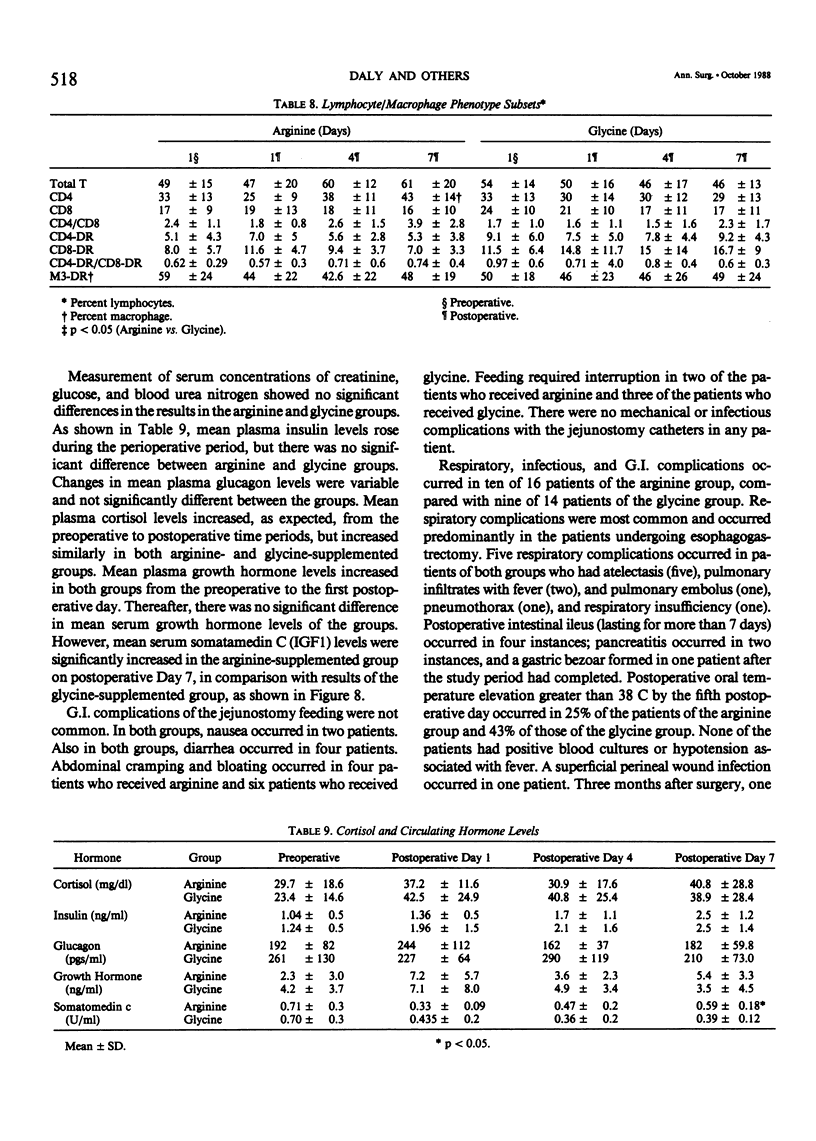
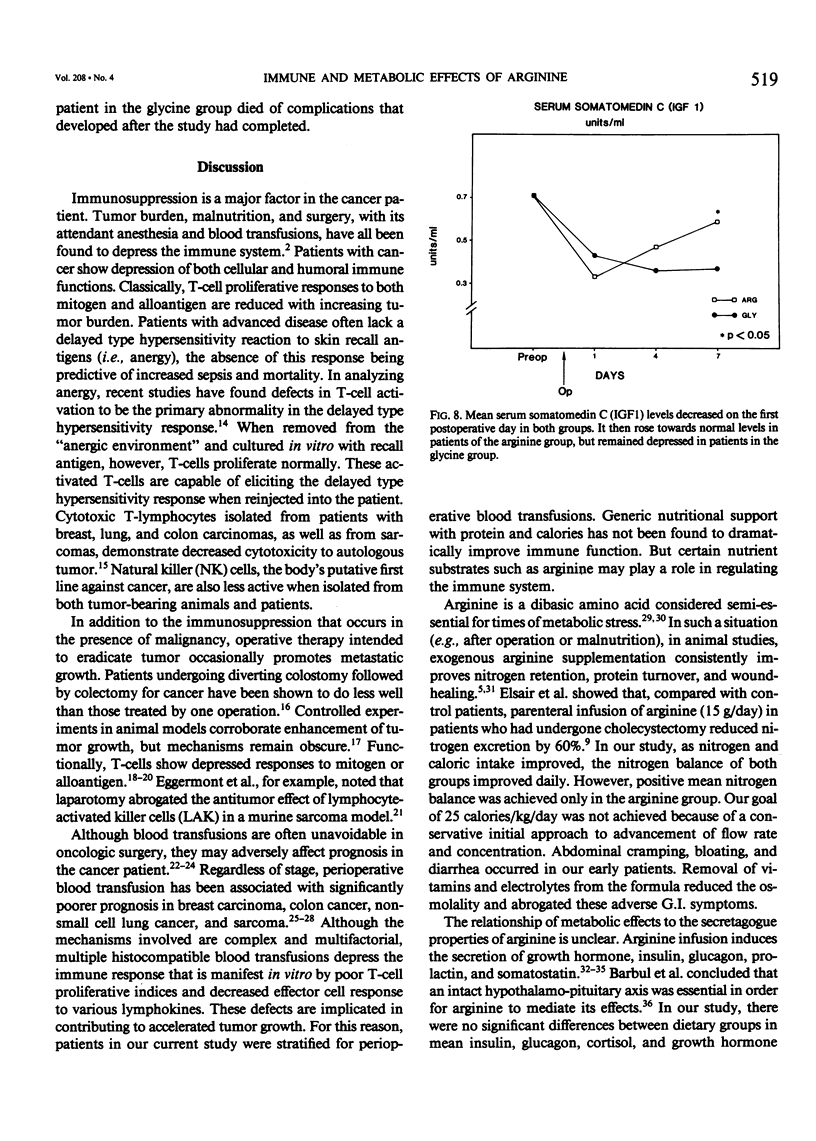
Abstract
Arginine enhances immune function and promotes nitrogen retention in animal models, but its immunomodulatory effects in surgical patients are unknown. This randomized, prospective trial evaluated the immune and metabolic effects of supplemental L-arginine (25 g/day, n = 16) or isonitrogenous L-glycine (43 g/day, n = 14) in 30 cancer patients undergoing major operation. Two groups of patients received either arginine or glycine for 7 days after surgery as a supplement to a graduated enteral diet. Nitrogen balance was measured daily, and immune parameters were determined both before and after surgery, on Days 1, 4, and 7. The T-lymphocyte response to concanavalin A (con A) and PHA and dual marker phenotype analysis of lymphocyte (CD2, CD4, CD4/DR, CD8, CD8/DR) and macrophage (M3/DR) subsets were determined. Mean age, degree of preoperative weight loss, disease stage, number of perioperative transfusions, and calorie and nitrogen intake were similar for the groups studied. Mean daily nitrogen balance (-2.3 g/day in the arginine group vs. -3.9 g/day in the glycine group) was not significantly different between the two groups, but positive mean nitrogen balance was achieved only in the arginine group between Days 5 and 7 after surgery. Supplemental arginine significantly enhanced the mean T-lymphocyte response (stimulation index) to con A from 45 +/- 26 on postoperative Day 1 to 72 +/- 47 and 87 +/- 49 on postoperative Days 4 and 7, compared with the values of 29 +/- 15, 27 +/- 20, and 33 +/- 34 in the glycine group at the same time points, respectively. Supplemental arginine increased mean CD4 phenotype (% T-cells) on postoperative Days 1 and 7 from 25 +/- 9 to 43 +/- 14, compared with the values of 30 +/- 14 and 29 +/- 13 in the glycine group (p less than 0.05). The beneficial effect of arginine on the immune system appeared distinct from its more moderate effect on nitrogen metabolism. As a nutrient substrate, arginine was nontoxic, and may benefit surgical patients who are at increased risk of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 1986 Mar-Apr;10(2):227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Barbul A., Rettura G., Levenson S. M., Seifter E. Wound healing and thymotropic effects of arginine: a pituitary mechanism of action. Am J Clin Nutr. 1983 May;37(5):786–794. doi: 10.1093/ajcn/37.5.786. [DOI] [PubMed] [Google Scholar]
