Abstract
We have previously shown that the tumor suppressor p53 can play a protective role against UV-induced apoptosis in human fibroblasts. In the present study, we investigated whether the protective function of p53 expression is established before or after UV irradiation. Using a stable human cell line expressing a murine temperature-sensitive p53 in which p53 function could be tightly and reversibly regulated, we found that functional p53 stimulated the induction of apoptosis when expressed for as little as 4–12 h after UV irradiation and that this induction was not dependent on de novo protein synthesis. In contrast, expression of p53 for 12 h or more before UV irradiation reduced the extent of apoptosis even when functional p53 expression was maintained after irradiation. The protection conferred by p53 required ongoing protein synthesis and correlated with enhanced recovery of mRNA synthesis. Together, these results suggest that p53 induces distinct proapoptotic and antiapoptotic signals and that these opposing activities can be separated both temporally and by their requirement for de novo protein synthesis. These findings may have important implications for the refinement of gene therapy approaches combining p53 with pharmacological agents that target transcription or translation.
INTRODUCTION
The p53 tumor-suppressor gene is the most commonly altered gene in malignancy (Hollstein et al., 1996). The tumor-suppressive function of p53 can be attributed in part to its participation in the cellular response to DNA damage. In response to DNA strand breaks or transcription blocking DNA damage, such as UV light–induced photoproducts, p53 accumulates through a posttranscriptional mechanism (Levine, 1997; Ljungman, 2000). The p53 protein can act as both an activator and a repressor of transcription (Levine, 1997). The transactivation function of p53 has been suggested to play a role in the regulation of the G1 and G2 cell cycle checkpoints (Levine, 1997; Bunz et al., 1998), the induction of apoptosis (Miyashita et al., 1994; Owen-Schaub et al., 1995; Sheikh et al., 1998b), and the stimulation of nucleotide excision repair (NER) (Hwang et al., 1999).
In addition to its role as a stress-inducible regulator of transcription, p53 may also be able to participate directly in NER and other cellular processes independent of its ability to act as a transcription factor (Caelles et al., 1994; Wang et al., 1995, 1996; Chen et al., 1996; McKay et al., 1999). Furthermore, p53 can regulate the basal level of expression of p53-responsive genes such as p21WAF1 and p48XPE in unstressed cells (Tang et al., 1998; Hwang et al., 1999). This implies that p53 may regulate cellular processes without being activated by DNA damage and thus may be biologically important even before sustaining DNA damage.
To further study the role of p53 in the regulation of apoptosis after exposure to UV light, a stable HT29 colon carcinoma subline expressing a temperature-sensitive allele of murine p53 (Merchant et al., 1996) was used. This temperature-sensitive p53 is fully active and nuclear at the permissive temperature of 32°C, whereas it is inactive and localized in the cytoplasm at the nonpermissive temperature of 38°C (Gannon and Lane, 1991; Ginsberg et al., 1991; Martinez et al., 1991). This system permitted the rapid switching from mutant to wild-type p53 conformation at the permissive temperature without inducing apoptosis and further permitted the rapid loss of p53 function at the restrictive temperature. Using this model system, we show that p53 expression before UV irradiation protected cells against UV irradiation, whereas p53 expression exclusively after UV irradiation stimulated UV-induced apoptosis. These results suggest that wild-type p53 expression in unstressed cells has an impact on the UV-induced p53 response.
MATERIALS AND METHODS
Cell Culture
The two cell lines used in this study, HT29-neo and HT29-tsp53 (formerly referred to as ts29-G cells), were derived from the human colon cancer cell line HT29 (Merchant et al., 1996). Both cell lines were maintained at 38°C (nonpermissive temperature) in RPMI-1640 supplemented with 10% FBS and antibiotics (penicillin/streptomycin).
UV Irradiation
Subconfluent cells, seeded 2 d before UV irradiation, were irradiated without medium at room temperature with a germicidal UV light (Philips, New York, NY) emitting at 254 nm at a rate of 0.6 J·m−2·s−1 (UVX radiometer, UVP, San Gabriel, CA).
Western Blots
Thirty micrograms of total cellular protein was loaded per well in 15% polyacrylamide gels. Proteins were transferred to Immobilon-P membrane (Millipore, Bedford, MA) overnight at 4°C. Detection of proteins was performed as described previously (Ljungman et al., 1999). The antibodies used were raised against p53 (p53 AB-2; Oncogene Research Products, Cambridge, MA), p21WAF1 (WAF1 AB-1; Oncogene Science), Bax (Bax N20; Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (clone AC-74; Sigma Chemical, St. Louis, MO).
Measurements of Apoptosis
Apoptosis was scored 48 h after UV irradiation either by assessing the fraction of cells with a sub-G1 DNA content by flow cytometry or by estimating the extent of DNA fragmentation with the use of agarose gel electrophoresis (Chung et al., 1998).
Bromodeoxyuridine Labeling and Flow Cytometry
Cells were incubated for either 15 min or 12 h with 30 μM bromodeoxyuridine (BrdU; Sigma) at 37°C to label nascent DNA synthesis, fixed, and analyzed by flow cytometry, as described previously (Chang et al., 1999). The antibodies used were anti-BrdU (diluted 1:100; PharMingen, San Diego, CA) and an FITC-conjugated anti-mouse immunoglobulin G (diluted 1:15; Sigma). The samples were assessed by two-parameter flow cytometry for FITC (DNA synthesis) and propidium iodide (DNA content) signals with the use of a Coulter Epics Elite Cell Sorter and the Multicycle Software package (Phoenix Flow Systems, San Diego, CA).
Measurements of mRNA Synthesis
Cells, incubated with 185 Bq/ml [14C]thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden) for 2 d to label cellular DNA, were sham-irradiated (control) or irradiated with 20 J/m2 UV light. Nascent mRNA was labeled for 30 min with [3H]uridine (Amersham Pharmacia Biotech) and was subsequently isolated from cell lysates with the use of the Straight A's mRNA isolation system (Novagen, Madison, WI). [3H]Uridine incorporation into poly(A) RNA was quantified with a scintillation counter as described previously (Ljungman et al., 1999).
