Abstract
Background
Malaria in pregnancy is characterised by the sequestration of Plasmodium falciparum-infected erythrocytes in placental intervillous spaces. Placental parasites express a specific phenotype, which allows them to cytoadhere to chondroitin sulfate A expressed by syncytiotrophoblasts. Malaria infection during pregnancy allows the acquisition of antibodies against placental parasites, these antibodies are thought to be involved in protection during subsequent pregnancies.
Methods
To investigate the development of a cellular response to placental parasites during pregnancy, peripheral blood mononuclear cells were collected from women at the time of their confinement. The study was performed in Cameroon where malaria transmission is perennial. In vitro cell proliferation and cytokine production were measured in response to non-malarial activators (concanavalin A and PPD), a recombinant protein from P. falciparum MSP-1, and erythrocytes infected by two P. falciparum lines, RP5 and W2. Like placental parasites, the RP5 line, but not W2, adheres to chondroitin sulfate A and to syncytiotrophoblasts.
Results
The proliferative response to all antigens was lower for cells obtained at delivery than 3 months later. Most interestingly, the cellular response to the RP5 line of P. falciparum was closely related to parity. The prevalence rate and the levels of response gradually increased with the number of previous pregnancies. No such relationship was observed with W2 line, or MSP-1 antigen.
Conclusions
This suggests the occurrence of an immune response more specific for the RP5 line in women having had multiple pregnancies, and who are likely to develop immunity to pregnancy-associated parasites. Both humoral and cellular mechanisms may account for the lower susceptibility of multigravidae to malaria.
Background
Malaria during pregnancy is a priority area for malaria research and control. Placental malaria is a common complication of pregnancy-associated malaria associated with foetal abortion and stillbirth, and is responsible for low birth weight of the offspring. Several epidemiological studies demonstrated these effects are most frequent and marked in primigravidae [1,2]. The factors responsible for the increase in malaria susceptibility during pregnancy, particularly during the first pregnancy, have not been fully elucidated.
Pregnancy-associated malaria is characterized by the sequestration of Plasmodium falciparum-infected erythrocytes in placental intervillous spaces. Placental parasites appear to express a specific phenotype defined by the adherence of P. falciparum-infected erythrocytes to the glycosaminoglycans chondroitin sulfate A (CSA) and hyaluronic acid, expressed by the syncytiotrophoblast [3,4]. Parasite adhesion to CSA is mediated by P. falciparum-variant surface antigens (VSAs) expressed on the surface of infected erythrocytes [5,6]. Placental parasites bind to CSA and not to other VSAs ligands, such as CD36 and ICAM-1, whereas the opposite is true for most parasite isolates from non-pregnant individuals [3,4,7]. The expression of this particular VSA by parasites binding to CSA in the placenta is thought to be the basis for the higher susceptibility of primigravidae. These women have not been exposed to and have no immunity to such parasites before becoming pregnant. The first pregnancy enables them to develop specific immunity, which protects them during subsequent pregnancies. This hypothesis is supported by the fact that multigravid pregnant women in endemic areas have antibodies to placental parasites acquired during previous malaria-infected pregnancies [8], and that these antibodies inhibit the cytoadherence of placental parasites to human syncytiotrophoblasts [9].
Pregnancy is characterised by a transient depression of cell-mediated immunity of the woman that allows foetal allograft retention, but also interferes with resistance of the mother to various infectious diseases [10]. Cellular immune responses to P. falciparum antigens are depressed in pregnant women in comparison with non-pregnant controls [11,12]. This pregnancy-induced immunosuppression may persist several months after delivery restoration of immune responses to P. falciparum antigens being observed within six months after delivery [13,14].
In this study, the cellular responses in women at the time of delivery as well as in women 3 months after delivery were assessed. In vitro lymphocyte proliferation and cytokine production were measured for cells obtained from women living in Yaounde, Cameroon, where malaria transmission is perennial. The results were analysed according to parasite infection of the placenta and gravidity status.
Materials and Methods
Study site, donors and blood sample collection
The study took place in Yaounde, Cameroon, a rainforest area with hyperendemic P. falciparum malaria and perennial transmission. Women delivering at the maternity wards of Nkoldongo and Djoungolo between June 1998 and January 1999 were enrolled after informed consent was obtained. To aid the identification of women with a placental malaria infection, a thick peripheral blood smear was obtained before delivery and examined for parasites. Following identification of an infected individual, the next uninfected woman of same parity presenting to the maternity wards was also enrolled. Term placentas and 10 ml heparinized venous blood samples were collected immediately after delivery. Thick smears of blood from the maternal side of the placenta were examined for the presence of pigment, parasites, or both. Another group of women were studied three (± 1) months after delivery, and 10 ml heparinized venous blood sample was drawn.
