Abstract
Transitional endoplasmic reticulum (tER) consists of confluent rough and smooth endoplasmic reticulum (ER) domains. In a cell-free incubation system, low-density microsomes (1.17 g cc−1) isolated from rat liver homogenates reconstitute tER by Mg2+GTP- and Mg2+ATP-hydrolysis–dependent membrane fusion. The ATPases associated with different cellular activities protein p97 has been identified as the relevant ATPase. The ATP depletion by hexokinase or treatment with either N-ethylmaleimide or anti-p97 prevented assembly of the smooth ER domain of tER. High-salt washing of low-density microsomes inhibited assembly of the smooth ER domain of tER, whereas the readdition of purified p97 with associated p47 promoted reconstitution. The t-SNARE syntaxin 5 was observed within the smooth ER domain of tER, and antisyntaxin 5 abrogated formation of this same membrane compartment. Thus, p97 and syntaxin 5 regulate assembly of the smooth ER domain of tER and hence one of the earliest membrane differentiated components of the secretory pathway.
INTRODUCTION
Elucidation of the molecular mechanisms controlling membrane biogenesis and transport in the early secretory pathway has been a consequence, in part, of advances in yeast genetic screens and various cell-free assays (Novick et al., 1980; Rothman, 1994). Genetic screens initially led to the demonstration of a role for the N-ethylmaleimide sensitive factor (NSF [Sec18p]) in secretion (Eakle et al., 1988; Wilson et al., 1989) and subsequently in multiple vesicle-transport steps (Graham and Emr, 1991; Rothman, 1994). NSF/s18p is one of the best characterized members of the ATPases Associated with different cellular Activities (AAA) ATPase family (Patel and Latterich, 1998) and along with its cofactor αSNAP/s17p and SNARE (SNAp REceptor) membrane proteins regulates the docking/fusion step of multiple cellular fusion events, including those within the secretion pathway and those within neuronal synapses (Rothman, 1994; Nichols and Pelham, 1998). SNARE membrane proteins along with NSF promote the physical linkage of juxtaposed membranes to atomic distances necessary for the subsequent coalescence of their respective phospholipid bilayers (Hanson et al., 1997; Woodman, 1997; Nichols and Pelham, 1998; Sutton et al., 1998; Skehel and Wiley, 1998).
The sequence similarity of NSF/s18p to a previously described cell division cycle gene, Cdc48p, prompted analysis of the role of the latter in the fusion of membranes in yeast. This led to the discovery that Cdc48p, but not NSF/s18p, regulated the fusion of endoplasmic reticulum (ER) membranes in yeast (Latterich et al., 1995). Vertebrate homologues with approximate 70% sequence identity to Cdc48p have been described in frog (p97 in Xenopus laevis, Peters et al., 1990), in porcine (Valosin-containing protein, Koller and Brownstein, 1987), in rat liver (Zhang et al., 1994; Rabouille et al., 1995), and recently in mice (Müller et al., 1999). As for NSF, p97 is now known to be implicated in a variety of membrane fusion events comprising the early secretion pathway of cells (Zhang et al., 1994; Rabouille et al., 1995; Acharya et al., 1995). The two AAA ATPases have been suggested to have two distinct roles, that of p97 being to control the fusion of homotypic membranes and that of NSF being to control fusion of heterotypic membranes (Rabouille et al., 1995; Acharya et al., 1995; Denesvre and Malhotra, 1996; Patel et al., 1998; Warren and Malhotra, 1998). Heterotypic fusion is thought to require NSF and the soluble NSF attachment proteins (α-SNAP [Sec17p]) (Rothman, 1994; Denesvre and Malhotra, 1996; Nichols and Pelham, 1998; Warren and Malhotra, 1998), whereas homotypic fusion of ER or Golgi membranes requires p97 (Cdc48p) and p47 (Latterich et al., 1995; Rabouille et al., 1995; Acharya et al., 1995; Kondo et al., 1997; Patel et al., 1998). p47 is a cofactor for p97-mediated membrane fusion (Kondo et al., 1997), and just as α-SNAP mediates the binding of NSF to SNARE proteins, p47 promotes p97 binding to cognate SNAREs (Rabouille et al., 1998).
Recently a cell-free assay has been shown to reconstitute the morphological transformations characteristic of the early secretion pathway (Lavoie et al., 1996; Lavoie et al., 1999). Using as starting material low-density microsomes (LDM) from rat liver, the formation of a smooth membrane tubular network emanating from rough ER cisternae has been reconstituted and shown to depend on distinct GTP-hydrolyzing and ATP-hydrolyzing steps. The GTP-hydrolyzing step was shown to reconstitute the rough ER cisternae, and the ATP-hydrolyzing step was shown to reconstitute the smooth tubular membrane domain of the reconstituted ER networks (Lavoie et al., 1996). The reconstituted ER networks were shown to correspond to transitional ER (tER) based on the following criteria: 1) morphological, they are composed of two continuous membrane domains, parallel rough cisternae, and interconnecting smooth tubules; 2) the smooth tubular membranes are enriched in the secretory cargo transferin and albumin as compared with the rough ER cisternae; and 3) the smooth tubular membranes are enriched in the recycling membrane proteins ERGIC 53/p58 and the p24 family member α2p24, as compared with the rough ER cisternae (Lavoie et al., 1999). Furthermore, upon the subsequent addition of cytosol, the smooth tubular domain of the tER transformed into pleomorphic vesiculotubular clusters (VTCs) (Lavoie et al., 1999). α2p24 was necessary for early events in the formation of the tER, and COPI coatomer was necessary for the latter cytosolic-dependent transformation of the tER into VTCs (Lavoie et al., 1999). In the present study, we demonstrate that p97 is the ATPase necessary for the early formation of the smooth tubular membrane domain of tER in vitro and show a requirement for the t-SNARE syntaxin 5 in the associated membrane fusion events of this transformation.
MATERIALS AND METHODS
Preparation of Microsomes from Rat Liver
Total microsomes were obtained by differential centrifugation of rat liver homogenates (Paiement et al., 1980). Subfractions enriched in rough and smooth vesicles were obtained from a step-gradient of sucrose employed to separate both rough microsomes and Golgi derivatives from total microsomes (Lavoie et al., 1996). For analytical density gradient centrifugations, fractions were loaded onto a 0.5–2.5 M sucrose gradient and centrifuged at 80,000 × g for 18 h. Fractions were collected and analyzed for their content of galactosyl transferase, mannosidase II, calnexin, α2p24 as previously described (Dominguez et al., 1998). Immunoblot studies of p97 and syntaxin 5 content of the gradient fractions were also carried out using antibodies described below.
Isolation of p97 and p47
p97 preparation: Cytosol from dog pancreas was precipitated with 20% (wt/vol) ammonium sulfate. The precipitate was collected by centrifugation and resuspended in low-salt buffer containing protease inhibitors, 1 mM dithiothreitol, 1 mM MgCl2, and 100 μM ATP. After dialysis in the same buffer, the protein mixture was separated by size exclusion using a Superdex-column, and fractions containing p97 were collected and separated by two rounds of anion exchange chromatography on a MonoQ-column at pH 7.4 and pH 6.4, respectively. The overall yield of purified p97 in the final fraction was ∼35% with a 400-fold enrichment factor. The purified p97 fraction contained associated p47 (as confirmed by immunoblotting). The p47/p97 ratio was ∼1/12th of that found in the original cytosol. Recombinant his6p47: Recombinant p47 was expressed as a his6-tagged fusion protein from a pQE30-vector (a kind gift of Drs. G. Warren and H. Kondo, ICRF, GB). After expression, bacteria were collected and lysed, and his6p47 was purified by metal-chelating chromatography using a nickle-agarose support.