RESULTS
Role for p53 in UV-induced Apoptosis
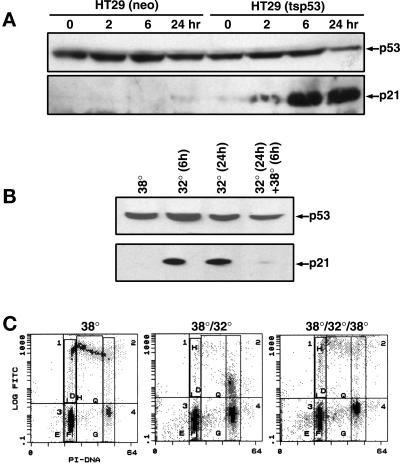
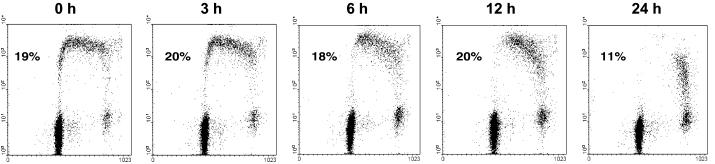
First, we examined whether the timing of functional p53 expression could be tightly regulated in the HT29-tsp53 cell system used in this study. At the nonpermissive temperature of 38°C, the p53-regulated protein p21WAF1 was not detected, but expression of this protein was induced within 2 h at the permissive temperature of 32°C. No measurable induction of p21WAF1 was observed in HT29-neo control cells (Figure 1A). p53 function, as assessed by the induction of p21WAF1, was found to be reversible at 38°C in HT29-tsp53 cells (Figure 1B). Examining the cell cycle distribution of these cells revealed the expected decrease in the percentage of cells entering S phase when incubated at 32°C (Figure 1C). Switching the temperature back to 38°C (Figure 1C) reversed the G1 arrest induced by functional p53 expression. Thus, transactivation and cell cycle arrest mediated by p53 can be tightly regulated and are reversible in this cell system.
Figure 1.
p53 function can be rapidly turned on and off in HT29-tsp53 cells by temperature switching. (A) Western blot showing the expression of p53 and p21WAF1 in HT29-neo and HT29-tsp53 cells after incubation of cells for different periods at 32°C. (B) Western blots showing stable expression of p53 and induction of p21WAF1 within 6 h at 32°C and the rapid loss of p21WAF1 when switching the HT29-tsp53 cells back to 38°C. (C) Two-parameter flow cytometry diagram of cells pulse labeled for 15 min with BrdU immediately before collection. BrdU incorporation (replicative DNA synthesis) is plotted on the Y axis and DNA content is plotted on the X axis. HT29-tsp53 cells were grown at 38°C (left panels), incubated at 32°C for 24 h (middle panels), or incubated at 32°C for 24 h and then returned to 38°C for 24 h (right panels).
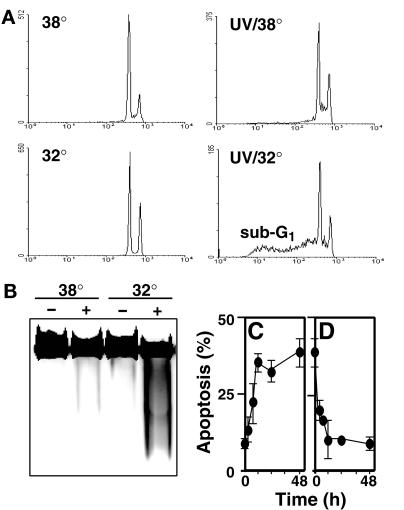
Although this allele of p53 induces apoptosis at the permissive temperature in many other cell lines (Caelles et al., 1994; Wagner et al., 1994), very little apoptosis was induced in HT29-tsp53 cells at the permissive temperature in the absence of DNA damage (Figure 2A) (Merchant et al., 1996). Whereas 20 J/m2 UV light only weakly induced apoptosis in HT29-tsp53 cells at the restrictive temperature of 38°C, expression of functional p53 exclusively after UV irradiation led to a significant increase in the induction of apoptosis (Figure 2, A and B). Because functional p53 expression was found to be readily reversible (Figure 1, B and C), this model system permitted us to investigate the minimal period of functional p53 expression that was required for the induction of apoptosis after UV irradiation. It was found that a 12-h incubation at 32°C immediately after UV irradiation was sufficient for p53 to contribute to UV-induced apoptosis (Figure 2C). Conversely, there was a significant decrease in the induction of apoptosis when the shift to 32°C was delayed by as little as 4 h (Figure 2D). Together, these results suggest that functional p53 contributes to UV-induced apoptosis when expressed within the first 4–12 h after UV irradiation.
Figure 2.
Post-UV expression of functional p53 enhances UV-induced apoptosis. (A) Flow cytometry diagram of propidium iodide–stained HT29-tsp53 cells maintained at 38°C (upper panels) or switched to 32°C (lower panels) at the time of mock irradiation (left panels) or UV irradiation with 20 J/m2 (right panels). Forty-eight hours after UV irradiation, cells were subjected to flow cytometry analysis, and cells with a sub-G1 DNA content were considered to be apoptotic. (B) HT29-tsp53 cells treated as in A were lysed in the wells of the agarose gel, and the extent of DNA fragmentation was assessed by electrophoresis. The black-and-white image of the ethidium bromide–stained gel has been reversed for clarity. (C) HT29-tsp53 cells were switched to 32°C at the time of UV irradiation and returned to 38°C at various times after UV treatment. Apoptosis was scored as in A and plotted as a function of post-UV incubation time at 32°C. (D) HT29-tsp53 cells were UV irradiated and maintained at 38°C for various periods before switching them to 32°C. Apoptosis was scored as in A and plotted as a function of time between UV irradiation and the switch to 32°C. In C and D, each point represents the mean ± SEM of two to eight independent experiments with background values subtracted. Mean background sub-G1 values varied between 6 and 11%.
