Abstract
Background
Neospora caninum is an important cause of infectious abortion and stillbirth in cattle world-wide. Infection is common and may frequently be passed from mother to calf (vertical transmission) with no signs of disease. Based on our previous observation that N. caninum-infection can be efficiently controlled with Toltrazuril-sulfone (Ponazuril) in experimentally infected mice, we addressed the question if efficacy could also be obtained in experimentally infected calves.
Material and Methods
The study included 19 calves and represents an initial explorative approach to document a basic effectiveness at first. Fifteen animals received each 2 x 108N. caninum trophozoites, half of the dose being injected intravenously and the other half subcutaneously. Efficacy of treatment was assessed using molecular detection of parasite DNA with PCR and pathological alterations by immunohistochemistry in different organs of the animals. Assessment included also clinical, serological and pathophysiological parameters.
Results
In those calves medicated with ponazuril (one, or six consecutive days, respectively, starting one day after infection), a complete abrogation of the parasite detectability was obtained in the brain and other organs, while 50% of non-treated calves became PCR-positive in brain and muscles. Clinically, ponazuril chemotherapy of infected calves – in comparison to non-treated infected animals – reduced symptoms (fever), but no differences were observed between treated and non-treated animals with regard to serum enzymes and metabolites. Efficacy of a six-day treament was also reflected by significantly lower anti-Neospora antibody concentrations developed after infection, when compared to non-treated animals.
Conclusion
Based on our findings in this initially explorative approach that indicate a basic effectiveness of ponazuril against experimental N. caninum infection in calves, we plan to follow our chemotherapeutical intervention strategy to control bovine neosporosis with a subsequent more extensive field study with naturally infected calves.
Background
Infectious organisms can cause significant losses in farm ruminant production as a result of abortion, embryonic damage or maternal infertility. One of the principal agents causing protozoal abortion or still-birth in cattle is Neospora caninum[1,2]. Infection affects approximately 12% of Swiss cattle [3] and may frequently be passed from mother to calf (vertical transmission) with no signs of disease. Problems occur when the parasite multiplies in the developing calf or the affected placenta and causes sufficient damage to trigger stillbirth or abortion, respectively. Preliminary research suggests that infection of the foetus early in gestation is more likely to be fatal to the conceptus than infection later in gestation [4]. However it also appears that infection is more likely to be transmitted in late rather than early pregnancy. Thus the majority of infections are not symptomatic and inapparent infections are maintained and linearly expanded in a herd. Control of bovine neosporosis is difficult. Effective vaccines to protect cattle from abortion or vertical parasite transmission are currently not available [1,5]. Pharmacologically active compounds are known to kill N. caninum[6]. Thus, toltrazuril and ponazuril proved high efficacy to prevent parasite dissemination and subsequent cerebral lesion formation in a murine experimental model [7]. To assess treatment efficacy, mice were clinically investigated for symptoms; histologically by searching respective cerebral lesions and molecular biologically by detection of parasite DNA using the polymerase-chain-reaction (PCR). Efficacy of treatment was demonstrated to eliminate the parasite, and to reduce but not to abrogate parasite-specific humoral immunity in mice.
With regard to controlling infection, disease or diaplacental parasite transmission in cattle, a chemotherapeutical approach has not been studied yet. Therefore, we designed now an initial explorative approach to document a basic effectiveness at first. This experiment represents a simple first step, to be followed with a more extensive, but also more expensive future study to provide a statistically evidence of the effect in large numbers of animals. To monitor treatment efficacy in the experimentally infected calves of this study, we used the same parameters as outlined above in the mouse model.
Materials and Methods
Parasites
Host Vero cells were maintained in 75 cm2 tissue culture flasks in 20 ml RPMI 1640 medium supplemented with 25 mM HEPES, 2 mM L-glutamine, 50 IU/ml penicillin, 50 μg/ml streptomycin and 10% fetal calf serum (FCS) (Gibco BRL, Life Technologies), and cultivation was as previously described [8]. Neospora caninum (NC-1 isolate) was cultivated in 75 or 175 cm2 culture flasks on Vero cells using the same medium but replacing FCS with 10% gamma-globulin-free horse serum (Gibco BRL, Life Technologies). Isolation and purification of in vitro-generated parasites were done as previously described [8]. Briefly, Vero cells infected with N. caninum were passaged through a 25G 5/8 needle, washed and run on PD-10 Sephadex G-25 M columns. Eluted tachyzoites were counted in a Neubauer-chamber and tested for viability by trypan blue exclusion. Tachyzoites were either directly used for infection of calves or stored frozen at -80°C for subsequent antigen processing. Crude somatic antigens from frozen N. caninum (SA-antigen) for the enzyme-linked immunosorbent assay (ELISA) was produced as described earlier [9].