Cell-free Incubation Conditions to Study tER Assembly
Unless otherwise indicated, the medium consisted of 0.25 ml containing 150 μg microsomal protein, 100 mM Tris-HCl pH 7.4, 5 mM MgCl2, 1 mM GTP, 2 mM ATP, an energy regenerating system (7.3 IU ml−1 creatine kinase, 2 mM creatine phosphate), 0.1 mM dithiothreitol, 0.02 mM phenylmethylsulfonyl fluoride, 0.09 μg ml−1 leupeptine, and 50 mM sucrose. This mixture was incubated at 37°C for 240 min. When the effect of antibody was studied, 10 μl of antiserum was added at different times of incubation. In studies with purified p97 and p47, these proteins were added to the incubation mixture at relative concentrations of 5 μg and 2.5 μg, respectively.
Measurement of Membrane-associated p97
LDM were incubated for different periods of time in complete medium, as defined above. After incubation, membranes were sedimented within the incubation medium by high-speed centrifugation (100,000 × g for 30 min). The proteins in the membrane pellets were dissolved directly in Laemmli buffer (Laemmli, 1970). The proteins in the supernatant fractions were concentrated by trichloroacetic acid precipitation. The trichloroacetic acid precipitates were neutralized with NaOH and dissolved in Laemmli buffer as done for the pellet proteins. Proteins were separated by SDS gradient PAGE, blotted onto nitrocellulose sheets, and p97 was detected by the immunoblot procedure previously described (Dominguez et al., 1991). p97 immunostaining on blots was scanned. Densitometric tracings were carried out and quantified using NIH Image software (Scion, Frederick, MD).
Electron Microscopy and Morphometry of ER Membranes
After incubation, membranes were fixed using 2.5% glutaraldehyde and recovered onto Millipore membranes by the random filtration technique of Baudhuin et al. (1967) and processed for electron microscopy (Paiement et al., 1980). Estimates of the lengths of embedded and sectioned rough and smooth membranes in the membrane networks were obtained from electron micrographs by morphometry using the membrane intersection counting procedure (Stäubli et al., 1969). The electron micrographs were fastened onto a measuring tablet (Graphic Master, Numonics, Montgomeryville, PA) and the Sigma-Scan measurement system (Jandel Scientific, San Rafael, CA) was employed to digitize morphometric data.
Preembedding Immunogold Labeling
The immunolocalization protocol was modified from that used by Dominguez et al. (1991) and is described in Lavoie et al. (1999).
Postembedding Immunogold Labeling
After incubation of membranes under assembly conditions, membranes were fixed using 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C. Cryoprotection, freezing, sectioning, immunolabeling, and contrasting were carried out as previously described by Dahan et al. (1994). For quantification of p97 labeling on cryosections, gold particles associated with rough membranes and smooth membranes comprising the ER networks were counted. Gold particles were counted over parallel juxtaposed ER cisternae (representing rough ER cisternae) and over the adjacent continuous mass of interconnecting membranes (corresponding to interconnecting smooth tubules). Surface area measurements of each compartment comprising the reconstituted ER networks were measured as previously described for ER membranes (Paiement et al., 1988; Lavoie et al., 1999). Gold particle densities were calculated as number of particles per compartment of ER network, and concentrations observed in each category of membranes were then expressed as average number of gold particles per surface area for each ER network.
Antibodies
Rabbit polyclonal antibodies against p97 used in the in vitro assays have been previously described (Zhang et al., 1994). Rabbit polyclonal antibodies against heat-denatured p97 from dog pancreatic cytosol, described above, were used in immunoblot studies. Affinity purified rabbit polyclonal antibodies against recombinant C-terminally HisX6-tagged cytoplasmic domain of syntaxin 5 were previously described (Subramaniam et al., 1997). Rabbit polyclonal antibodies against ribophorin II were a gift from G. Kreibich (Department of Cell Biology, New York University, School of Medicine, New York, NY).
RESULTS
Selective Effect of ATP Hydrolysis on Fusion of the Smooth but Not the Rough Membranes During Reconstitution of tER
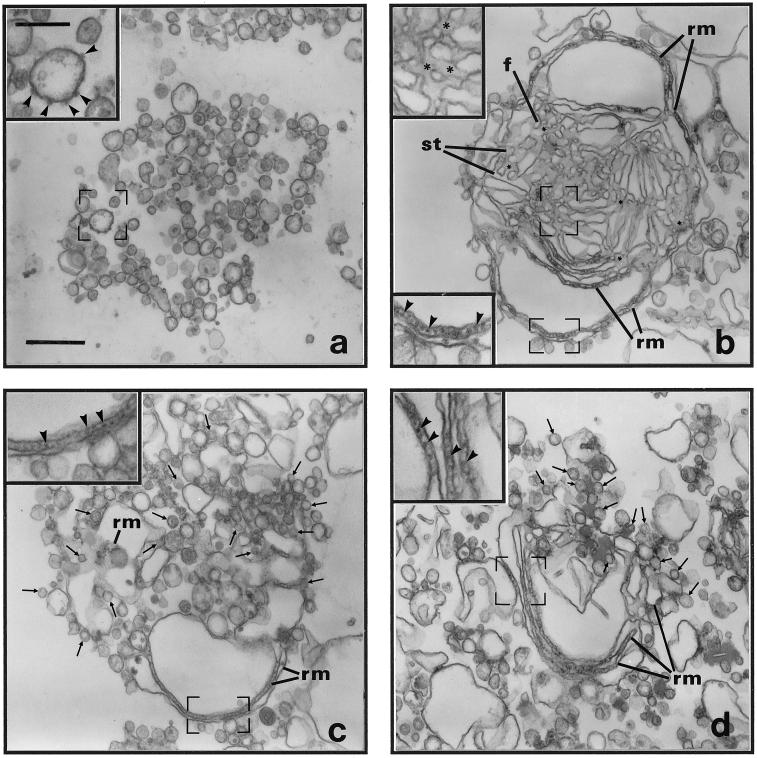
As reported previously (Lavoie et al., 1996; Lavoie et al., 1999), the incubation of LDM from rat liver (Figure 1a) with Mg2+GTP and Mg2+ATP leads to the fusion of rough and smooth vesicles and the selective and specific assembly of membrane networks consisting of parallel rough ER cisternae continuous with interconnecting smooth tubules (Figure 1b). This in vitro reconstituted membrane network was previously defined as tER based on (1) morphological criteria, i.e., a segregated smooth tubular compartment in direct continuity with rough ER cisternae, (2) the higher content of secretory cargo in the smooth tubular compartment, and (3) the higher content of the recycling membrane proteins ERGIC 53/p58 and the p24 family member α2p24 (Lavoie et al., 1999). For consistency, in this paper we use the same morphological criteria to define the in vitro reconstituted ER membrane networks as reconstituted tER; they correspond to structures which contain two membrane domains: one domain is characterized by the presence of parallel rough cisternae and the second one with which it is continuous is characterized by the presence of interconnecting smooth tubules. Omission of ATP from the incubation medium or incubation with Mg2+GTP and Mg2+ATP and the ATP hexokinase, d-Hexose-6-phosphotransferase, permitted fusion of rough vesicles, leading to formation of reconstituted membrane networks which contained only parallel rough membrane cisternae but inhibited fusion of smooth vesicles, and these were now observed in close apposition to the rough ER cisternae (arrows, Figure 1, c and d). Remarkably, N-ethylmaleimide (NEM) also inhibited smooth tubule formation (Figure 2). Thus, using rat liver membranes, formation of the smooth tubular membrane domain of tER but not that of the parallel rough membrane domain requires ATP hydrolysis and is NEM sensitive.
Figure 1.