p53 Can Protect against UV-induced Apoptosis
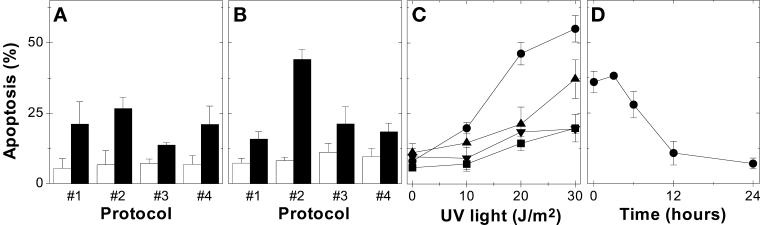
The results presented above could be perceived to contradict recent reports that suggest that p53 is protective against UV-induced apoptosis (McKay and Ljungman, 1999; Wani et al., 1999). It is important to note that in those studies, wild-type p53 was present both before and after UV irradiation, whereas we found that p53 contributed to apoptosis when p53 was in mutant conformation before UV irradiation but was functional after UV irradiation. Therefore, we performed a series of temperature-shift experiments (Table 1) to test the effect of previous p53 expression on UV-induced apoptosis. Whereas these temperature shifts had very little effect on UV-induced apoptosis in HT29-neo control cells (Figure 3A), profound differences were observed in HT29-tsp53 cells (Figure 3B). p53 expression exclusively before UV irradiation failed to sensitize cells to subsequent UV exposure (compare protocols 1 and 3). In fact, previous expression of functional p53 inhibited UV-induced apoptosis mediated by functional p53 expressed during the post-UV incubation period (compare protocols 2 and 4). This effect of previous p53 expression was observed over a broad range of UV doses (Figure 3C). Interestingly, very little protection against the subsequent UV exposure was conferred by incubation of HT29-tsp53 cells for 3–6 h at 32°C (Figure 3D), even though p21WAF1 was induced within 2 h (Figure 1A). Therefore, expression of neither functional p53 nor p21WAF1 appeared to be sufficient for protection against UV-induced apoptosis. We conclude that p53 expression can stimulate UV-induced apoptosis when expressed exclusively after UV irradiation, whereas p53 can protect against UV-induced apoptosis when expressed before UV irradiation.
Table 1.
Temperature-shift protocols used in this study
Temperature during a 24-h period before UV irradiation.
Temperature after UV irradiation.
Figure 3.
Previous expression of functional p53 protects cells against UV-induced apoptosis. HT29-neo (A) or HT29-tsp53 (B) cells were mock irradiated (white bars) or UV irradiated with 20 J/m2 (black bars), and apoptosis was scored 48 h later by flow cytometry. The numbers below the bars represent the temperature-shift protocols described in Table 1. (C) The effect of UV dose on the induction of apoptosis in HT29-tsp53 cells was determined for the same temperature-shift protocols: 1 (▪), 2 (●), 3 (▴), and 4 (▾). Each value represents the mean ± SEM of at least three independent experiments. (D) HT29-tsp53 cells were incubated at 32°C for various periods before being exposed to UV irradiation (20 J/m2). The cells were maintained at 32°C after UV irradiation, and apoptosis was scored as the percentage of cells with a sub-G1 DNA content measured 48 h after treatment. Each point represents the mean ± SEM of three to seven independent experiments with background values subtracted. The mean of the background values from the different experiments varied between 7 and 16%.
The Protective Function of p53 Does Not Appear to Involve a Sustained G1 Arrest
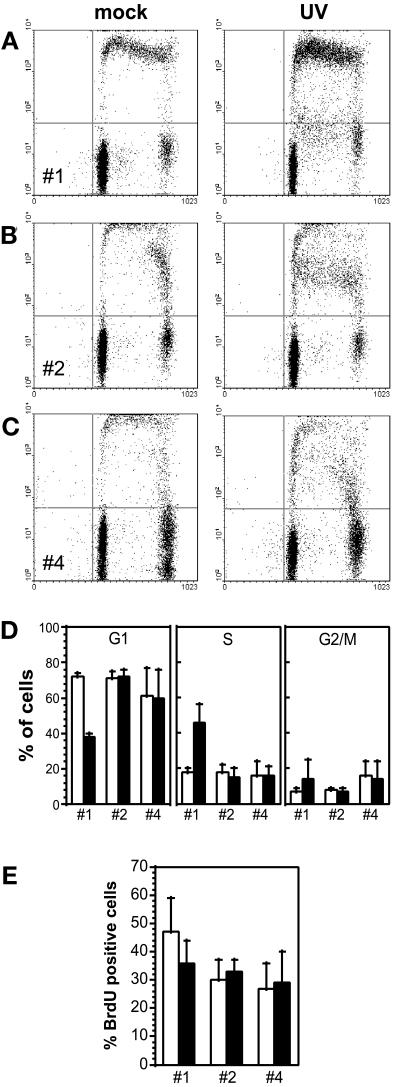
It is thought that p53 may protect cells exposed to DNA-damaging agents by arresting them in the G1 phase of the cell cycle, allowing cells more time for repair before DNA replication (Lane, 1992; Bissonnette and Hunting, 1998; McKay et al., 1998). To address whether the protective function of p53 was related to the induction of a sustained cell cycle arrest, we assessed the cell cycle distribution of HT29-tsp53 cells with the use of two-parameter flow cytometry. UV irradiation of HT29-tsp53 cells maintained at the restrictive temperature led to a significant increase in the S-phase population of cells 24 h after UV irradiation (Figure 4). Irradiation of cells subjected to temperature-shift protocols 2 and 4 did not alter the proportion of cells in the G1, S, or G2/M phase of the cell cycle (Figure 4D). Furthermore, there were no significant differences in cell cycle distribution of HT29-tsp53 cells subjected to protocols 2 and 4 despite the fact that these protocols greatly affected sensitivity to UV-induced apoptosis (Figure 3). We also performed similar experiments in which BrdU was maintained in the medium during the entire post-UV incubation period. No significant difference was detected in the proportion of cells entering S phase during the 12-h period in which cells were committed to p53-dependent apoptosis (Figure 4E). Therefore, the results suggest that the protective effect of previous p53 expression against UV-induced apoptosis does not appear to involve the establishment of a sustained G1 cell cycle arrest.