Antigens
Concanavalin A (CA; Sigma, St Louis, Mo) was used at a final concentration of 10 μg/ml. The recall antigen tuberculin purified protein derivative (PPD; Statens Serum Institut, Copenhagen, Denmark) was used at a final concentration of 12.5 μg/ml. The Escherichia coli-expressed rMSP119 protein based on P. falciparum Merozoite Surface Protein 1 (MSP-1), was cleaved from a GST-fusion protein and used at a final concentration of 5 μg/ml [15].
Two P. falciparum lines were used as antigens following in vitro culture. Erythrocytes infected with the RP5 P. falciparum line (a gift from Dr. J. Gysin, Laboratoire de Génétique et d'Immunologie, Marseille, France) bind to CSA and not to other known receptors of P. falciparum [16] and, consequently, bind to human syncytiotrophoblasts [17], as do pregnancy-associated parasites [8]. Fortnightly, the binding phenotype was selected and checked using CSA-coated Petri dishes [9]. The unselected W2 P. falciparum line does not bind to CSA, nor to human syncytiotrophoblasts (data not shown). Infected erythrocytes were obtained from unsynchronised in vitro cultures at a 5 % haematocrit in RPMI 1640 Hepes and 10 % heat-inactivated human AB serum. Cells were harvested at 5 % parasite density by centrifugation and used to stimulate cultures at 10 % haematocrit. A 100 μl aliquot, corresponding to 5.106 parasitized erythrocytes, was placed in each well.
Isolation and culture of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated on Ficoll-Paque® (Pharmacia, Uppsala, Sweden), and cell viability was confirmed by trypan-blue staining. Purified PBMC were suspended at 106 cells/ml in buffered RPMI 1640 containing 10 % human serum, and 100 μl aliquots were placed in triplicate in round bottom 96-well plates. Antigens, infected erythrocytes, non-infected erythrocytes (NIE), or medium alone were added in 100 μl amounts at the indicated concentrations. Plates were incubated at 37°C in a humidified chamber with 5% CO2 for 3 or 6 days. The 3- and 6-day culture supernatants from each triplicate were pooled and stored at -80°C.
After 6 days, 110 μl were removed from culture supernatants and 50 μl fresh medium containing 0.5 μCi methyl-[3H]-thymidine (specific activity 2 Ci/mmole, Amersham, Les Ulis, France) were added to each well. After a further 16 hr, cells were collected on glass-fiber filters and incorporated radioactivity was measured. Stimulation indices (SI) were calculated by dividing the geometric mean cpm of antigen-stimulated cultures by the geometric mean cpm of unstimulated cultures. A positive response for all antigens was defined as a SI = 2.5 [12].
Measurement of cytokine levels in supernatants
IL-2, IL-4, IL-10, and IFN-γ concentrations were measured in culture supernatants by a sandwich ELISA method, using manufacturer's instructions (CLB Amsterdam, The Netherlands). The detection limit was 5 pg/mL for IL-2, 0.4 pg/mL for IL-4, 3 pg/mL for IL-10, and 2 pg/mL for IFN-γ. Values below this threshold were assigned a value of half the detection limit. For statistical analysis, cytokine concentrations in supernatants of unstimulated cultures or in the presence of NIE were subtracted from the values obtained for supernatants of antigen-stimulated cultures, and resulting values were used to define responders and non-responders.
Statistical analyses
Differences in prevalence rates between groups were tested by the Chi-square test or the Chi-square test for trend. Differences between groups were analysed by non-parametric methods (Mann-Whitney U-test). A logistic regression using the maximum likelihood ratio method (LR procedure of BMDP) was used to adjust immune responses simultaneously on parity status and infection or age. The significance limit was p = 0.05.