Nucleotide-dependent assembly of transitional ER (tER). LDM were unincubated (a) or incubated in complete medium containing 5 mM MgCl2, 2 mM ATP, and 1 mM GTP for 240 min at 37°C (b). (c) Membranes after incubation in the absence of ATP. (d) Membranes after incubation in complete medium plus the ATP hexokinase, d-Hexose-6-phosphotransferase (30 U). Membrane networks containing branching and anastomosing smooth tubules in continuity with peripheral rough ER cisternae were observed only under conditions promoting the hydrolysis of ATP. Microsomes were treated for morphological analysis by electron microscopy as described in MATERIALS AND METHODS. (a–d) Insets represent higher magnification micrographs of membranes within the regions outlined by frames. Arrowheads indicate ribosomes on membranes, and arrows point to smooth vesicles apposed to rough ER cisternae; all images are of the same magnification; all insets are of equivalent magnification. (b) Asterisks indicate tritubular junctions, f indicates fenestrations between smooth tubules, and st points to examples of smooth ER tubules. (b–d) rm indicates parallel rough ER membranes. (a) scale bar represents 500 nm. (a inset) scale bar represents 200 nm.
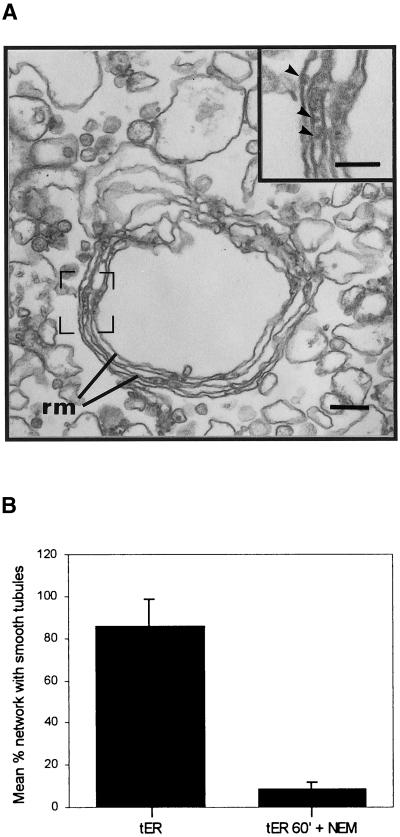
Figure 2.
Smooth tubule formation in tER is NEM sensitive. LDM were incubated for 60 min (as in Figure 1b) and in the additional presence of 2 mM NEM for 180 min. (a) Membrane networks essentially devoid of interconnecting smooth tubules were observed; scale bar represents 200 nm. Structures labeled rm correspond to parallel rough ER membranes. (a inset) Arrowheads point to ribosomes; scale bar represents 100 nm. (b) Quantitation of the effect of NEM. The amount of membrane networks with rough ER membranes and distinct smooth ER tubules were counted for control incubations minus NEM (tER) as well as for NEM-treated membranes (tER 60′ + NEM).
Inhibition of Formation of Smooth ER Domain of tER by Antibodies to p97
Latterich and Schekman (1994) using ER and nuclear membranes from yeast described ATP hydrolysis-dependent steps in both ER-ER and ER-nuclear envelope fusion. They identified the AAA protein Cdc48p but not NSF as responsible for this ATP-hydrolysis–dependent fusion (Latterich et al., 1995; Patel et al., 1998). The mammalian homologue of Cdc48p is p97 (Koller and Brownstein, 1987; Zhang et al., 1994; Rabouille et al., 1995; Acharya et al., 1995; Müller et al., 1999) and has been implicated in tER budding and ER to Golgi transport in rat hepatocytes (Zhang et al., 1994), as well as in the reformation of Golgi-flattened cisternae (Rabouille et al., 1995; Acharya et al., 1995). Hence, p97 was tested as the candidate ATPase responsible for the formation of smooth tubular networks in the assay described here.
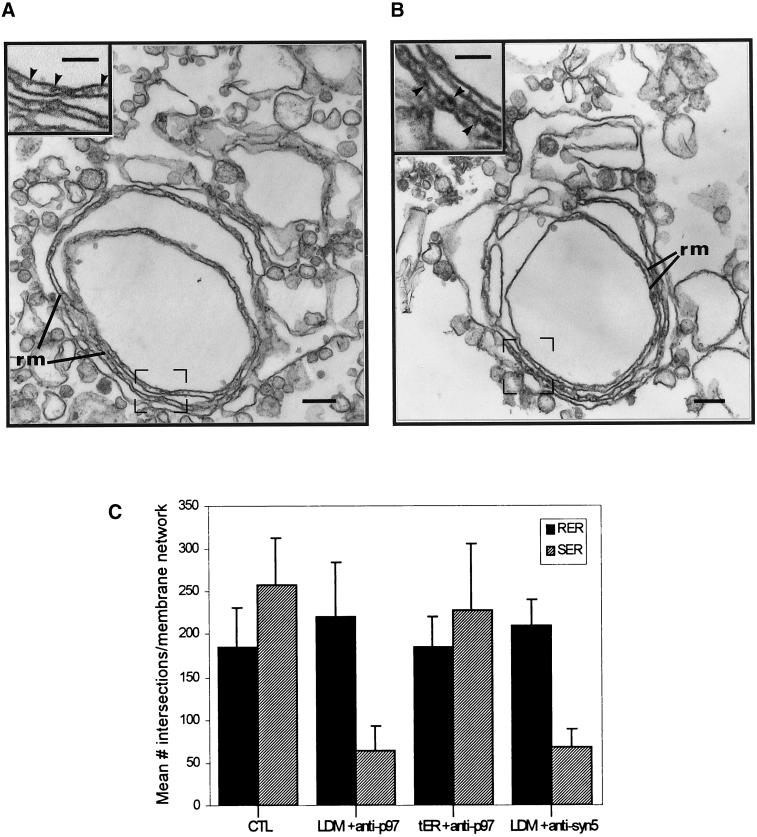
When LDM were incubated with Mg2+GTP and Mg2+ATP in the presence of antibodies to p97, rough vesicles fused to reconstitute membrane networks made up of only parallel rough ER cisternae (Figure 3, a and c). The absence of interconnecting tubules within these networks was attributed to the inhibition of fusion of smooth vesicles to form the smooth tubule domain of reconstituted tER. The relative proportion of rough and smooth membranes associated with the reconstituted networks is an index of the amount of membrane fusion and was calculated by morphometry using membrane length measurements after incubation in the absence or presence of antibody. Quantitation confirmed the effect of the antibodies on reconstitution of the two ER membrane domains. Whereas anti-p97 inhibited fusion of smooth vesicles and thus assembly of smooth ER tubules, these antibodies had no effect on the fusion of rough vesicles and thus on the assembly of rough ER membranes within reconstituted membrane networks (Figure 3, a and c). As control, addition of anti-p97 antibodies to preassembled tER networks had no effect on the relative amounts of rough and smooth ER membranes within tER networks. Hence, once the tER was assembled, the subsequent addition of antibodies to p97 had no effect on either of the two membrane domains of tER (Figure 3c).
Figure 3.
Antibodies to p97 and syntaxin 5 inhibit assembly of smooth tubules in tER. LDM were incubated as for Figure 1b. (a) Antibodies to p97 were present in the incubation medium. (b) Antibodies to syntaxin 5 were present in the incubation medium. (c) Quantitation of p97 and syntaxin 5 antibody effects by morphometry. The amount of rough ER membranes (RER) and smooth endoplasmic reticulum tubules (SER) in reconstituted membrane networks was calculated as indicated in MATERIALS AND METHODS. Microsomes were incubated under control (CTL) conditions as described for Figure 1b or identically in the presence of antibodies to p97 (LDM + anti-p97) or in the presence of antibody to syntaxin 5 (LDM + anti-syn 5). As an additional control, microsomes were preincubated 180 min to promote transitional ER formation and then incubated an additional 60 min in the presence of anti-p97 antibodies (tER + anti-p97). (a and b) Structures labeled rm correspond to parallel rough ER membranes. Insets show high-magnification electron micrographs of parallel rough ER membranes within regions outlined by frames. Scale bars represent 200 nm. (a and b insets) Scale bars represent 100 nm.