Figure 4.
Protection conferred by p53 does not appear to be related to sustained G1 arrest. (A–D) Twenty-four hours after either mock irradiation or UV irradiation with 20 J/m2, BrdU pulse-labeled HT29-tsp53 cells (15-min pulse) were subjected to two-parameter flow cytometry analysis as described in Figure 1. (A) Cells were maintained at 38°C throughout the experiment (protocol 1; Table 1). (B) Cells were switched to 32°C immediately after UV irradiation (protocol 2). (C) Cells were switched to 32°C 24 h before UV irradiation and maintained at 32°C during the post-UV incubation period (protocol 4). (D) The cell cycle distributions (as in A–C) of mock-irradiated (white bars) or UV-irradiated (black bars) HT29-tsp53 cells subjected to protocols 1, 2, and 4 were determined from multiple experiments. (E) HT29-tsp53 cells were subjected to protocols 1, 2, and 4 (Table 1), BrdU labeled continuously for 12 h after mock irradiation or UV irradiation with 20 J/m2, and subjected to two-parameter flow cytometry analysis. Results are expressed as the percentage of cells that entered S phase within 12 h after UV irradiation. Each point in D and E represents the mean ± SE of two to four experiments.
To determine whether differences in the cell cycle distribution of HT29-tsp53 cells at the time of irradiation could account for differences in the apoptotic response, a detailed time course for the establishment of cell cycle arrest in HT29-tsp53 cells was obtained (Figure 5). A clear time-dependent decrease in the proportion of cells entering S phase was observed in HT29-tsp53 cells when incubated at 32°C. This G1 arrest was first evident within 6 h at 32°C, which correlated with maximal induction of p21WAF1 (Figure 1). Importantly, even though G1 arrest was detected within 6 h at 32°C, significant protection against UV-induced apoptosis required 12 or more hours of functional p53 expression (Figure 3D). Thus, we find no clear correlation between the establishment of G1 arrest and resistance to UV-induced apoptosis in these cells.
Figure 5.
Protection conferred by p53 does not correlate with previous establishment of G1 arrest. Two-parameter flow cytometry analysis of the cell cycle distribution of unirradiated HT29-tsp53 cells at different times after switching to the permissive temperature of 32°C. Numbers represent the percentages of cells in S phase.
p53 and the Recovery of Transcription
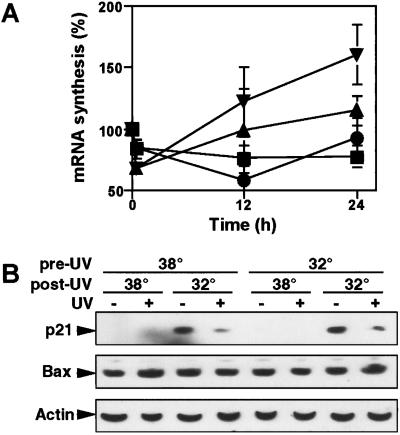
The ability of cells to recover mRNA synthesis after UV irradiation has been found to be inversely correlated with the severity of the apoptotic response after exposure of cells to UV light (Ljungman and Zhang, 1996; McKay et al., 1998; McKay and Ljungman, 1999). Furthermore, Li-Fraumeni syndrome cells lacking normal p53 function have been shown to be defective in the recovery of mRNA synthesis after UV irradiation and are hypersensitive to UV-induced apoptosis (McKay and Ljungman, 1999). Therefore, we hypothesized that the antiapoptotic function of p53 may be related to an enhanced recovery of mRNA synthesis after UV irradiation. Consistent with this hypothesis, we show that the recovery of mRNA synthesis was more efficient in HT29-tsp53 cells maintained at 32°C than in cells maintained at 38°C (Figure 6A). This is rather striking given the fact that NER is extremely sensitive to decreases in temperature (Hjertvik et al., 1998). Importantly, previous expression of functional p53 stimulated the recovery of mRNA synthesis, whereas switching cells to the permissive temperature at the time of UV irradiation failed to stimulate this recovery process (Figure 6A). Furthermore, previous expression of functional p53 resulted in a modest stimulation of the recovery of transcription when p53 was in mutant conformation during the post-UV recovery period. These results suggest that functional p53 participates in the recovery of mRNA synthesis after UV irradiation but that this participation is indirect and is established before UV irradiation, perhaps through the regulation of one or more rate-limiting steps involved in this recovery process.
Figure 6.
p53 stimulates the recovery of transcription after UV irradiation. (A) Cells were subjected to the temperature-shift protocols described in Table 1: 1 (▪), 2 (●), 3 (▴), and 4 (▾). At various times after UV irradiation, nascent RNA was labeled for 30 min with [3H]uridine. Polyadenylated RNA was isolated with the use of oligo-dT beads and quantified with a scintillation counter. (B) The expression of p21WAF1 and Bax was assessed 6 h after UV irradiation by Western blot analysis in HT29-tsp53 cells subjected to the different temperature-shift protocols (Table 1). Expression of actin was used as a loading control.