Results
A total of 47 women were enrolled at delivery, and 41 (including 15 of these) were investigated 3 months after delivery. Among the former, 17 were primigravidae (mean age: 18.8 ± 1.7 years; 7 having presented with a placental infection) and 30 multigravidae (mean age: 26.1 ± 4.2 years; 15 having presented with a placental infection). Among the latter, 17 were primigravidae (mean age: 20.8 ± 3.6 years; 9 having presented with a placental infection) and 24 multigravidae (mean age: 26.1 ± 5.0 years; 12 having presented with a placental infection). The two groups of women investigated at delivery and 3 months after delivery were similar, as regards to age, parity, area of residence, and season of bleeding (all p > 0.2).
In the presence of concanavalin A and PPD, lymphocytes from most women proliferated whether these cells were collected at delivery or 3 months later. Both the prevalence rates and the mean levels in the proliferation assay were higher 3 months after delivery than at delivery (Table 1). The prevalence rate of women whose cells proliferated in response to MSP-1 or RP5, but not to W2, was also significantly higher 3 months after delivery than at delivery. Similarly, the mean level of responses was higher. No differences were found on proliferation responses at delivery according to parity and malaria status (all p > 0.1), whereas these differences in response to RP5 after delivery appeared to be related to both the occurrence of a P. falciparum infection of the placenta, and to the parity status of the woman. Three months after delivery, cell proliferation in response to RP5 was observed in 53 % of the mothers who had a parasite-positive placenta at delivery, while cells from only 17 % of the other women proliferated (p = 0.02) (see Fig. 1A). The mean levels of responses to RP5 were also increased in women who had presented with a malarial infection of the placenta three months earlier (2.4 (1.4 – 4.0) vs. 1.4 (1.0 – 1.9), Mann-Whitney U-test, p = 0.07). Likewise, cells from 50 % of multiparous women proliferated in response to RP5, while those of only 18 % of primiparous proliferated (p = 0.04) (Fig. 1B). This difference was also observed for the mean level of response, which was higher in multiparous women (2.3 (1.4 – 3.7) vs. 1.4 (1.0 – 2.0), Mann-Whitney U-test, p = 0.08). Logistic regression demonstrated that both placental infection (p = 0.03) and parity (p = 0.05) were independent predictors of the proliferative response to RP5. No such difference was observed with either the MSP-1 antigen or the W2 P. falciparum strain.
Table 1.
In vitro proliferative responses of peripheral blood mononuclear cells collected at delivery and 3 months later from Cameroonian womena.
| Delivery (n = 47) | 3 months after (n = 41) | Pb | Pc | |||
| %+ | Mean | %+ | Mean | %+ | Mean | |
| ConA | 76.6 | 7.0 (5.1–9.7) | 88.9 | 9.3 (6.5–13.4) | 0.13 | 0.25 |
| PPD | 85.1 | 10.4 (7.5–14.6) | 100.0 | 24.4 (18.6–32.0) | 0.016 | 0.0005 |
| MSP1 | 8.1 | 0.9 (0.7–1.1) | 32.4 | 1.6 (1.1–2.3) | 0.002 | 0.05 |
| RP5 | 17.9 | 1.2 (0.9–1.7) | 35.1 | 1.9 (1.4–2.5) | 0.05 | 0.09 |
| W2 | 10.9 | 1.1 (0.9–1.4) | 16.2 | 0.9 (0.6–1.3) | 0.47 | 0.44 |
a Data are expressed as prevalence rates and geometric means (95 % confidence interval) of stimulation indices. >b P value of the difference in prevalence rates, Chi2 test c P value of the difference in mean levels, Mann-Whitney U-test
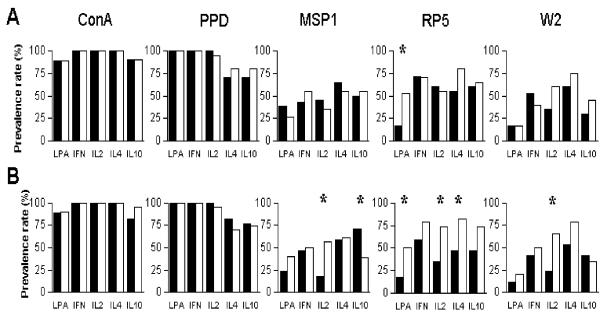
Figure 1.
In vitro proliferative (LPA) and cytokine prevalence rates of response of peripheral blood mononuclear cells from Cameroonian women 3 months after delivery. Panel A, women having presented (n = 20, white) or not (n = 21, black) with a P. falciparum infection of the placenta; panel B, primiparae (n = 17, black) and multiparae (n = 24, white). Stars indicate significant differences (Chi2 test). ConA: concanavalin A; PPD: tuberculin purified protein derivative; MSP1: recombinant protein from P. falciparum merozoite surface protein-1; RP5 and W2: erythrocytes infected with the RP5 and W2 P. falciparum lines, respectively.