Requirement of the SNARE Protein Syntaxin 5 in Formation of the Smooth Tubular Domain of tER
The t-SNARE syntaxin 5 has been demonstrated to be required for the formation of pre-Golgi intermediates in permeabilized cells reconstituting ER-to-Golgi transport (Rowe et al., 1998), as well as in p97-mediated events in the reconstitution of Golgi-flattened cisternae in vitro (Rabouille et al., 1998). To test for a syntaxin 5 requirement, LDM were incubated in the presence of antibodies to the cytoplasmic domain of syntaxin 5. Under these conditions, reconstituted membrane networks consisted only of parallel rough cisternae, and these were devoid of associated smooth tubules (Figure 3, b and c). This is identical to that observed with antibodies to p97. Preincubation of the antibodies to syntaxin 5 with purified glutathione-S-transferase (GST)-syntaxin 5 abolished the effect of the antibodies on reconstitution. Hence, quantitation revealed that 20 of 22 ER networks reconstituted in the presence of antisyntaxin 5 antibodies were devoid of associated smooth tubules. After preincubation of the antibodies with GST-syntaxin 5, only 6 of 22 ER networks were observed without smooth ER tubules, a 3-fold difference. Furthermore, we have previously shown that antibodies to the cytoplasmic tail of the abundant ER membrane protein calnexin have no effect on smooth tubule assembly (Lavoie et al., 1999). Thus, p97 and syntaxin 5 are required for the fusion of smooth vesicles and consequently for the assembly of the smooth ER tubular domain within transitional ER.
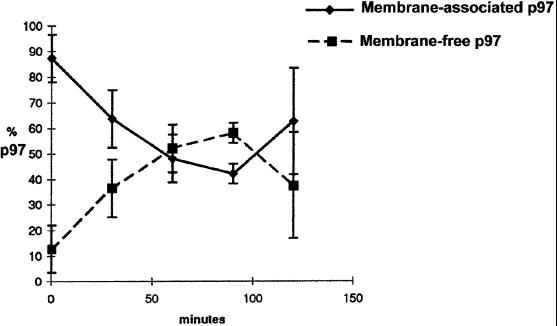
Dissociation of p97 from Membranes During Fusion
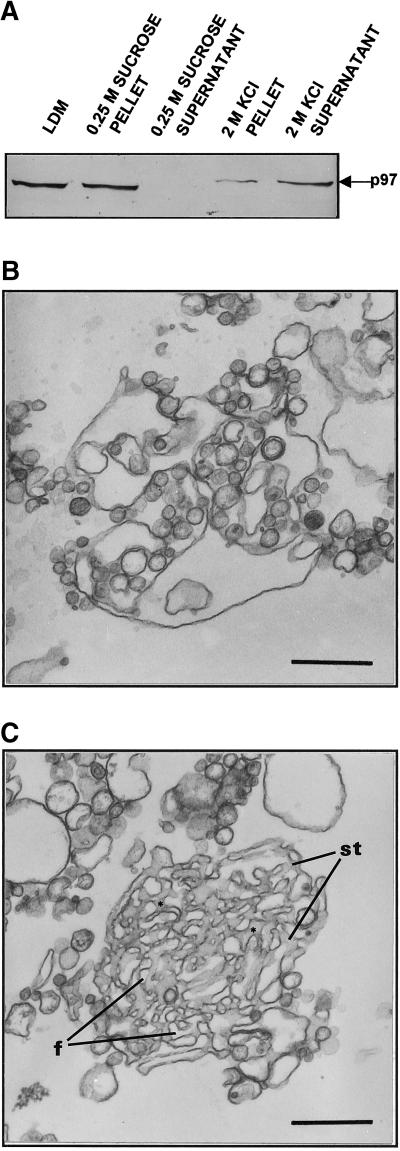
Release of p97 from ER membranes was studied during incubation conditions that led to tER formation. Sedimentation of incubated membranes during tER formation revealed a time-dependent membrane dissociation of p97 (Figure 4). However, the requirement of p97 for membrane fusion was established by further analysis. High-salt (KCl) washing of LDM released > 90% of membrane-associated p97 (Figure 5a) as well as all ribosomes (not shown). In four separate fractionation experiments, only 6.1 ± 3.4% (mean ± SD) of membrane-associated p97 was detectable on LDM after treatment with 2 M KCl. When KCl-treated LDM were incubated in the presence of Mg2+GTP and Mg2+ATP, the microsomes did fuse but the assembled ER membrane networks were devoid of interconnecting smooth tubules (Figure 5b). When purified p97/p47 was added back to the incubation mixture, KCl-treated microsomes were able to reconstitute membrane networks containing interconnecting smooth tubules (Figure 5c).
Figure 4.
Release of membrane-associated p97. LDM were incubated as in Figure 1b for different periods of time. After incubation, membranes were sedimented within the incubation medium by high-speed centrifugation (100,000 g for 30 min). The pellet and supernatant proteins were separated by SDS polyacrylamide gels, p97 was detected by immunoblot, and the amount of p97 was determined by densitometry.
Figure 5.
Addition of exogenous p97/p47 stimulates smooth tubule assembly within tER. (a) Release of endogenous p97 from LDM by treatment with 2 M KCl. After treatment either with 0.25 M sucrose or 2 M KCl in 0.25 M sucrose, microsomes were sedimented by high-speed centrifugation to form a microsomal pellet and supernatant. p97 content was then compared in the microsomal pellet and the supernatant by immunoblot analysis. (b) Dilated ER cisternae assembled using KCl-treated LDM and incubation as in Figure 1b. (c) tER comprised of interconnecting smooth tubules (st) and fenestrations (f) reconstituted after incubation of KCl-treated LDM in the same medium as in Figure 5b but containing exogenous p97/p47 (5 μg p97, 2.5 μg p47, 150 μg microsomal protein). (b and c) Microsomes were incubated as described for Figure 1b; scale bars represent 500 nm.
Results were confirmed by quantitation. The number of reconstituted membrane networks with recognizable interconnecting smooth tubules was determined after incubation under different conditions. Of the reconstituted ER membrane networks produced by untreated microsomes incubated in the presence of Mg2+GTP and Mg2+ATP, 85.4 ± 3.6% were comprised of interconnecting smooth tubules. Using the same incubation conditions, only 12.5 ± 10.8% of membrane networks produced using KCl-treated microsomes were comprised of recognizable interconnecting smooth tubules. In contrast, KCl-treated microsomes incubated in the presence of Mg2+GTP and Mg2+ATP plus purified p97 and p47 protein led to the assembly of ER membrane networks, of which 75.0 ± 6.3% contained interconnecting smooth tubules. Assembly of membrane networks containing smooth tubules in the presence of p97 was selectively abolished by preincubation of purified p97 protein with anti-p97 antibodies (our unpublished results). Thus, p97 promoted specific fusion of membranes of a subcompartment of the ER which is involved in the assembly of smooth ER tubules. p97 is a positive regulator of membrane fusion in this system, with dissociation of p97 occurring coincident with membrane fusion.