To investigate whether UV exposure impaired the expression of p53-responsive genes, the expression of p21WAF1 and Bax was assessed 6 h after UV irradiation of HT29-tsp53 cells subjected to each of the temperature-shift protocols described in Table 1. UV irradiation was found to block the p53-mediated induction of p21WAF1 (Figure 6B, lane 4). As reported previously, UV irradiation led to a loss of p21WAF1 proteins that had been accumulated by previous p53 expression (Figure 6B, lane 8) (McKay et al., 1998; Wang et al., 1999). Interestingly, Bax protein levels were not altered by p53 expression or UV irradiation (Figure 6B, lanes 1, 3, and 7). These results, together with previous reports (Lu et al., 1996; McKay et al., 1998), suggest that transactivation of p53-responsive genes such as p21WAF1 is attenuated soon after exposure to 20 J/m2 UV light.
Protein Synthesis and p53-mediated Apoptosis
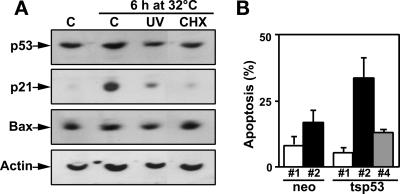
Overexpression of functional p53 did not in itself lead to the induction of apoptosis in HT29-tsp53 cells, but apoptosis was stimulated by functional p53 expression after UV irradiation under conditions in which transcription was impaired. We hypothesized that p53-mediated apoptosis could be transactivation-independent and could result from the inefficient expression of proteins that normally protect against the proapoptotic function of p53. Therefore, we sought to determine whether apoptosis was induced by UV irradiation in the presence of the protein synthesis inhibitor cycloheximide (CHX). CHX alone induced apoptosis in HT29-tsp53 but not HT29-neo cells, and previous expression of functional p53 protected against CHX-stimulated apoptosis (Figure 7A). Like UV light, CHX inhibited the p53-mediated induction of p21WAF1 (Figure 7B). We suggest that p53 contributes to both proapoptotic and antiapoptotic pathways in these cells. The antiapoptotic function of p53 appears to involve gene and protein expression and needs to be established before cell treatment, whereas the proapoptotic activity of p53 is transactivation- and translation-independent.
Figure 7.
CHX blocks p21WAF1 expression and potentiates p53-mediated apoptosis. (A) Cells were switched to 32°C at the time of UV or CHX treatment, and p53, p21WAF1, Bax, and actin levels were assessed by Western blot analysis 6 h later. (B) HT29-neo and HT29-tsp53 cells were subjected to protocols 1 (white bars), 2 (black bars), and 4 (gray bars) with CHX for 48 h. The induction of apoptosis in mock-treated controls was subtracted from that determined for each treatment. CHX significantly stimulated the induction of apoptosis in HT29-tsp53 cells subjected to protocol 2. Values are expressed as means ± SEM from two to five independent experiments.
DISCUSSION
The Timing of p53 Expression Affects UV-induced Apoptosis
Using an HT29 colon carcinoma–derived cell line expressing a temperature-sensitive allele of p53, we found that the timing of p53 expression affected whether p53 contributed to or inhibited UV-induced apoptosis. As reported previously (Merchant et al., 1996), apoptosis was not induced at the permissive temperature in HT29-tsp53 cells. However, p53 significantly enhanced UV-induced apoptosis when expressed exclusively after UV irradiation. We demonstrated that this proapoptotic effect required p53 expression during the first 4–12 h after UV irradiation. In distinct contrast, functional p53 expression for 12 h or more before UV irradiation protected cells against UV-induced apoptosis even when p53 expression was maintained after irradiation (Figure 3). This is the first demonstration that previous expression of p53 can have an impact on the response to subsequent cellular stresses. We conclude that p53 participates in UV-induced apoptosis soon after UV irradiation in these cells but that the proapoptotic effect of p53 can be overcome by previous expression of functional p53.
Overexpression of p53 is thought to transactivate hundreds of genes (Farmer et al., 1992; Zambetti et al., 1992; Smith and Fornace, 1997). Although expression of the proapoptotic protein Bax is regulated by p53 (Miyashita et al., 1994), we did not observe significant changes in Bax expression in the HT29-tsp53 cells at the permissive temperature. Wild-type p53 has also been reported to regulate the level of expression of the death receptors Fas and TRAIL (Owen-Schaub et al., 1995; Sheikh et al., 1998b). UV light has been suggested to promote apoptosis through the stimulation of death receptor signaling (Rehemtulla et al., 1997; Aragane et al., 1998; Sheikh et al., 1998a; Hill et al., 1999), and thus, one might expect that enhanced expression of these receptors by expression of functional p53 would sensitize cells to subsequent UV exposure. In contrast to this prediction, we found that previous expression of functional p53 conferred protection against UV-induced apoptosis. Therefore, it is unlikely that p53-enhanced expression of Bax, Fas, or TRAIL contributed to UV-induced apoptosis in these cells.
Several previous studies have shown that p53 can induce apoptosis without a requirement for the transactivation of target genes (Caelles et al., 1994; Wagner et al., 1994; Haupt et al., 1995; Chen et al., 1996; Wang et al., 1996). Our study shows that p53 expression induced apoptosis under conditions in which transcription (Figure 5), translation (Figure 6), or both were impaired, supporting a transactivation- and translation-independent mechanism of p53-mediated apoptosis. In fact, not only did p53-mediated apoptosis occur in the absence of de novo protein synthesis, but inhibition of protein synthesis in HT29-tsp53 cells was found to stimulate p53-mediated apoptosis. Thus, our results support a model (McKay et al., 1998) in which a sustained high cellular level of wild-type p53 under conditions in which it is unable to enhance the expression of p53-regulated proteins triggers apoptosis.