Calves
The study included 19 male calves with a mean body weight of 85 kg (range 60 – 108 kg) and a mean age of 70 days (range 42 to 98 days old) at the day of infection. All animals tested were serologically negative (N. caninum-Indirect-Fluorescence-Antibody-Tests (IFAT) and N. caninum-ELISA, according to [10]) prior to infection. The calves had free access to hay and water ad libitum. Furthermore, they had access to a drinking-automate (UFA AG, Sursee, Switzerland) that provided to each calf a body weight-dependent quantity of fresh milk obtained daily from N. caninum seronegative lactating cows. One-hundred liter fresh milk was complemented with 55 grams of a pre-mix (UFA top-fit PreVitin, Sursee, Switzerland). Calves were sacrificed by deactivation of the Medulla oblongata and subsequent bleeding. Organs were carefully removed and subsequently dissected by the use of disposable tools in order to avoid any cross-contamination. All calves were housed and handled under standard animal experimentation conditions respecting the rules of the Swiss regulation of animal experimentation; the experiments were accepted and validated by the governmental regulation committee and obtained the permission no. 7/01.
Experimental infection
Each calf received 2 x 108N. caninum trophozoites for infection. The dose was divided into 1 x 108N. caninum trophozoites suspended in 5 ml sterile phosphate-buffered-saline (PBS) to be injected intravenously (i.v.) and an identical dose injected subcutaneously (s.c.). Non-infected control animals received an appropriate number of Vero-cells i.v. and s.c.. In order to monitor the infectivity status of the tachyzoites used for experimental infection of calves, 2 mice were additionally infected with 2 x 106 parasites i.p.. These mice were sacrificed 29 days post infectionem (p.i.) and investigated for the presence of N. caninum as previously reported [9].
Study design and experimental ponazuril therapy
With regard to infection and treatment schedules, the calves were assigned into the following groups:
Group 1 (2 calves): non-infected and non-medicated controls
Group 2 (2 calves): non-infected controls with medication (medication: 6 consecutive days of chemotherapy starting at 1 day post infectionem, d.p.i.)
Group 3 (6 calves): infected and non-medicated (positive) controls
Group 4 (5 calves): infected and medicated (medication: 1 day of chemotherapy starting at 1 d.p.i.)
Group 5 (4 calves): infected and medicated (medication: 6 consecutive days of chemotherapy starting at 1 d.p.i.)
Medication was performed perorally with 20 mg ponazuril (toltrazuril-sulfone) per kg body weight, using a 5% ponazuril suspension. The first medication dose was applied 24 h after infection, and, if repeated, every subsequent 24 hours for six days.
Per group, half of the calves were sacrificed on day 45 and the other on day 90 p.i., respectively (for group 5, three animals on day 45 and two on day 90 p.i.).
N. caninum-specific PCR
N. caninum – specific PCR was performed with 10 samples isolated from all sacrificed calves. Each brain sample was independently homogenized with a stirrer and DNA was isolated from 50 to 100 μl homogenate using the DNeasy™ kit (Qiagen, Basel, Switzerland) according to the manufacturer's recommendations. Neospora-specific PCR was done as described before [10-12]. For PCR analyses and per animal, a total of 10 different samples were collected from the brain (4 samples from the main cranial lobe, 4 from the main caudal lobe, 2 from the cerebellum), 3 from the heart and 3 from the muscles (one from the masseter, one from the diaphragma and one from the tongue), see Table 1. In addition, single probes were collected from the GI-tract, the liver, the spleen, the lungs, the pancreas and from popliteal lymphnodes. These probes were only PCR-investigated for infected and non-treated calves.
Table 1.