- Barbul A., Sisto D. A., Wasserkrug H. L., Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981 Aug;90(2):244–251. [PubMed] [Google Scholar]
- Barbul A., Wasserkrug H. L., Seifter E., Rettura G., Levenson S. M., Efron G. Immunostimulatory effects of arginine in normal and injured rats. J Surg Res. 1980 Sep;29(3):228–235. doi: 10.1016/0022-4804(80)90165-1. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Fluck P. A., Hurst N. P. Depression of lymphocyte responses after surgical trauma. Br J Exp Pathol. 1973 Dec;54(6):597–607. [PMC free article] [PubMed] [Google Scholar]
- Blumberg N., Agarwal M. M., Chuang C. Relation between recurrence of cancer of the colon and blood transfusion. Br Med J (Clin Res Ed) 1985 Apr 6;290(6474):1037–1039. doi: 10.1136/bmj.290.6474.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Sunkara P. S. Increased ornithine decarboxylase activity and polyamine biosynthesis are required for optimal cytolytic T lymphocyte induction. Cell Immunol. 1987 Mar;105(1):110–117. doi: 10.1016/0008-8749(87)90060-8. [DOI] [PubMed] [Google Scholar]
- Burrows L., Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982 Sep 18;2(8299):662–662. doi: 10.1016/s0140-6736(82)92764-7. [DOI] [PubMed] [Google Scholar]
- Daly J. M., Dudrick S. J., Copeland E. M., 3rd Intravenous hyperalimentation. Effect on delayed cutaneous hypersensitivity in cancer patients. Ann Surg. 1980 Nov;192(5):587–592. doi: 10.1097/00000658-198011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont A. M., Steller E. P., Sugarbaker P. H. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery. 1987 Jul;102(1):71–78. [PubMed] [Google Scholar]
- Furlanetto R. W., Marino J. M. Radioimmunoassay of somatomedin C/insulin-like growth factor I. Methods Enzymol. 1987;146:216–226. doi: 10.1016/s0076-6879(87)46023-0. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Häcker-Shahin B., Dröge W. Putrescine and its biosynthetic precursor L-ornithine augment the in vivo immunization against minor histocompatibility antigens and syngeneic tumor cells. Cell Immunol. 1986 May;99(2):434–443. doi: 10.1016/0008-8749(86)90251-0. [DOI] [PubMed] [Google Scholar]