The p53 protein can exert both protective and apoptosis-enhancing functions after exposure to DNA-damaging agents. The protective roles of p53 in inducing cell cycle arrest and stimulating NER are thought to be dependent on the p53-mediated transactivation of genes such as p21WAF1 and p48 (Tang et al., 1998; Hwang et al., 1999). However, some DNA-damaging agents induce DNA lesions that interfere with the elongation of RNA polymerase II, and thus, the induction of protective gene products by p53 may not occur efficiently (Figures 5 and 6) (McKay et al., 1998). In fact, we found that 20 J/m2 UV light severely attenuated the p53-mediated induction of p21WAF1 in HT29-tsp53 cells (Figure 5). The protective effect of the expression of functional p53 before, but not after, UV irradiation (Figure 3) most likely resulted from the ability of p53 to transactivate genes before sustaining DNA damage.
Although p21WAF1 can be protective against p53-mediated apoptosis under certain circumstances (Polyak et al., 1996; Gorospe et al., 1997) and the expression of p21WAF1 was induced within 2 h at the permissive temperature, the protection conferred by p53 required 12 or more hours of functional p53 expression. Therefore, it is unlikely that either p53 or p21WAF1 per se was directly involved in the protection exerted by the pre-UV expression of functional p53. These results suggest that the expression of one or more alternative p53-regulated proteins is involved in this protective function of p53.
The Enhanced Recovery of Transcription is a Pre-UV Function of p53
The recovery of mRNA synthesis after the induction of RNA polymerase II–blocking lesions by UV light (Sauerbier and Hercules, 1978; Protic-Sabljic and Kraemer, 1985; Donahue et al., 1994) is greatly affected by the transcription-coupled repair capacity of cells (Ljungman and Zhang, 1996; McKay and Ljungman, 1999). Although it remains somewhat controversial, the wild-type p53 status of a cell may be important for the efficient execution of preincision steps in both the transcription-coupled repair (Wang et al., 1995; Mirzayans et al., 1996; McKay et al., 1997; Barley et al., 1998; McKay and Ljungman, 1999; Rey et al., 1999; Therrien et al., 1999) and global genomic repair (Ford and Hanawalt, 1995, 1997; Smith et al., 1995; McKay et al., 1999; Therrien et al., 1999; Wani et al., 1999) pathways of NER. In addition, it has been reported that DNA is more relaxed (less supercoiled) after UV irradiation in p53-deficient cells (Aranda-Anzaldo et al., 1999), suggesting that DNA repair–induced strand breaks persist longer in p53-deficient cells. Thus, both preexcision and postexcision events in the NER pathway may be enhanced in wild-type p53-expressing cells, resulting in a more rapid recovery of transcription and a reduced rate of apoptosis after exposure to UV light. In addition, it is possible that postrepair processes required for the efficient recovery of transcription are stimulated by p53 (McKay and Ljungman, 1999).
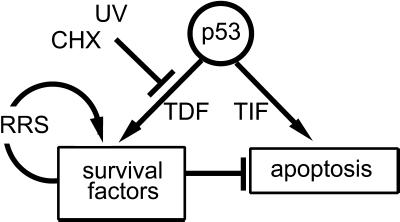
It has been reported previously that the recovery of transcription after UV irradiation is defective in p53-compromised cells (Ford and Hanawalt, 1995; Mirzayans et al., 1996; McKay et al., 1997; Barley et al., 1998; McKay and Ljungman, 1999) and that these cells are hypersensitive to UV-induced apoptosis (McKay and Ljungman, 1999; Wani et al., 1999). In these previous studies, the model system used did not permit the issue of the timing of p53 expression to be addressed. Defects in NER and the recovery of transcription have been detected in cells with compromised p53 function very soon after UV irradiation (Wang et al., 1995; McKay and Ljungman, 1999; Therrien et al., 1999). However, the induction of p53 and its downstream gene products is delayed by several hours after UV irradiation (Lu and Lane, 1993; Ljungman and Zhang, 1996; Dumaz et al., 1997; McKay et al., 1998). Furthermore, p53 and its target genes are not strongly induced by UV light at doses used to assess NER and the recovery of transcription (Ljungman and Zhang, 1996; Dumaz et al., 1997; McKay et al., 1998). Therefore, the “induction” of p53 and its downstream gene products after UV irradiation does not appear to be essential for these recovery processes. Importantly, recent reports suggest that p53 can regulate the level of expression of p53-responsive gene products under nonstressed conditions (Tang et al., 1998; Hwang et al., 1999). Thus, it is plausible that p53 stimulates the recovery of transcription by regulating the basal level of expression of one or more gene product(s) that is (are) rate limiting for this recovery process. In support of this model, we found that functional p53 expression was required before UV irradiation to facilitate the recovery of transcription in HT29-tsp53 cells. This pre-UV function of p53 would be expected to permit more efficient recovery of gene expression after UV irradiation (Figure 8).
Figure 8.
In this model, the apoptosis-promoting functions of high levels of functional p53 are balanced by the p53-mediated transactivation of survival factors. When expression of these survival factors is attenuated by either UV light or CHX, p53 will induce transactivation-independent apoptosis. In addition, p53 expression before UV irradiation increases the efficiency of survival-promoting functions such as the recovery of mRNA synthesis. We also suggest that previous expression of p53 protects cells against UV- or CHX-induced apoptosis by increasing the expression of p53-regulated survival-promoting factors before cellular stress. These protective functions are not fully independent because stimulating the recovery of transcription after UV irradiation will also permit the recovery of post-UV expression of p53-regulated survival factors, thus decreasing the induction of apoptosis. TDF, transactivation-dependent function; TIF, transactivation-independent function; RRS, recovery of mRNA synthesis.