PCR-results with a total of 380 tissue samples obtained from non-infected (control) and N. caninum-infected calves without and with ponazuril therapy (1T = medication for one day; 6T = medication for 6 consecutive days). Bold numbers indicate the number of positive samples per total number of samples analyzed; – means that no sample was PCR-positive. Infected animals without therapy were euthanized on day 45 p.i. (nos. 1–3) and 90 p.i. (nos. 4–6)
| without infection | infection, without therapy | infection, with therapy | ||||||||
| () = number of calves tested | without therapy (2) | with therapy_6T (2) | no.1 (1) | no.2 (1) | no.3 (1) | no.4 (1) | no.5 (1) | no.6 (1) | with therapy_1T (5) | with therapy_6T (4) |
| brain | - / 20 | - / 20 | - / 10 | 2 / 10 | - / 10 | - / 10 | 1 / 10 | - / 10 | - /50 | - /40 |
| heart | - / 6 | - / 6 | - / 3 | - / 3 | 1 / 3 | - / 3 | - / 3 | - / 3 | - /15 | - /12 |
| muscles* | - / 6 | - / 6 | - / 3 | 1 / 3 | - / 3 | - / 3 | 1 / 3 | - / 3 | - /15 | - /12 |
| liver | - / 8 | - / 8 | - / 4 | - / 4 | - / 4 | - / 4 | - / 4 | - / 4 | - /20 | - /16 |
*muscles: per animal, one sample from the M. masseter, one from the diaphragma and one from the tongue, respectively.
Histology and immunohistochemistry
Sections of formalin fixed and paraffin-embedded tissues were haemalaun-eosin stained for microscopic examination or were used for immunohistochemistry as described elsewhere [10]. Samples from the brain, heart, lung, liver and kidney were examined.
Serology
Antibodies against N. caninum were detected by an enzyme-linked immunosorbent assay (ELISA) using a soluble somatic antigen [10]. N. caninum-seropositivity was primarily defined by an ELISA-value ≥ 1 antibody units [12].
Clinical and pathophysiological parameters
Rectal temperature
The body temperature of calves was rectally assessed in the morning between 08:00 h and 08:30 h, this in parallel to the drawing of blood samples.
Gain of body weight
Body weight of each individual animal was assessed once per week, starting 4 weeks before initiation of infection and or medication, until the end of experiments.
Determination of blood metabolites and enzymes
Plasma levels of glucose, urea, non-estered fatty acids (NEFA) and triglycerides, enzymes [(aspartate-amino-transferase (AST), gamma-glutamyle-transferase (γ-GT), creatine-kinase (CK) and glutamate-dehydrogenase (GLDH)] were assessed in blood samples taken at 0, 1, 2, 3 and 4 d.p.i, using appropriate test kits purchased from Hoffmann-La-Roche (Basel, Schweiz) (kit nos. # 07 3685 6, # 07 3679 1, # 07 3671 6, # 07 3641 4, # 07 3655 4 and # 07 3647 3), from Wako Chemicals (Neuss, Germany) (kit no. # 994–75409) and from Boehringer (Ingelheim, Germany) (kit no # 124311), respectively. Kit performance occurred in a Cobas Mira plus analyzer (Roche, Basel, Switzerland). Prior to testing, plasma samples were stored at -20°C.
Ponazuril serum levels
For each animal, several (see Fig. 3) serum samples were stored frozen (-20°C) for a subsequent determination of the ponazuril serum level. Ponazuril was determined by HPLC with fluorescence detection after post column derivatisation with UV-light at the laboratory for Animal Health, Bayer AG, Crop Protection Development, Leverkusen, together with pharmacokinetic calculations, both according to the method previously described in Furr and Kennedy [13]. Half life times were calculated by analysis with the software package TOPFIT 2.0 using the non compartment model.
Figure 3.
Concentration of ponazuril in serum from calves either given a 1-day-dose (four calves nos. 1T.1 – 1T.4) or a daily dose for 6 consecutive days at 20 mg/kg body weight (five calves nos. 6T.1 – 1T.5). Lacking lines and markers indicate that no sample was analyzed for this time point.
Statistical analyses
Due to only explorative status of this first study, and the thus minimal number of calves used for each experimental group, we did not use statistics to assess infection intensity and extensity data. The study classifies therefore as a preliminary descriptive experiments, results expressing tendencies. However, for serological data analysis, the software package Revelation® from Microtech (Embrach, Switzerland) could be used for statistics.