Localization of p97 and Syntaxin 5 in ER Subcompartments
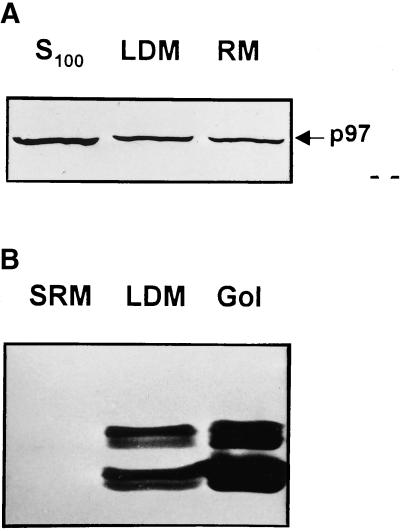
The distribution of p97 was compared with that of syntaxin 5 in subcellular fractions and analytical gradients. As expected, by subcellular fractionation, p97 was found in high concentration in rat liver cytosol and in significant but similar proportions in classical rough microsomes and LDM (Figure 6a). Quantitation by densitometry using purified p97 as reference protein revealed as much as 1.5% of total protein associated with LDM was p97 protein (our unpublished results). Surprisingly, when syntaxin 5 content was examined, it was barely detectable in classical rough microsomes, and the amount detected in LDM was high when compared with that detected in a purified Golgi membrane fraction (Figure 6b). Thus, p97 was similarly distributed between rough and smooth microsomes, and syntaxin 5 was more concentrated in smooth microsomes and Golgi-enriched membranes.
Figure 6.
Content of p97 and syntaxin 5 in ER membranes. (a) Immunoblot analysis of p97 in rat liver cytosol (S100), low-density microsomes (LDM), and rough microsomes (RM). (b) Immunoblot analysis of syntaxin 5 in rough microsomes stripped of associated ribosomes (SRM), LDM, and Golgi Apparatus fraction (Gol). Equivalent amounts of fraction protein, purified as described in MATERIALS AND METHODS, were loaded in each lane, and the proteins indicated were detected with appropriate antisera.
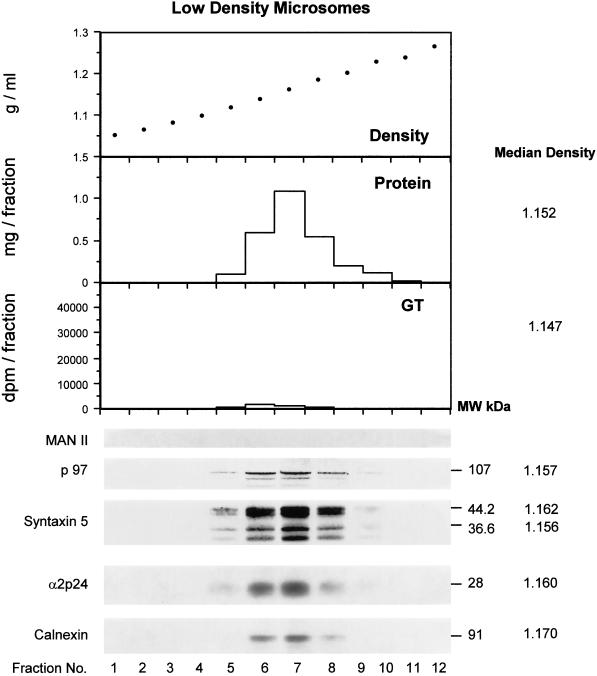
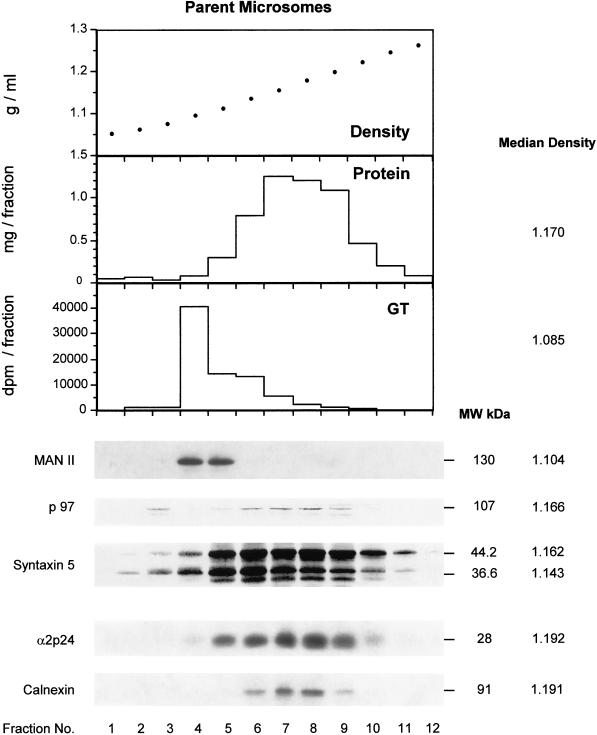
A more detailed comparison of the distribution of p97 and syntaxin 5 was obtained using analytical gradients of LDM as well as of parent microsomes (Figure 7). In LDM (Figure 7a), p97 revealed a distribution coincident with total protein with near identical median density. Syntaxin 5 revealed a major isoform of 44.2 kDa and a minor isoform at 36.6 kDa as reported previously by Hui et al., (1997). These proteins showed a similar distribution to p97. Calnexin and the terminally glycosylated p24 family member α2p24 (Dominguez et al., 1998; Lavoie et al., 1999) also revealed a similar distribution. Golgi contamination was negligible as evaluated by two markers. Little uridine diphosphogalactose:ovomucoid galactosyl transferase as assessed by enzyme assay was detected, and no mannosidase II was observed by immunoblot analysis (Figure 7a). By comparison, the parent microsomes used to make the LDM revealed a high content of the same Golgi markers (Figure 7b). Remarkably, a low content of membrane-associated p97 coincided with the distribution of Golgi markers in parent microsomes (Figure 7b). In contrast to LDM, the two major polypeptides recognized by the antisyntaxin 5 antibody revealed a different subcellular distribution with the lower molecular weight 36.6 kDa isoform at a median density of 1.143, compared with 1.162 for the higher molecular weight isoform of 44.2 kDa in the parent microsomal fraction (Figure 7b). This study conclusively rules out Golgi contamination as a possible explanation for the presence of p97 or syntaxin 5 in the LDM fraction. Indeed, even in parent microsomes, the distribution of p97 and syntaxin 5 was distinct to that of the Golgi markers. In LDM, total protein, p97, syntaxin 5, α2p24, and calnexin all showed similar distributions and median densities, whereas in parent microsomes, calnexin and α2p24 revealed higher median densities. Hence, LDM represent a biochemically distinct subset of microsomes with biochemical features expected of fragmented tER.
Figure 7.
Analytical fractionation by isopycnic density gradient centrifugation of LDM (a) and the parent microsomes (b) from which they were derived. Shown are the densities of each fraction, the protein distribution, the distribution of the Golgi markers β1, 4 galactosyl transferase (GT, measured by enzyme assay) and the marker mannosidase II (Man II, measured by immunoblot analysis). The distribution of p97 and syntaxin 5 isoforms are indicated as are the distributions of the primarily cis Golgi-located protein α2p24 and the primarily ER-located protein calnexin. The median density of the constituents are indicated on the right next to the molecular masses of the respective proteins.
Gold immunolabeling was also carried out. After in vitro reconstitution of tER, immunolabeling of p97 revealed no detectable immunoreactivity consistent with its dissociation from the membranes during the conditions of the in vitro incubations (see Figure 4). In contrast, labeling of the membrane protein syntaxin 5 was readily visualized (Figure 8, a and b). Using a preembedding immunolabeling protocol (Figure 8a), gold particles were found over interconnecting smooth tubules at a higher density to that over parallel membranes of the rough ER cisternae. By postembedding immunolabeling using cryosections (Figure 8b), gold particles were also abundant over smooth interconnecting tubules, although some syntaxin 5 was evident over parallel membranes of rough portions of the tER (Figure 8b). Although the preembedding immunolabeling method permitted better recognition of the rough and smooth ER subcompartments in tER (Figure 8a), quantitation with this method was not pursued due to potential problems of antibody penetration between closely apposed ER membranes. Quantitation of gold particle distribution in tER was preferred using cryosections because this method ensured unhampered access to syntaxin 5 epitopes in tER. A slightly higher density of gold particles was observed over smooth interconnected membrane tubules compared with that over parallel rough ER cisternae. A 1.38-fold concentration was observed for syntaxin 5 in the smooth ER compartment, as compared with the surrounding rough membranes (Table 1), and the distributions were judged significantly different between these two compartments (0.01 < p < 0.05, n = 41). As control, quantitation revealed labeling of ribophorin II, a rough ER marker, to be much higher in the rough ER cisternae (Table 1). The markers for the ER–Golgi intermediate compartment, α2p24 and p58, were previously observed to be enriched in the smooth ER tubular compartment, with values calculated at 2.1-fold and 1.6-fold, respectively, over that in rough ER cisternae (Lavoie et al., 1999). These values of enrichment of protein markers are similar to those recently reported for the KDEL receptor and for the SNARE rBET1 in transitional ER in the intact pancreatic acinar cell (Martinez-Menárquez et al., 1999).