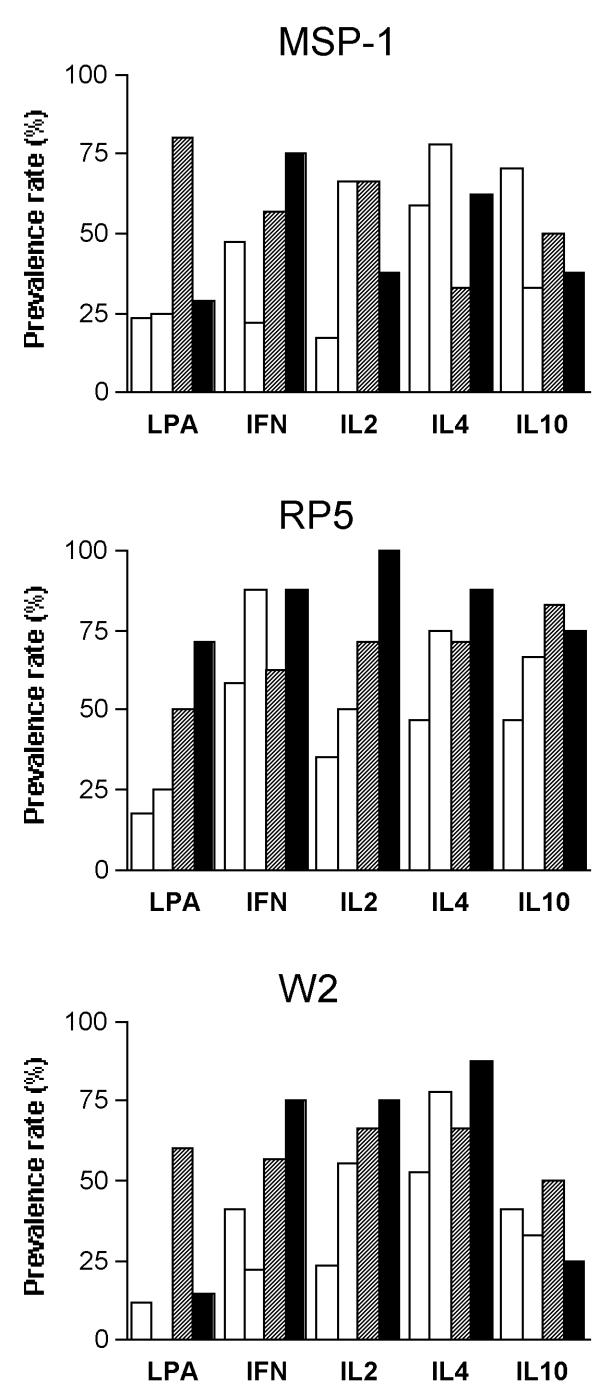
The in vitro production of IFN-γ, IL-2, IL-10, and IL-4 was investigated three months after delivery and compared according to parity and to placenta malaria status at delivery. No difference in cytokine response was observed with respect to placenta malarial status at delivery (i. e. with or without placental infection) (Fig. 1A). However, considering the parity status (primiparous versus multiparous) differences were observed in cytokine production, especially in response to RP5 (Fig. 1B). The prevalence rates of IL-2 production were increased in multiparous women (all p = 0.05) (Fig. 1B) in response to the three malaria antigens, while no differences were observed with concanavalin A or PPD. The prevalence of production of IL-2 and IL-4 by cells from multiparous women was twice as high (both p < 0.02), and a similar trend was observed for IFN-γ and IL-10, although the differences did not reach significance (p = 0.16 and 0.08 respectively). Moreover, the prevalence rate of responses to RP5 gradually increased with parity (Fig. 2), this increase being significant for lymphocyte proliferation and IL2 and IL4 production (Chi2 test for trend, p = 0.01, 0.002 and 0.05, respectively). Such parity-related changes were not observed with the other malarial antigens. Among multiparous women, except in the case of IL2 production in presence of W2, these responses did not increase with parity (Fig. 2). The prevalence rates of IL-10 response to MSP-1 decreased with parity (p = 0.04).
Figure 2.