Results
Infection and effect of treatment
The infection intensity and extensity was analyzed by PCR specific for N. caninum DNA (Table 1) and by immunohistochemistry, including brain, heart and muscle samples from all 19 calves of the experiment. In non-medicated control-infected calves, the presence of N. caninum-DNA was confirmed by PCR in three out of six calves, affecting the brain and muscles in two, and the heart only in another animal. All 11 infected and ponazuril-treated calves remained negative in PCR for all samples tested. As all samples derived from the GI-tract, the liver, the spleen, the lungs, the pancreas and lymphnodes were PCR-negative in infected and non-treated animal, thus we did not perform the respective PCR with the other calves used in the experiment. In the histological analyses of the calf brains, no multifocal cerebral micro-lesions could be detected, neither in PCR-negative nor in PCR-positive samples. In immunohistochemistry, no individual N. caninum tachyzoites could be detected in the same respective brain samples.
Serology
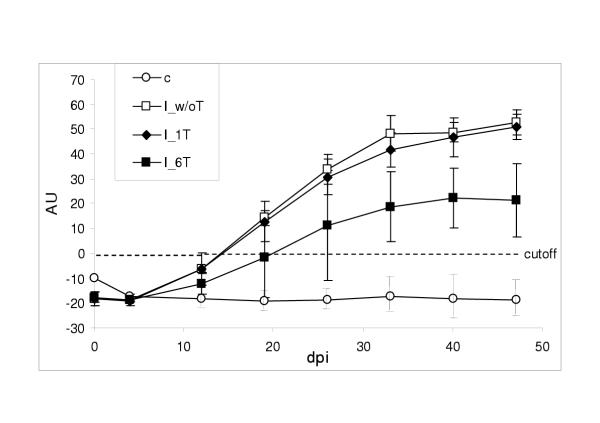
The humoral immune responses of the different animal groups are shown in Fig. 1. Non-medicated infected calves developed a relatively high anti-N. caninum antibody concentration, the corresponding seroconversion started by the day range 10–20 p.i.. The calf group that had received ponazuril treatment for a period of 6 consecutive days exhibited a significantly lower anti-N. caninum antibody response and a delay in seroconversion when compared to infected (and non-medicated) controls. Conversely, the anti-N. caninum antibody concentrations of infected and one-day-medicated calves were only slightly below those of non-treated animals, differences were non-significant .
Figure 1.
Anti-Neospora caninum serum IgG measured by ELISA in non-infected and non-medicated negative control calves (c), infected and non-medicated positive control calves (I_w/oT), infected and one-day medicated calves (I_1T) and infected and six-day medicated calves (I_6T). Number of animals per group as in Table 1. AU = antibody units; dotted line (cut-off) indicates the negative/positive threshold determined by the mean plus 3 S.D. of measurements from non-infected control calves. Error bars display standard deviations.
Clinical and pathophysiological parameters
Rectal temperature
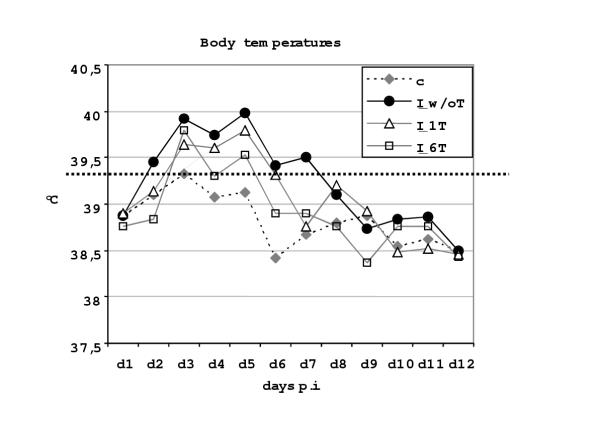
The body temperature of calves was assessed daily for the first 20 days, subsequently weekly until the end of experiments. Fig. 2 shows the average body temperature for the different groups of animals for the first 12 days p.i., as in the subsequent periods no average elevations could be seen anymore. Due to the relative low number of animals, a statistical evaluation of the significance of differences could not be performed. Nevertheless, a clear tendency was observed in the two ponazuril-treated groups, which both exhibited lower average body temperatures for the first 5 days p.i. (average peak maximum 39.8°C for treated and 40.0°C for non-treated calves) and also a shorter time period of elevated (≥ 39.2°C; [14]) body temperature (average 3–4 days for treated and 6 days for non-treated calves, respectively).
