution of cells containing mRNAs encoding substance P and neurokinin B in the rat central nervous system. J Comp Neurol. 1988;272:90–113. doi: 10.1002/cne.902720107. [DOI] [PubMed] [Google Scholar]
- Bouras C, Magistretti PJ, Morrison JH. An immunohistochemical study of six biologically active peptides in the human brain. Hum Neurobiol. 1986;5:213–226. [PubMed] [Google Scholar]
- Chigr F, Najimi M, Leduque P, Chayvialle JA, Bouvier R, Kopp N. Anatomical distribution of substance P-immunoreactive neurons in human brainstem during the first postnatal year. Brain Res Bull. 1991;26:515–523. doi: 10.1016/0361-9230(91)90089-3. [DOI] [PubMed] [Google Scholar]
- del Fiacco M, Dessi ML, Levanti MC. Topographical localization of substance P in the human post-mortem brainstem. An immunohistochemical study in the newborn and adult tissue. Neuroscience. 1984;12:591–611. doi: 10.1016/0306-4522(84)90075-7. [DOI] [PubMed] [Google Scholar]
- Mai JK, Stephens PH, Hopf A, Cuello AC. Substance P in the human brain. Neuroscience. 1986;17:709–739. doi: 10.1016/0306-4522(86)90041-2. [DOI] [PubMed] [Google Scholar]
- Nomura H, Shiosaka S, Tohyama M. Distribution of substance P-like immunoreactive structures in the brainstem of the adult human brain: an immunocytochemical study. Brain Res. 1987;24:365–370. doi: 10.1016/0006-8993(87)91396-5. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Fodor M. Distribution of neuropeptides in the human lower brainstem (pons and medulla oblongata). Adv Behav Biol. 1995;43:101–113. [Google Scholar]
- Rikard-Bell GC, Törk I, Sullivan C, Scheibner T. Distribution of substance P-like immunoreactive fibres and terminals in the medulla oblongata of the human infant. Neuroscience. 1990;34:133–148. doi: 10.1016/0306-4522(90)90308-q. [DOI] [PubMed] [Google Scholar]
- Mileusnic D, Magnuson DJ, Hejna MJ, Lorens JB, Lorens SA, Lee JM. Age and species-dependent differences in the neurokinin B system in rat and human brain. Neurobiol Aging. 1999;20:19–35. doi: 10.1016/s0197-4580(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Shults CW, Yajima H, Gullner H-G, Chase TN, O'Donohue TL. Demonstration and distribution of kassinin-like material (substance K) in the rat central nervous system. J Neurochem. 1985;45:552–558. doi: 10.1111/j.1471-4159.1985.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Tateishi K, Matsuoka Y, Hamaoka T. Establishment of highly specific radioimmunoassays for neurokinin A and neurokinin B and determination of tissue distribution of these peptides in rat central nervous system. Regul Peptides. 1989;24:245–257. doi: 10.1016/0167-0115(89)90221-8. [DOI] [PubMed] [Google Scholar]
- de León M, Coveñas R, Narváez JA, Tramu G, Aguirre JA, González-Barón S. Somatostatin-28 (1-12)-like immunoreactivity in the cat diencephalon. Neuropeptides. 1991;19:107–117. doi: 10.1016/0143-4179(91)90140-e. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Joh TH, Cotton RG, Howe PR, Geffen LB, Blessing WW. Distribution of substance P-like immunoreactive neurons in the human medulla oblongata: co-localization with monoamine-synthesizing neurons. Synapse. 1988;2:353–370. doi: 10.1002/syn.890020403. [DOI] [PubMed] [Google Scholar]
- Bouras C, Magistretti PJ, Morrison JH, Constantinidis J. An immunohistochemical study of pro-somatostatin derived peptides in the human brain. Neuroscience. 1987;22:781–800. doi: 10.1016/0306-4522(87)92959-9. [DOI] [PubMed] [Google Scholar]
- Mai JK, Triepel J, Metz J. Neurotensin in the human brain. Neuroscience. 1987;22:499–524. doi: 10.1016/0306-4522(87)90349-6. [DOI] [PubMed] [Google Scholar]
- Guntern R, Vallet PG, Bouras C, Constantinidis J. An improved immunohistostaining procedure for peptides in human brain. Experientia. 1989;45:159–161. doi: 10.1007/BF01954858. [DOI] [PubMed] [Google Scholar]
- Haines DE. Neuroanatomy: An Atlas of Structures, Sections and Systems. Baltimore, Urban & Schwarzenberg. 1987.
- Paxinos G, Törk I, Halliday G, Mehler WR. Human homologs to brainstem nuclei identified in other animals as revealed by acetylcholinesterase activity. In: Paxinos G, editor. The Human Nervous System. San Diego, Academic Press; 1990. pp. 149–202. [Google Scholar]
- Marcos P, Coveñas R, Narváez JA, Aguirre JA, Tramu G, González-Barón S. Neuropeptides in the cat amygdala. Brain Res Bull. 1998;45:261–268. doi: 10.1016/s0361-9230(97)00343-2. [DOI] [PubMed] [Google Scholar]