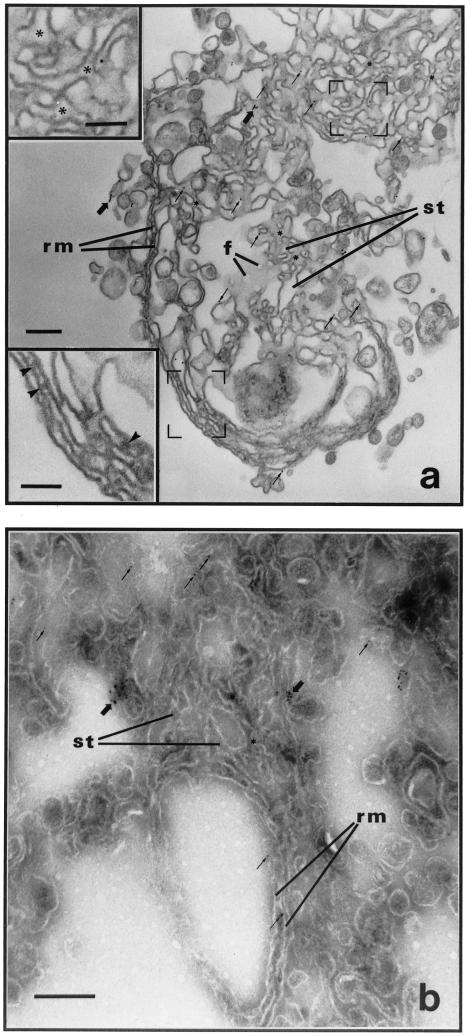
Figure 8.
Localization of syntaxin 5 within reconstituted tER. LDM were incubated in the presence of mixed nucleotides to form tER. Antibodies to syntaxin 5 were applied and gold immunolabeling studied using the preembedding (a) and postembedding (b) immunolabeling procedures described in the MATERIALS AND METHODS. (a and b) Gold particle labeling (arrows) is predominantly distributed over interconnecting smooth tubular portions of tER. Structures labeled rm correspond to parallel rough ER membranes, and those labeled st correspond to smooth ER tubules. Large arrows point to clusters of gold particle labeling, whereas small arrows point to single gold particle label. Arrowheads point to ribosomes on parallel rough membranes, and asterisks indicate tritubular junctions in smooth ER tubules. Scale bars represent 200 nm. (a insets) High-magnification micrographs of membranes within the regions framed; scale bars represent 100 nm.
Table 1.
Amount of syntaxin 5 and ribophorin in reconstituted tER
| Protein | Networks (n) | Gold particles (n) | Surface area of SERa (%) | Labeling over SER (%) | Concentration in SERb |
|---|---|---|---|---|---|
| Syntaxin 5 | 41 | 401 | 73.9 | 79.8 | 1.38X |
| Ribophorin II | 11 | 462 | 75.7 | 59.5 | 0.47X |
SER corresponds to the smooth ER tubules of the reconstituted tER.
To calculate concentrations, percent antigen labelling densities were determined for the rough and smooth ER compartments, and these values were compared to the relative amounts of surface areas occupied by each compartment as described previously (Lavoie et al., 1999).
DISCUSSION
As reported previously (Lavoie et al., 1996; Lavoie et al., 1999) and in this paper, the incubation of LDM from rat liver (Figure 1a) with Mg2+GTP and Mg2+ATP leads to the fusion of rough and smooth vesicles and the selective and specific assembly of membrane networks consisting of parallel rough ER cisternae continuous with interconnecting smooth tubules (Figure 1b). This in vitro reconstituted membrane network was previously defined as tER based on (1) morphological criteria, i.e., it is made up of continuous rough and smooth membrane domains; (2) the smooth tubular network is enriched in content of the secretory cargo, albumin, and transferin; (3) the smooth tubular network is enriched in the content of the recycling membrane proteins ERGIC 53/p58 and the p24 family member α2p24; and (4) the smooth tubular domain of the reconstituted membrane networks can transform into pleiomorphic VTCs after incubation of preassembled tER in the presence of cytosol with the cytosol requirement attributed, at least in part to COPI coatomer (Lavoie et al., 1999). Thus, reconstituted tER defined by our cell-free incubation system consists of continuous rough and smooth membrane domains; it contains molecular markers of the ER and the Golgi intermediate compartments; and one of the subdomains of the tER, the smooth tubular domain, has the capacity to transform into VTCs. In this paper, data is provided suggesting that p97 and syntaxin 5 are required for assembly of the smooth tubular domain of the tER.
The rough ER membrane domain which is continuous with the smooth tubular membrane domain of the tER defined by our cell-free incubation system is different from classical rough ER membrane which is recovered from tissue homogenates as high-density rough microsomes. Although both types of rough ER membranes can undergo GTP-dependent fusion, the fusion events are different. For example, antibodies to α2p24 inhibit fusion of the partially rough ER comprising transitional ER but not that of classical rough ER (Lavoie et al., 1999). Fusion of classical rough ER requires prior removal of associated ribosomes (Paiement et al., 1980; Paiement and Bergeron, 1983), and that of transitional rough ER does not (Lavoie et al., 1996). Classical rough ER does not fuse with transitional rough ER when the two types of rough ER are mixed with nucleotides (unpublished observations). Furthermore, classical rough ER contains barely detectable syntaxin 5, whereas LDM, which have the capacity to reconstitute tER, contain significant amounts of this SNARE protein (see Figure 6 in this paper). Hence, the fusion machinery associated with the rough membrane domain of transitional ER is suggested to be different from that associated with the rest of the ER, and this may be related to the capacity of this subcompartment to permit formation of a smooth tubular ER domain and, eventually, ER exit sites.
In situ rough and smooth portions of tER appear continuous. So why would membrane fusion be needed to generate the smooth tubular domain of tER? Perhaps vesicle fragments generated from rough and smooth ER membranes during homogenization express in vitro fusion properties that reflect the innate capacity of a subcompartment of the ER to fuse and form tubules. ER tubule formation may be necessary to allow ER differentiation in preparation for formation of ER cargo exit sites. Consistent with this is the observation that the cargo proteins transferin and albumin are enriched in the smooth tubule compartment of rat ER (Lavoie et al., 1999). The data in this paper implicate both p97 and syntaxin 5 in smooth tubule formation. Alternatively, the smooth tubular domain of tER could represent a retrograde fusion compartment for vesicles in transit from post-ER VTCs and/or cis Golgi compartments. The detection of terminally glycosylated α2p24 in LDM (Lavoie et al., 1999) is consistent with this possibility.