Figure 2.
Average rectal temperatures followed for the first 12 days p.i. in non-infected negative control calves (c), infected and non-medicated positive control calves (I_w/oT), infected and one-day medicated calves (I_1T) and infected and six-day medicated calves (I_6T). The dotted horizontal bar indicates the upper level of the normal body temperature in this age class.
Gain of body weight
Body weight of each animal was assessed weekly from the time point of purchase (approximately 4–5 weeks prior to experiments) until the end of all experiments (euthanasia of calves). In order to address potential differences between the different animal groups, the groups were comparatively clustered in time periods before experiments (all calves were then equally hold and nourished = pre-experimental control phase) and experimental time periods. All groups showed an average gain in body weight that was not different from the other groups (Table 2).
Table 2.
Gain in body weight (average per group) in relation to the initial weight of each calf at its time point of purchase. Animal groups included non-infected (control) and N. caninum-infected calves without and with ponazuril therapy (1T = medication for one day; 6T = medication for 6 consecutive days). Δ1 refers to the gain of body weight in the period between purchase and initiation of experiments (day of infection with or without treatment) and Δ2 to the time period between start and end of experiments.
| non-infected controls | infection, without therapy | infection, with therapy_1T | infection, with therapy_6T | |
| Δ1 (%) | 1.2 | 1.3 | 1.6 | 1.5 |
| Δ2 (%) | 2.2 | 2.2 | 2.4 | 1.9 |
(%) value 1 refers to the initial body weight assesed at the time point of purchase.
Determination of blood metabolites and enzymes
Serum levels of glucose, urea, non-esterified fatty acids and triglycerids, enzymes [(aspartate-amino-transferase (AST), gamma-glutamyle-transferase (γ-GT), creatine-kinase (CK) and glutamate-dehydrogenase (GLDH)] were assessed in blood samples obtained from all animals on days 0, 1, 2, 3 and 4 p.i.. Within the first four days p.i., no differences became apparent between any groups of animals, respective to infection and medication status.
Ponazuril serum levels
Ponazuril serum levels were determined for a selected number of animals, according to the list and time schedule shown in Fig. 3. The oral absorption of ponazuril was rapid, and serum concentrations became apparent at the second day of treatment (= day 3.p.i.). A marked difference in the maxima and length of detectable ponazuril concentrations was seen between the one-day and the six-day-medication. Serum concentrations in the 1-day-dose had reached a steady state between days 5 and 7 p.i.. Six-day medication led to an accumulation of the compound in the serum, so that the maximal concentration peaks were reached between days 8 and 12 p.i.. Accumulation was documented by an approximate 3 to 4-fold increase of the concentration maxima in 6-day-treated versus 1-day-treated calves. Following discontinuation of chemotherapy, at latest on day 47 p.i. for the 1-day-treatment and on day 82 for the 6-day-treatment, the serum concentrations had dropped below 1 mg/l. From the 8 animals investigated in this part of the study, the mean half life time, calculated upon concentrations of the active substance, was 9.8 days.
Discussion
Neosporosis is a disease affecting predominantly the fetal development in bovine hosts, it may also cause neuromuscular dysfunction in infected new born calves and pups [2]. In cattle, the outcome of a N. caninum infection appears largely dependent upon the effectiveness of the immune system of the mother animal [15]. Thus, in a certain number of cases, immunity appears insufficient to prevent vertical transmission to the bovine fetus [15].
Chemotherapeutical treatment of neosporosis may be an issue, provided that an appropriate drug is made available. In this respect, we have previously described the use of a mouse model to demonstrate the efficacy of toltrazuril and ponazuril for the prevention of parasite dissemination and subsequent cerebral lesion formation [7]. We now addressed – in a first step – the question, if the same drug would exhibit a basic effect in the experimentally infected bovine host. This question was tackled in view to justify and plan future trials for the treatment of experimentally and naturally acquired bovine neosporosis.