In conclusion, we have demonstrated that p53 strongly promotes apoptosis in HT29-tsp53 cells when gene expression is blocked by UV-induced DNA damage or CHX treatment but that this induction of apoptosis is strongly attenuated by the previous expression of functional p53. Therefore, the timing of p53 expression affects whether p53 contributes to or inhibits apoptosis induced by UV light or CHX treatment in these cells. Although the exogenous p53 expression in this cell system is very high and there may be some cell type specificity in the p53 response, together with our earlier work (McKay and Ljungman, 1999) these results suggest that previous p53 expression confers protection against UV-induced apoptosis. This protection is most likely due to the regulation of one or more gene products that inhibit UV-induced apoptosis, and this arises at least in part through the stimulation of protective processes such as DNA repair and the recovery of transcription. We suggest that p53 induction in the context of either blocked transcription or translation would promote apoptosis (Figure 8). Importantly, these results suggest that the timing of p53 expression may have significant clinical implications for combining p53 gene therapy with therapeutic agents that target transcription or translation of tumor cells.
ACKNOWLEDGMENTS
We thank Dr. Jonathan Maybaum for the cell lines used in this study. We are also grateful for the excellent technical assistance of the staff at the Flow Cytometry Core Unit (University of Michigan). This work was supported by funds from the Department of Radiation Oncology (University of Michigan), by funds from the University of Michigan Comprehensive Cancer Center's institutional grant from the American Cancer Society, and by a grant from the National Institutes of Health (CA82376-01).
REFERENCES
- Aragane Y, Kulms D, Metze D, Wilkes G, Pöppelmann B, Luger T, Schwarz T. Ultraviolet light induces apoptosis via direct activation of CD95 (fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda-Anzaldo A, Orozco-Velasco F, Garcia-Villa E, Gariglio P. p53 is a rate-limiting factor in the repair of higher-order DNA structure. Biochim Biophys Acta. 1999;1446:181–192. doi: 10.1016/s0167-4781(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Barley RDC, Enns L, Paterson MC, Mirzayans R. Aberrant p21(WAF1)-dependent growth arrest as the possible mechanism of abnormal resistance to ultraviolet light cytotoxicity in Li-Fraumeni syndrome fibroblast strains heterozygous for TP53 mutations. Oncogene. 1998;17:533–543. doi: 10.1038/sj.onc.1202271. [DOI] [PubMed] [Google Scholar]
- Bissonnette N, Hunting DJ. p21-induced cycle arrest in G1 protects cells from apoptosis induced by UV-irradiation or RNA polymerase II blockage. Oncogene. 1998;16:3461–3469. doi: 10.1038/sj.onc.1201899. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Caelles C, Helmberg A, Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- Chang D, Chen F, Zhang FF, McKay BC, Ljungman M. Dose-dependent effects of DNA-damaging agents on p53-mediated cell cycle arrest. Cell Growth Differ. 1999;10:155–162. [PubMed] [Google Scholar]
- Chen XB, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;10:2438–2451. doi: 10.1101/gad.10.19.2438. [DOI] [PubMed] [Google Scholar]
- Chung DH, Zhang FF, Chen F, McLaughlin WP, Ljungman M. Butyrate attenuates BCLXL expression in human fibroblasts and acts in synergy with ionizing radiation to induce apoptosis. Radiat Res. 1998;149:187–194. [PubMed] [Google Scholar]
- Donahue BA, Yin S, Taylor JS, Reines D, Hanawalt PC. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc Natl Acad Sci USA. 1994;91:8502–8506. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumaz N, Duthu A, Ehrhart JC, Drougard C, Appella E, Anderson CW, May P, Sarasin A, DayaGrosjean L. Prolonged p53 protein accumulation in trichothiodystrophy fibroblasts dependent on unrepaired pyrimidine dimers on the transcribed strands of cellular genes. Mol Carcinog. 1997;20:340–347. [PubMed] [Google Scholar]
- Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992;358:83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Ford JM, Hanawalt PC. Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc Natl Acad Sci USA. 1995;92:8876–8880. doi: 10.1073/pnas.92.19.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Hanawalt PC. Expression of wild-type p53 is required for efficient global genomic nucleotide excision repair in UV-irradiated human fibroblasts. J Biol Chem. 1997;272:28073–28080. doi: 10.1074/jbc.272.44.28073. [DOI] [PubMed] [Google Scholar]
- Gannon J, Lane D. Protein synthesis required to anchor a mutant p53 protein which is temperature-sensitive for nuclear transport. Nature. 1991;349:802–806. doi: 10.1038/349802a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Michael-Michalovitz D, Ginsberg D, Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991;11:582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorospe M, Cirielli C, Wang XT, Seth P, Capogrossi MC, Holbrook NJ. p21(Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene. 1997;14:929–935. doi: 10.1038/sj.onc.1200897. [DOI] [PubMed] [Google Scholar]
- Haupt Y, Rowan S, Shaulian E, Vousden KH, Oren M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- Hill LL, Ouhtit A, Loughlin SM, Kripke ML, Ananthaswamy HN, Owen-Schaub LB. Fas ligand: a sensor for DNA damage critical in skin cancer etiology. Science. 1999;285:898–900. doi: 10.1126/science.285.5429.898. [DOI] [PubMed] [Google Scholar]
- Hjertvik M, Erixon K, Ahnstrom G. Repair of DNA damage in mammalian cells after treatment with UV and dimethyl sulfate: discrimination between nucleotide and base excision repair by their temperature dependence. Mutat Res. 1998;407:87–96. doi: 10.1016/s0921-8777(97)00062-1. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Shomer B, Greenblatt M, Soussi T, Hovig E, Montesano R, Harris CC. Somatic point mutations in the p53 gene of human tumors and cell lines: updated compilation. Nucleic Acids Res. 1996;24:141–146. doi: 10.1093/nar/24.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BJ, Ford JM, Hanawalt PC, Chu G. Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc Natl Acad Sci USA. 1999;96:424–428. doi: 10.1073/pnas.96.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208–225. doi: 10.1038/sj.neo.7900073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman M, Zhang F. Blockage of RNA polymerase as a possible trigger for UV light-induced apoptosis. Oncogene. 1996;13:823–831. [PubMed] [Google Scholar]