The ER–Golgi transitional elements are usually described as pre-Golgi intermediates composed of vesicular tubular clusters physically distinct from the ER (Saraste and Kuismanen, 1992; Hauri and Schweizer, 1992; Balch et al., 1994; Griffiths et al., 1995; Bannykh et al., 1996; Rowe et al., 1998). However, other studies have suggested that the intermediate compartments are smooth tubular networks in continuity with the rough ER. Indeed, the budding compartment of mouse hepatitis virus (Krijnse-Locker et al., 1994), the site of accumulation of the E1 glycoprotein of the rubbella virus (Hobman et al., 1992), the site of concentration and assembly of chondroitin sulfate proteogycan precursors (Vertel et al., 1989), and the site of accumulation of vesicular stomatitis virus glycoproteins in cells injected with anti-β-COP (Pepperkok et al., 1993) all showed smooth tubular networks in continuity with rough ER cisternae. These have been classified as intermediate compartments or as hypertrophied transitional ER lying along the main route of exocytic traffic. The reconstituted network of anastomosing smooth tubules we describe as being continuous with rough membrane cisternae (Lavoie et al., 1996, 1999, and this paper) exhibits similar morphological and biochemical characteristics to the intermediate compartments described above. Because the addition of cytosol to our in vitro system transforms only the tubular network into free-standing vesicular tubular clusters (Lavoie et al., 1999), the LDM fraction represents a pre-Golgi intermediate compartment.
An ER pool of syntaxin 5 has been described by Rowe et al. (1998) to be required for the formation of post-ER pre-Golgi intermediates in an ER-to-Golgi transport assay which monitored the glycosylation of vesicular stomatitis virus (VSV) protein G cargo (Rowe et al., 1998). However, antisyntaxin 5 in these studies did not inhibit the Sar1-dependent formation of vesicles budding off the ER. Our work defines an important role for syntaxin 5 in a step before the budding step and not analyzed previously (Rowe et al., 1998), namely in the prior formation of the tER. This membrane fusion event is proposed to be largely homotypic via smooth membranes present in the LDM starting preparation. The involvement of p97 as the activating ATPase necessary for this fusion event was supported by the following criteria: 1) an NEM-sensitive ATP hydrolysis event was required for the formation of the smooth tubular membrane domain of the tER in vitro; 2) the membrane fusion event leading to smooth tubule formation was inhibited by antibodies specific to p97; 3) membranes depleted of p97 by high-salt wash were incapable of forming the smooth tubular compartment by membrane fusion; and 4) the addition of purified p97/p47 to the salt-washed membranes reconstituted, by an ATP-hydrolysis–requiring event, the ability to reform smooth membrane tubules.
In one current model of SNARE-mediated membrane fusion events, the ATPase activity of p97 would activate syntaxin 5, (Nichols and Pelham, 1998; Ungermann et al., 1998; Bock and Scheller, 1999; Yu et al., 1999). However, as shown in Figure 4, p97 dissociates from LDM coincident with membrane fusion. This suggests an additional regulatory step involved in fusion and allows modulation of the number of rounds of fusion ultimately limiting the size of the tER compartment which is known to vary widely within different cell types (Vertel et al., 1989; Hobman et al., 1992; Pepperkok et al., 1993; Krijnse-Locker et al., 1994; Bannykh et al., 1996). Modulation of the number of rounds of fusion could explain why the amount of membrane associated with in vitro reconstituted tER using liver microsomes is always the same (Lavoie et al., 1999). The factors regulating membrane association of p97 are likely crucial for this modulation and are currently being studied.
Latterich and colleagues (Patel et al., 1998) clearly showed that the yeast homologue of p97, Cdc48p, fuse yeast microsomes using the ER t-SNARE, Ufe1p. Our results suggest that p97 promotes fusion of smooth microsomes from rat liver using the t-SNARE, syntaxin 5. The difference in the results obtained with the two different systems could be explained by the different capacities of p97 and Cdc48p to interact with different SNAREs. Although p97 and Cdc48p exhibit a high degree of identity, their capacity to interact with different SNAREs has yet to be defined. Alternatively different SNAREs, along with GTPases and tethering cofactors, may control fusion in different subcompartments of the ER. Consistent with this possibility is the fact that we observed barely detectable amounts of syntaxin 5 in classical rough ER microsomes but high amounts of this SNARE protein in microsomes containing rough ER which is capable of assembling tER (see Figure 6 in the present paper). The mammalian homologue for Ufe1p (yet to be identified) might also be involved in p97-dependent ER fusion, but this has not been shown and the ER subcompartment in which this occurs has yet to be defined. Likewise, in the yeast cell, it is not clear whether Ufe1p and the p97 homologue Cdc48p promote ER fusion in all ER subcompartments. Indeed, yeast cells contain two topologically distinguishable ER subcompartments: one, a reticular network of interconnecting cisternae distributed throughout the cytoplasm which is continuous with the nucleus; and two, a peripheral cisterna tightly apposed to the plasma membrane (Rose et al., 1989; Preuss et al., 1991; Rossanese et al., 1999). Whether Ufe1p and Cdc48p regulate fusion of ER derivatives from either or both of these yeast subcompartments is not clear. A third possibility is that syntaxin 5 is in a heteromeric complex with the mammalian Ufe1p homologue. Finally, Saccharomyces cerevisiae has been demonstrated to be devoid of a localized transitional ER (Rossanese et al., 1999); therefore, the Cdc48p(p97)–Ufe1p pathway in this yeast may not be conserved in mammalian cells.
ER reconstitution exhibits temporal and spatial similarities to Golgi reconstitution. Vesiculated ER membranes containing a mixture of both rough and smooth membrane derivatives reconstitute ER via two separate fusion events, an initial GTP-dependent step permitting reconstitution of the rough ER subcompartment (Lavoie et al., 1996; Lavoie et al., 1999), followed by a second p97-dependent step leading to the reconstitution of the smooth ER subcompartment (this paper). In the case of Golgi reconstitution, vesiculated Golgi membranes reconstitute Golgi stacks via two distinct membrane fusion events: an initial NSF-dependent fusion step, followed by a subsequent p97-dependent step (Acharya et al., 1995). Furthermore, the spatial organization of the smooth and rough ER subcompartments generated in vitro (e.g., a central core of interconnecting smooth tubules continuous with peripherally located rough membrane cisternae) is analogous to that of Golgi stacks (consisting of a central core continuous with peripheral rims) generated from mitotic Golgi fragments in vitro (Rabouille et al., 1995). In both cases, p97 promotes the homotypic fusion of a central core of membranes, interconnecting tubules for tER (this paper), and a central core of Golgi stacks (Rabouille et al., 1995).
The results presented here are unexpected, given the role previously ascribed to p97/p47 and syntaxin 5 in Golgi cisternal reassembly of mitotic fragments from mitotic cells (Warren and Malhotra, 1998). Two main views have been proposed to explain the morphological transformations accompanying the Golgi complex during mitosis. In one view, Golgi fragments retain their molecular composition during mitosis and reassemble by homotypic membrane fusion during telophase to form Golgi cisternae (Warren and Malhotra, 1998). In the second view, Golgi cisternae are thought to coalesce into the ER during mitosis by a process analogous to that occurring during treatment with brefeldin A. After mitosis, ER–Golgi differentiation is thought to occur by membrane partitioning in a manner resembling brefeldin A washout (Zaal et al., 1999). The second model is consistent with cisternal maturation-progression (Morré and Keenan, 1997; Nichols and Pelham, 1998; Lippincott-Schwartz et al., 1998) and predicts that molecules regulating the biogenesis of the early secretory pathway would be required for the differentiation of Golgi cisternae from hybrid membranes in early interphase. The results as documented here show that p97/p47 and syntaxin 5 are required for the formation of the earliest differentiated compartments of the early secretory pathway, i.e., that of the smooth tubular domain of the transitional ER.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council of Canada to J.P. and J.J.M.B. C.L. was a recipient of a studentship from the Medical Research Council of Canada.