With regard to our experimental design, the high costs of experimental calves did not allow to directly plan experiments with number of calves allowing statistical analyses. Thus, the project was basically designed as an explorative study, only allowing to document descriptively the effect of the treatment in experimentally infected calves. Conversely to our previous study in mice, where we had started chemotherapy 6 hours prior to infection, we now provided a 24 hour cycle to the parasite to establish and multiply within the animals, before medication was started. Chemotherapy itself was either performed as a short term, one-day medication or as a 6-consecutive-day medication. Assessment of serum levels of ponazuril showed that the two medication schedules considerably influenced the maximal serum concentrations in the animals. The relatively long half-life time of ponazuril led to an accumulation of the compound in 6-day-treated animals, so that the maximal peak was approximately 3 to 4-times higher than in 1-day-medicated calves. Also the duration of ponazuril detectability in serum was markedly longer in 6-day-treated animals. Nevertheless, no differences appeared between the two treatment schedules as in none of the treated animals N. caninum became detectable by PCR. With regard to the non-medicated control calves, which had received the same infection dose, we had to notify that only 3 out of 6 animals reached the infection stage where the parasite became detectable by PCR in the brain and muscles. Calves therefore seemed to exhibit, constitutively, a relatively low susceptibility to experimental infection. Similar observations had already been reported by others [16]. Nevertheless, the absence of any DNA detectability in treated calves versus the 50% detectability in non-treated calves indicate efficacy of ponazuril to suppress parasite dissemination and establishment in peripheral organs such as brain and muscles. Histology and immunohistochemistry, probably because of the low infection intensity, did not result in the demonstration of parasites and respective lesions in the PCR positive tissues. Uggla et al. [16] had orally infected calves with N. caninum-tachyzoites. Similarly to our results, although their animals seroconverted upon infection, and some became positive by PCR, neither pathognomonic tissue damages nor parasites themselves could be demonstrated by histology and immunohistochemistry.
In our mouse model [7], we had shown that toltrazuril/ponazuril treatment, in parallel to abrogating parasite dissemination, also predominantly influenced the kinetics of the humoral immune response. Similarly now in calves, experimentally infected and non-treated animals developed a marked humoral immune response, seroconversion occurred between days 10 and 20 p.i., and maximal serum antibody concentrations were reached between days 30 and 40 p.i.. One-day medication of infected calves – although abrogating parasite detectability by PCR – did not change the time point of seroconversion and the antibody concentration levels when compared to non-treated animals. Conversely, the 6-day-treatment markedly reduced the concentration maximum of serum antibodies, and delayed the time point of seroconversion by approximately one week. These findings indicate that the one-day treatment may not have killed the parasite as efficiently or rapidly as the 6-day treatment. A higher antibody concentration may be linked either to a higher antigen concentration (indicating post-infectional parasite multiplication despite treatment) or to a longer lasting stimulation with identical antigen concentrations (indicating a longer survival of the inoculated parasite population). This question will require appropriate experiments to be addressed.
When tackling clinical parameters during the first phase of the experiments, it became apparent that non-treated as well as treated calves responded with fever to the infection. However, treated animals had milder fever as expressed by average lower peak levels and shorter fever periods. As the first fever occurred only 1–2 days after experimental infection in non-treated and 2–3 days p.i. in treated animals, we can exclude endotoxin activity by inoculated N. caninum tachyzoites. Principally, endotoxin induced fever is expected to occur a few hours after inoculation of e.g. lipopolysaccharides (LPS) [14]. Inoculation of an appropriate number of Vero-cells in non-infected control animals did not result in fever, indicating that fever was indeed related to the parasite inoculum. In consequence, we can conclude that ponazuril therapy inhibited the parasite proliferation in a way to reduce fever. To elucidate the fever aspect more in details, we will have to perform experimental inoculation of the same dose but of killed parasites (such as done in the previous mouse experiments; [7]). With these experiments we will be able to show if fever is directly associated to viable trophozoites or not.