- Lundy J., Lovett E. J., 3rd, Wolinsky S. M., Conran P. Immune impairment and metastatic tumor growth: the need for an immunorestorative drug as an adjunct to surgery. Cancer. 1979 Mar;43(3):945–951. doi: 10.1002/1097-0142(197903)43:3<945::aid-cncr2820430324>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Meakins J. L., Pietsch J. B., Bubenick O., Kelly R., Rode H., Gordon J., MacLean L. D. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977 Sep;186(3):241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merimee T. J., Lillicrap D. A., Rabinowitz D. Effect of arginine on serum-levels of human growth-hormone. Lancet. 1965 Oct 2;2(7414):668–670. doi: 10.1016/s0140-6736(65)90399-5. [DOI] [PubMed] [Google Scholar]
- O'Mahony J. B., McIrvine A. J., Palder S. B., See-Young L., Saporoschetz I. B., Wilmore D. W., Mannick J. A. The effect of short term postoperative intravenous feeding upon cell-mediated immunity and serum suppressive activity in well nourished patients. Surg Gynecol Obstet. 1984 Jul;159(1):27–32. [PubMed] [Google Scholar]
- Palmer J. P., Walter R. M., Ensinck J. W. Arginine-stimulated acute phase of insulin and glucagon secretion. I. in normal man. Diabetes. 1975 Aug;24(8):735–740. doi: 10.2337/diab.24.8.735. [DOI] [PubMed] [Google Scholar]
- Park S. K., Brody J. I., Wallace H. A., Blakemore W. S. Immunosuppressive effect of surgery. Lancet. 1971 Jan 9;1(7689):53–55. doi: 10.1016/s0140-6736(71)90777-x. [DOI] [PubMed] [Google Scholar]
- Polk H. C., Jr, George C. D., Wellhausen S. R., Cost K., Davidson P. R., Regan M. P., Borzotta A. P. A systematic study of host defense processes in badly injured patients. Ann Surg. 1986 Sep;204(3):282–299. [PMC free article] [PubMed] [Google Scholar]
- Rakoff J. S., Siler T. M., Sinha Y. N., Yen S. S. Prolactin and growth hormone release in response to sequential stimulation by arginine and synthetic TRF. J Clin Endocrinol Metab. 1973 Nov;37(5):641–644. doi: 10.1210/jcem-37-5-641. [DOI] [PubMed] [Google Scholar]
- Riddle P. R., Berenbaum M. C. Postoperative depression of the lymphocyte response to phytohaemagglutinin. Lancet. 1967 Apr 8;1(7493):746–748. doi: 10.1016/s0140-6736(67)91364-5. [DOI] [PubMed] [Google Scholar]
- Rode H. N., Christou N. V., Bubenik O., Superina R., Gordon J., Meakins J. L., MacLean L. D. Lymphocyte function in anergic patients. Clin Exp Immunol. 1982 Jan;47(1):155–161. [PMC free article] [PubMed] [Google Scholar]