REFERENCES
- Acharya U, Jacobs R, Peters JM, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;82:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P, Evrard P, Berthet J. Electron microscopic examination of subcellular fractions: I. The preparation of representative samples from suspensions of particles. J Cell Biol. 1967;32:181–191. doi: 10.1083/jcb.32.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Scheller RH. SNARE proteins mediate lipid bilayer fusion. Proc Natl Acad Sci USA. 1999;96:12227–12229. doi: 10.1073/pnas.96.22.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan S, Ahluwalia JP, Wong L, Posner BI, Bergeron JJM. Concentration of intracellular hepatic apolipoprotein E in Golgi apparatus saccular distensions and endosomes. J Cell Biol. 1994;127:1859–1869. doi: 10.1083/jcb.127.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denesvre C, Malhotra V. Membrane fusion in organelle biogenesis. Curr Opin Cell Biol. 1996;8:519–523. doi: 10.1016/s0955-0674(96)80030-5. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Lanoix J, Paiement J. Localizations of ras antigenicity in rat hepatocyte plasma membrane and rough endoplasmic reticulum fractions. Exp Cell Res. 1991;192:137–147. doi: 10.1016/0014-4827(91)90168-t. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud J-P, Thomas DY, Bergeron JJM, Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakle KA, Bernstein M, Emr SD. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988;8:4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Emr S. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Pepperkok R, Locker JK, Kreis TE. Immunocytochemical localization of beta-COP to the ER-Golgi boundary and the TGN. J Cell Sci. 1995;108:2839–2856. doi: 10.1242/jcs.108.8.2839. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hauri H-P, Schweizer A. The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol. 1992;4:600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobman TC, Woodward L, Farquhar MG. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-Golgi compartment. J Cell Biol. 1992;118:795–811. doi: 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui N, Nakamura N, Sonnichsen B, Shima DT, Nilsson T, Warren G. An isoform of the Golgi t-SNARE, syntaxin 5, with an endoplasmic reticulum retrieval signal. Mol Biol Cell. 1997;8:1777–1787. doi: 10.1091/mbc.8.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller KJ, Brownstein MJ. Use of a cDNA clone to identify a supposed precursor protein containing valosin. Nature. 1987;325:542–545. doi: 10.1038/325542a0. [DOI] [PubMed] [Google Scholar]
- Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J, Ericsson M, Rottier PJM, Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latterich M, Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994;78:87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Latterich M, Fröhlich K-U, Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;82:885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Lavoie C, Lanoix J, Kan FWK, Paiement J. Cell-free assembly of rough and smooth endoplasmic reticulum. J Cell Sci. 1996;109:1415–1425. doi: 10.1242/jcs.109.6.1415. [DOI] [PubMed] [Google Scholar]
- Lavoie C, Paiement J, Dominguez M, Roy L, Dahan S, Gushue JN, Bergeron JJM. Roles for α2P24 and COPI in ER cargo exit site formation. J Cell Biol. 1999;146:285–299. doi: 10.1083/jcb.146.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Cole NB, Donaldson JG. Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance. Histochem Cell Biol. 1998;109:449–462. doi: 10.1007/s004180050247. [DOI] [PubMed] [Google Scholar]
- Martinez-Menárquez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Morré DJ, Keenan TW. Membrane flow revisited. Bioscience. 1997;47:489–498. [Google Scholar]
- Müller JM, Meyer HH, Ruhrberg C, Stamp GW, Warren G, Shima DT. The mouse p97 (CDC48) gene: genomic structure, definition of transcriptional regulatory sequences, gene expression, and characterization of a pseudogene. J Biol Chem. 1999;274:10154–10162. doi: 10.1074/jbc.274.15.10154. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Pelham HRB. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta. 1998;1404:9–31. doi: 10.1016/s0167-4889(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Paiement J, Bergeron JJM. Localization of GTP-stimulated core glycosylation to fused microsomes. J Cell Biol. 1983;96:1791–1796. doi: 10.1083/jcb.96.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiement J, Beaufay H, Godelaine D. Coalescence of microsomal vesicles from rat liver: a phenomenon occurring in parallel with enhancement of the glycosylation activity during incubation of stripped rough microsomes with GTP. J Cell Biol. 1980;86:29–37. doi: 10.1083/jcb.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiement J, Kan FWK, Lanoix J, Blain M. Cytochemical analysis of the reconstitution of endoplasmic reticulum after microinjection of rat liver microsomes into Xenopus oocytes. J Histochem Cytochem. 1988;36:1263–1273. doi: 10.1177/36.10.2843593. [DOI] [PubMed] [Google Scholar]
- Patel S, Latterich M. The AAA team: related ATPases with diverse functions. Trends Cell Biol. 1998;8:65–71. [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Peters J-M, Walsh MJ, Franke WW. An abundant and ubiquitous homo-oligomeric ring-shaped ATPase particle related to the putative vesicle fusion proteins Sec18p and NSF. EMBO J. 1990;9:1757–1767. doi: 10.1002/j.1460-2075.1990.tb08300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D. Structure of the yeast endoplasmic reticulum: localization of ER proteins using immunofluorescence and immunoelectron microscopy. Yeast. 1991;7:891–911. doi: 10.1002/yea.320070902. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters J-M, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;82:905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Kondo H, Newman R, Hui N, Freemont P, Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92:603–610. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- Rose MD, Misra LM, Vogel JP. KAR2, a karyogamy gene, is the yeast homologue of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O'Connor J, Williamson EK, Glick BS. Golgi structure correlates with transitional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol. 1999;145:69–81. doi: 10.1083/jcb.145.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rowe T, Dascher C, Bannykh S, Plutner H, Balch WE. Role of vesicle-associated syntaxin 5 in the assembly of pre-Golgi intermediates. Science. 1998;279:696–700. doi: 10.1126/science.279.5351.696. [DOI] [PubMed] [Google Scholar]
- Saraste J, Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- Stäubli W, Hess R, Weibel ER. Correlated morphometric and biochemical studies on the liver cell: II. Effects of phenobarbital on rat hepatocytes. J Cell Biol. 1969;42:92–112. doi: 10.1083/jcb.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam VN, Loh E, Hong W. N-ethylmaleimide-sensitive factor (NSF) and a-soluble NSF attachment proteins (SNAP) mediate dissociation of GS28-syntaxin 5 Golgi SNAP receptors (SNARE) complex. J Biol Chem. 1997;272:25441–25444. doi: 10.1074/jbc.272.41.25441. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 X resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Vertel BM, Velasco A, LaFrance S, Walters L, Kaczman-Daniel K. Precursors of chondroitin sulfate proteoglycan are segregated within a subcompartment of the chondrocyte endoplasmic reticulum. J Cell Biol. 1989;109:1827–1836. doi: 10.1083/jcb.109.4.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Malhotra V. The organization of the Golgi apparatus. Curr Opin Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Wilcox CA, Flynn GC, Chen E, Kuang WJ, Henzel WJ, Block MR, Ullrich A, Rothman JE. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989;339:355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Woodman PG. The roles of NSF, SNAPs and SNAREs during membrane fusion. Biochim Biophys Acta. 1997;1357:155–172. doi: 10.1016/s0167-4889(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Yu RC, Jahn R, Brunger AT. NSF N-terminal domain crystal structure: models of NSF function. Mol Cell. 1999;1:97–107. doi: 10.1016/s1097-2765(00)80191-4. [DOI] [PubMed] [Google Scholar]
- Zaal KJ, Smith CL, Polishchuk RS, Altan N, Cole NB, Ellenberg J, Hirschberg K, Presley JF, Roberts TH, Siggia E, Phair RD, Lippincott-Schwartz J. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ashendel CL, Becker GW, Morré DJ. Isolation and characterization of the principal ATPase associated with transitional endoplasmic reticulum of rat liver. J Cell Biol. 1994;127:1871–1883. doi: 10.1083/jcb.127.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]