Bovine N. caninum infection, although not demonstrating any acute-phase symptoms, may result in chronic phase consequences, as indicated by reduced gain of body weight in seropositive versus seronegative calves [18]. In our experiments, however, no respective differences became apparent. While planning the experiments, we hypothesized that a putative intensive parasite dissemination in non-treated calves should result in tissue and organ damages, respectively, and thus also in the modification of blood parameters. Therefore, we analyzed serum glucose, GLDH and γ-GT to address liver functions, AST and CK for muscle damages, urea for kidney damages and triglycerides and NEFA to check the lipid status. All results were within the normal range of concentrations [19-21], thus indicating the absence of any pathology that could have been demonstrated with the respective laboratory tools [22]. These findings support again our hypothesis on the relatively low susceptibility of calves for experimental N. caninum infection. Therefore, even if the parasite became disseminated and subsequently established in the brain and muscles of at least 3 from 6 infected and non-treated calves, the clinical consequences of this infection became not detectable.
Conclusions
Based upon our findings in this initially explorative approach that indicate a basic effectiveness of ponazuril against experimental N. caninum infection in calves, we plan to follow our chemotherapeutical intervention strategy to control bovine neosporosis with a subsequent more extensive field study. This will now tackle naturally infected calves derived from N. caninum-positive mothers. This field study will be designed in view to include a large number of animals of appropriate statistical significance. Parameters to asses efficacy of treatment will include the same as used in this study. Conceptually, there is a general acceptance that healthy newborn calves from N. caninum-positive mothers are suspected to stay life-long chronically infected [23], with reactivations occurring during pregnancy. Elimination of the parasite after birth by chemotherapy may provide negative off-spring and thus N. caninum-free breeding lines. Furthermore, as the milk yield and gain of body weight appeared to be reduced in persistently infected cows [18], creation of Neospora-free breeding lines may also improve other economic aspects.
Authors' contributions
Author 1 (SK) carried out the experimental infection and treatment studies, and performed PCR, ELISA and histological/immunohistochemical analyses. Author 2 (HS) participated in the clinical investigations and the molecular and serological assays. Author 3 (JB) carried out the pathophysiological testings. Author 4 (RK) performed the determination of the ponazuril serum levels. Author 4 (GG) participated in the design of the study. Author 5 (BG) conceived the study and its design and supervised its coordination.
Acknowledgments
Acknowledgements
This work was supported, in part, by a grant of Swiss Federal Office of Science and Education (grant no. BBW C01.0122 in the frame of COST 854). The authors wish to thank Mrs. Ursula Mäusle for technical assistance.
Contributor Information
Sandra Kritzner, Email: bruno.gottstein@ipa.unibe.ch.
Heinz Sager, Email: heinz.sager@ipa.unibe.ch.
Jürg Blum, Email: juerg.blum@itz.unibe.ch.
Ralph Krebber, Email: gisela.greif.gg@bayer-ag.de.
Gisela Greif, Email: gisela.greif.gg@bayer-ag.de.
Bruno Gottstein, Email: bruno.gottstein@ipa.unibe.ch.
References
- Dubey JP. Neosporosis in cattle: biology and economic impact. J Am Vet Med Assoc. 1999;214:1160–1163. [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/S0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- Gottstein B, Hentrich B, Wyss R, Thür B, Bruckner L, Müller N, Kaufmann H, Waldvogel A. Molekular- und immundiagnostische Untersuchung zur bovinen Neosporose in der Schweiz. Schweiz Arch Tierheilk. 1999;141:59–68. [PubMed] [Google Scholar]
- Hemphill A, Gottstein B. An European perspective on Neospora caninum . Int J Parasitol. 2000;30:877–924. doi: 10.1016/S0020-7519(00)00072-2. [DOI] [PubMed] [Google Scholar]