In vitro proliferative and cytokine prevalence rates of response to MSP1 (recombinant protein from P. falciparum Merozoite Surface Protein-1), or to erythrocytes infected with the RP5 or the W2 P. falciparum strain from peripheral blood mononuclear cells collected 3 months after first (n = 17, white), second (n = 9, grey), third (n = 7, stripped), or = fourth (n = 8, black) delivery in Cameroonian women.
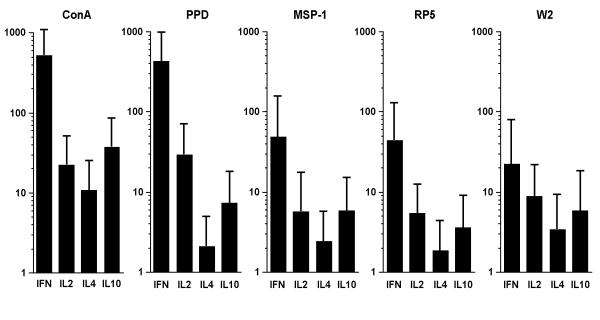
The geometric mean levels of production of all cytokines in response to concanavalin A, as well as those of IFN-γ and IL-2 in response to PPD were 10 times higher than responses to the three malaria antigens (Fig. 3). The level of production of these cytokines in response to RP5 was also increased also two to six times higher in cells from multiparous women, and the difference was significant for IL-2 and IL-4 (5.1 vs 1.5 pg/ml, 5.3 vs 0.5 pg/ml respectively; all p < 0.04). In contrast, the responses to the other antigens were only moderately affected by the parity status. IL2 production in the presence of RP5 or W2 also increased with age in an univariate analysis, but logistic regression demonstrated that parity remained the only independent predictor of the IL2 response to RP5 (p = 0.02) and W2 (p = 0.005).
Figure 3.
In vitro cytokine production in response to Con A: concanavalin A; PPD: tuberculin purified protein derivative; MSP1: recombinant protein from P. falciparum Merozoite Surface Protein-1; RP5 and W2: erythrocytes infected with the RP5 and W2 P. falciparum strains, respectively from peripheral blood mononuclear cells collected 3 months after delivery in Cameroonian women. Figure shows the geometric means of responders and the 95% CI.
Discussion
Pregnancy is an immunological balancing act in which the mother's immune system has to remain tolerant to the foetus and yet maintain immune competence for defense against microorganisms. Several studies have documented a decrease of cell-mediated immune responses to malaria parasite antigens during pregnancy [9,13,18] and early post-partum [11,14] in association with increased susceptibility to malaria. Consequently, the exploration of P. falciparum specific immune responses performed during this time is unlikely to produce results that are easy to interpret. Our rationale for not investigating cytokine secretion at delivery, but for performing full analysis of the cellular immune response three months after delivery, was that we expected that immunosuppression would be abolished by this time. Indeed, we confirmed an important systemic lowering of the malaria-specific and non-specific cellular responses, as the proliferative response of peripheral blood mononuclear cells was highly reduced at delivery, as compared to three months later.
Regarding the relationship between parity status and cellular responses to the three P. falciparum antigens used, different profiles were observed. IL2 prevalence rate in response to all malaria antigens gradually increased with the number of previous parities, independent of age, suggesting the construction of T cell memory during successive pregnancies. In response to the RP5 line, the prevalence rate and the level of response increased with the number of previous parities in most of the assays used. This relationship was only evident for IL2 in responses to the other P. falciparum line or a recombinant protein based on MSP-1, an antigen shared by all P. falciparum lines. This demonstrates the occurrence of an immune response that is more specific for a parasite phenotype expressed by the RP5 line in women who have had repeated pregnancies. The RP5 line of P. falciparum expresses the specific cytoadherence properties of the pregnancy-associated parasites, whereas the W2 line does not cytoadhere to syncytiotrophoblast (Maubert, unpublished data). However, P. falciparum lines might not be the most appropriate tool to dissect the immune response, as numerous antigens are implicated.