- Rose W. C. THE NUTRITIVE SIGNIFICANCE OF THE AMINO ACIDS AND CERTAIN RELATED COMPOUNDS. Science. 1937 Oct 1;86(2231):298–300. doi: 10.1126/science.86.2231.298. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Seipp C. A., White D. E., Wesley R. Perioperative blood transfusions are associated with increased rates of recurrence and decreased survival in patients with high-grade soft-tissue sarcomas of the extremities. J Clin Oncol. 1985 May;3(5):698–709. doi: 10.1200/JCO.1985.3.5.698. [DOI] [PubMed] [Google Scholar]
- Saito H., Trocki O., Wang S. L., Gonce S. J., Joffe S. N., Alexander J. W. Metabolic and immune effects of dietary arginine supplementation after burn. Arch Surg. 1987 Jul;122(7):784–789. doi: 10.1001/archsurg.1987.01400190050010. [DOI] [PubMed] [Google Scholar]
- Seifter E., Rettura G., Barbul A., Levenson S. M. Arginine: an essential amino acid for injured rats. Surgery. 1978 Aug;84(2):224–230. [PubMed] [Google Scholar]
- Sitren H. S., Fisher H. Nitrogen retention in rats fed on diets enriched with arginine and glycine. 1. Improved N retention after trauma. Br J Nutr. 1977 Mar;37(2):195–208. doi: 10.1079/bjn19770021. [DOI] [PubMed] [Google Scholar]
- Tartter P. I., Burrows L., Papatestas A. E., Lesnick G., Aufses A. H., Jr Perioperative blood transfusion has prognostic significance for breast cancer. Surgery. 1985 Feb;97(2):225–230. [PubMed] [Google Scholar]
- Tartter P. I., Martinelli G., Steinberg B., Barron D. Changes in peripheral T-cell subsets and natural-killer cytotoxicity in relation to colorectal cancer surgery. Cancer Detect Prev. 1986;9(3-4):359–364. [PubMed] [Google Scholar]
- Utsumi M., Makimura H., Ishihara K., Morita S., Baba S. Determination of immunoreactive somatostatin in rat plasma and responses to arginine, glucose and glucagon infusion. Diabetologia. 1979 Nov;17(5):319–323. doi: 10.1007/BF01235888. [DOI] [PubMed] [Google Scholar]
- Waymack J. P., Rapien J., Garnett D., Tweddell J. S., Alexander J. W. Effect of transfusion on immune function in a traumatized animal model. Arch Surg. 1986 Jan;121(1):50–55. doi: 10.1001/archsurg.1986.01400010056007. [DOI] [PubMed] [Google Scholar]
- Waymack J. P., Robb E., Alexander J. W. Effect of transfusion on immune function in a traumatized animal model. II. Effect on mortality rate following septic challenge. Arch Surg. 1987 Aug;122(8):935–939. doi: 10.1001/archsurg.1987.01400200085016. [DOI] [PubMed] [Google Scholar]
- Weese J. L., Ottery F. D., Emoto S. E. Do operations facilitate tumor growth? An experimental model in rats. Surgery. 1986 Aug;100(2):273–277. [PubMed] [Google Scholar]