- Andrianarivo AG, Rowe JD, Barr BC, Anderson ML, Packham AE, Sverlow KW, Choromanski L, Loui C, Grace A, Conrad PA. A POLYGEN(TM)-adjuvanted killed Neospora caninum tachyzoite preparation failed to prevent foetal infection in pregnant cattle following i.v./i.m. experimental tachyzoite challenge. Int J Parasitol. 2000;30:985–990. doi: 10.1016/S0020-7519(00)00088-6. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Rippey NS, Cole RA, Parsons LC, Dubey JP, Tidwell RR, Blagburn BL. Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am J Vet Res. 1994;55:976–981. [PubMed] [Google Scholar]
- Gottstein B, Eperon S, Dai WJ, Cannas A, Hemphill A, Greif G. Efficacy of toltrazuril and ponazuril against experimental Neospora caninum infection in mice. Parasitol Res. 2001;87:43–48. doi: 10.1007/s004360000306. [DOI] [PubMed] [Google Scholar]
- Hemphill A, Gottstein B, Kaufmann H. Adhesion and invasion of bovine endothelial cells by Neospora caninum . Parasitol. 1996;112:183–197. doi: 10.1017/s0031182000084754. [DOI] [PubMed] [Google Scholar]
- Eperon S, Broennimann K, Hemphill A, Gottstein B. Susceptibility of B-cell deficient C57BL/6 (μMT) mice to Neospora caninum infection. Parasite Immunol. 1999;21:225–236. doi: 10.1046/j.1365-3024.1999.00223.x. [DOI] [PubMed] [Google Scholar]
- Sager H, Fischer I, Furrer K, Strasser M, Waldvogel A, Boerlin P, Audigé L, Gottstein B. A Swiss case-control-study to assess Neospora caninum-associated bovine abortions by PCR, histopathology and serology. Vet Parasitol. 2001;102:1–15. doi: 10.1016/S0304-4017(01)00524-6. [DOI] [PubMed] [Google Scholar]
- Müller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiology. 1996;34:2850–2852. doi: 10.1128/jcm.34.11.2850-2852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein B, Hentrich B, Wyss R, Thür B, Busato A, Stärk KDC, Müller N. Molecular and immunodiagnostic investigations on bovine neosporosis in Switzerland. Intern J Parasitol. 1998;28:679–691. doi: 10.1016/S0020-7519(98)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr M, Kennedy T. Cerebrospinal fluid and serum concentrations of ponazuril in horses. Vet Therapeutics. 2001;2:232–237. [PubMed] [Google Scholar]
- Rosenberger G. Die klinische Untersuchung des Rindes. Paul Parey: Berlin, Hamburg. 1990;3 [Google Scholar]
- Innes EA, Buxton D, Eperon S, Gottstein B. Immunology of Neospora caninum infection in cattle and mice. Int J Parasitol. 2000;30:896–900. [Google Scholar]
- Uggla A, Stenlund S, Holmdahl OJ, Jakubek EB, Thebo P, Kindahl H, Bjorkman C. Oral Neospora caninum inoculation of neonatal calves. Int J Parasitol. 1998;28:1467–1472. doi: 10.1016/S0020-7519(98)00110-6. [DOI] [PubMed] [Google Scholar]
- Kinsberger M, Bruckmaier RM, Blum JW. Metabolic, endocrine and hematological responses to E. coli endotoxin admininstration in 1-week old calves. J Vet Med A. 1994;41:530–547. doi: 10.1111/j.1439-0442.1994.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Barling KS, McNeill JW, Thompson JA, Paschal JC, McCollum FT, Craig TM, Adams LG. Association of serologic status for Neospora caninum with postweaning weight gain and carcass measurements in beef calves. J Am Vet Med Assoc. 2000;217:1356–1360. doi: 10.2460/javma.2000.217.1356. [DOI] [PubMed] [Google Scholar]
- Blum JW, Flueckiger N. Early metabolic and endocrine effects of perorally administered beta-adrenoceptor agonists in calves. Eur J Pharmacol. 1988;151:177–187. doi: 10.1016/0014-2999(88)90798-4. [DOI] [PubMed] [Google Scholar]
- Hugi D, Bruckmaier RM, Blum JW. Insulin resistance, hyperglycemia, glucosuria, and galactosuria in intensively milk-fed calves: dependency on age and effects of high lactose intake. J Anim Sci. 1997;75:469–482. doi: 10.2527/1997.752469x. [DOI] [PubMed] [Google Scholar]
- Zimmerli UV, Blum JW. Acute and longterm metabolic, endocrine, respiratory, cardiac and skeletal muscle activity changes in response to perorally administrated-adrenoceptor agonists in calves. J Anim Physiol Anim Nutr. 1990;63:157–172. [Google Scholar]
- Kraft W, Dürr U. Klinische Labordiagnostik in der Tiermedizin. Schattaluer: Stuttgart, New York. 1999;4 [Google Scholar]
- Long MT, Baszler TV, Mathison BA. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum . J Parasitol. 1998;84:316–320. [PubMed] [Google Scholar]