- Ljungman M, Zhang FF, Chen F, Rainbow AJ, McKay BC. Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene. 1999;18:583–592. doi: 10.1038/sj.onc.1202356. [DOI] [PubMed] [Google Scholar]
- Lu X, Burbidge SA, Griffin S, Smith HM. Discordance between accumulated p53 protein level and its transcriptional activity in response to UV radiation. Oncogene. 1996;13:413–418. [PubMed] [Google Scholar]
- Lu X, Lane DP. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993;75:765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Martinez J, Georgoff I, Martinez J, Levine A. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991;5:151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- McKay B, Ljungman M. Role for p53 in the recovery of transcription and protection against apoptosis induced by ultraviolet light. Neoplasia. 1999;1:276–284. doi: 10.1038/sj.neo.7900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BC, Francis MA, Rainbow AJ. Wildtype p53 is required for heat shock and ultraviolet light enhanced repair of a UV-damaged reporter gene. Carcinogenesis. 1997;18:245–249. doi: 10.1093/carcin/18.2.245. [DOI] [PubMed] [Google Scholar]
- McKay BC, Ljungman M, Rainbow AJ. Persistent DNA damage induced by ultraviolet light inhibits p21(waf1) and bax expression: implications for DNA repair, UV sensitivity and the induction of apoptosis. Oncogene. 1998;17:545–555. doi: 10.1038/sj.onc.1201963. [DOI] [PubMed] [Google Scholar]
- McKay BC, Ljungman M, Rainbow AJ. Potential roles for p53 in nucleotide excision repair. Carcinogenesis. 1999;20:1389–1396. doi: 10.1093/carcin/20.8.1389. [DOI] [PubMed] [Google Scholar]
- Merchant AK, Loney TL, Maybaum J. Expression of wild-type p53 stimulates an increase in both Bax and Bcl-x(L) protein content in HT29 cells. Oncogene. 1996;13:2631–2637. [PubMed] [Google Scholar]
- Mirzayans R, Enns L, Dietrich K, Barley RDC, Paterson MC. Faulty DNA polymerase delta/epsilon-mediated excision repair in response to gamma radiation or ultraviolet light in p53-deficient fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Carcinogenesis. 1996;17:691–698. doi: 10.1093/carcin/17.4.691. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Krajewsky S, Krajewska M, Wang H, Lin H, Hoffman B, Lieberman D, Reed J. Tumor suppressor p53 is a regulator of bcl-2 and bax in gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- Owen-Schaub LB, et al. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol Cell Biol. 1995;15:3032–3040. doi: 10.1128/mcb.15.6.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- Protic-Sabljic M, Kraemer K. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci USA. 1985;82:6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- Rey O, Lee S, Park NH. Impaired nucleotide excision repair in UV-irradiated human oral keratinocytes immortalized with type 16 human papillomavirus genome. Oncogene. 1999;18:6997–7001. doi: 10.1038/sj.onc.1203180. [DOI] [PubMed] [Google Scholar]
- Sauerbier W, Hercules K. Gene and transcription mapping by radiation effects. Annu Rev Genet. 1978;12:329–363. doi: 10.1146/annurev.ge.12.120178.001553. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Antinore MJ, Huang Y, Fornace AJ., Jr Ultraviolet-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene. 1998a;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr, el-Deiry WS. p53-dependent, and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998b;58:1593–1598. [PubMed] [Google Scholar]
- Smith ML, Chen IT, Zhan QM, O'Connor PM, Fornace AJ. Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene. 1995;10:1053–1059. [PubMed] [Google Scholar]
- Smith ML, Fornace AJ. p53-mediated protective responses to UV irradiation. Proc Natl Acad Sci USA. 1997;94:12255–12257. doi: 10.1073/pnas.94.23.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Zhao K, Pizzolato JF, Fonarev M, Langer JC, Manfredi JJ. Constitutive expression of the cyclin-dependent kinase inhibitor p21 is transcriptionally regulated by the tumor suppressor protein p53. J Biol Chem. 1998;273:29156–29163. doi: 10.1074/jbc.273.44.29156. [DOI] [PubMed] [Google Scholar]
- Therrien JP, Drouin R, Baril C, Drobetsky EA. Human cells compromised for p53 function exhibit defective global and transcription-coupled nucleotide excision repair, whereas cells compromised for pRb function are defective only in global repair. Proc Natl Acad Sci USA. 1999;96:15038–15043. doi: 10.1073/pnas.96.26.15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AJ, Kokontis JM, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21(waf1/cip1) Genes Dev. 1994;8:2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- Wang JA, Fan S, Yuan RQ, Ma YX, Meng Q, Goldberg ID, Rosen EM. Ultraviolet radiation down-regulates expression of the cell-cycle inhibitor p21WAF1/CIP1 in human cancer cells independently of p53. Int J Radiat Biol. 1999;75:301–316. doi: 10.1080/095530099140483. [DOI] [PubMed] [Google Scholar]
- Wang XW, et al. The XPB and XPD DNA helicases are components of the p53-mediated apoptosis pathway. Genes Dev. 1996;10:1219–1232. doi: 10.1101/gad.10.10.1219. [DOI] [PubMed] [Google Scholar]
- Wang XW, et al. p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat Genet. 1995;10:188–195. doi: 10.1038/ng0695-188. [DOI] [PubMed] [Google Scholar]
- Wani MA, Zhu QZ, El-Mahdy M, Wani AA. Influence of p53 tumor suppressor protein on bias of DNA repair and apoptotic response in human cells. Carcinogenesis. 1999;20:765–772. doi: 10.1093/carcin/20.5.765. [DOI] [PubMed] [Google Scholar]
- Zambetti G, Bargonetti J, Walker K, Prives C, Levine A. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 1992;6:1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]