Pregnancy-associated parasites adhere to CSA expressed on the placental endothelium, representing a phenotype that is not shared with parasites isolated from non-pregnant hosts, but that is expressed by the RP5 line. Several molecules on the surface of infected erythrocytes suggested as acting as counter-receptors for these epithelial molecules and the major adherence receptor appears to be PfEMP-1 [6]. PfEMP-1 molecules are highly antigenic and are encoded by a diverse multigene family, and expression on the surface of erythrocytes can switch during the course of a clonal infection [19]. The hypothesis [3] that the strains of P. falciparum implicated in placental malaria are representative of a unique sub-population, was recently supported by the demonstration that the expressed PfEMP-1 in infected placental parasite samples are closely related to each other [20]
Among pregnant women, primiparous women with a non-infected placenta have the highest likelihood of never having been infected with pregnancy-associated parasites. However, even in these women, an infection may have occurred during early pregnancy, explaining why some of them had raised responses to the RP5 strain after delivery. The higher proportion of multiparous women that responded to the RP5 strain probably reflects a pregnancy-associated parasite-specific immunization during a previous parasitized pregnancy. This likelihood of having been infected, as well as the proportion of responders, increases with parity. In line with our data, a previous report demonstrated that the acquisition of antibodies against the RP5 line and against CSA-adhering parasites correlated with the number of past pregnancies [8,21]. Moreover, antibodies directed against CSA-adhering parasites and acquired during an infected pregnancy inhibited the cytoadherence of CSA-adhering parasites to human syncytiotrophoblasts [22]. It is likely that women having multiple pregnancies, who develop an immune response to RP5, are developing immunity to pregnancy-associated parasites, thereby decreasing the risk of pregnancy-associated malaria during subsequent pregnancies. Moore et al. [23] proposed a model in which re-circulation of memory T lymphocytes from the intervillous blood to local lymphoid tissue facilitates the maintenance of local immunity. Both cellular and humoral components may account for the lower susceptibility of multigravidae to malaria.
Understanding the mechanisms involved in protection of multigravidae to placental malaria will have consequences in public health management, both in terms of drug-based control measures and malaria vaccines. Indeed, to limit costs, some countries advise restricting drug prophylaxis against malaria during pregnancy to the primiparae, the most sensitive group of women. As the lower susceptibility of multiparae may rely on an earlier exposure and immunization against placental parasites, a total prevention of malaria during the first pregnancy may inhibit the development of immune responses against pregnancy-associated parasites. However, no difference in susceptibility to malaria was observed during the second pregnancy of women who had or had not received drug prophylaxis during their first pregnancy [24]. This is probably because, in areas in which malaria is endemic, prophylaxis is rarely taken before the fifth or sixth month of pregnancy, and its efficacy is frequently impaired by drug resistance. It is likely that drug prophylaxis will limit placental colonization, without inhibiting the development of specific immunity.
The consequence of our findings has implications for the design of a malaria vaccine. Bull and colleagues reported that protection against disease is dependent on a variant-specific immune response directed against VSAs that probably include members of the PfEMP-1 family [25]. This supports the idea that PfEMP-1 could be used to develop an anti-disease vaccine, although the large size of the PfEMP-1 family raises doubts about the feasibility of this approach. In the case of pregnancy-associated malaria, the number of antigenic variants may be very small, and therefore PfEMP-1 may be a good vaccine candidate for pregnancy-associated malaria. Further studies using specific antigen from placental malaria parasites will provide additional information about the development of both cellular and humoral immunity in pregnant women.
Authors' contributions
Author : 1 N F conceived of the study, carried out the studies, the immunology tests, the immunoassays and the statistical analysis and drafted the manuscript.
Author 2: G T participated in the immunological tests.
Author 3 : B M participated in the immunological tests.
Author 4 : M M participated in the immunoassays
Author 5 : I S produced the recombinant protein preparation
Author 6 : MC participated in the design of the study and statistical analysis
Author 7 : A A H produced the recombinant protein preparation
Author 8 : GC participated in the design of the study
Author 9 : PD participated in the design of the study, statistical analysis, drafted the manuscript and coordinated the study.
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgments
This work was supported by IRD, AUPELF/UREF, INSERM, and EU Contracts TS3CT94 0272 and QLK2-CT-1999-01293. B. M. was the recipient of a fellowship from the French Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche.
We acknowledge the strong support of the staff of the maternity clinics of Nkolndongo and of the Djoungolo Hospital, who were in charge of the collection of biological samples and other data. We are very grateful to the mothers. We are also grateful to André Garcia for help in statistical analysis, and to Christian Boudin for his support and scientific advice.
Contributor Information
Nadine Fievet, Email: Nadine.Fievet@ird.sn.
Germaine Tami, Email: oceac@comnet.com.
Bertrand Maubert, Email: bmaubert@free.fr.
Marlène Moussa, Email: marlene@magic.fr.
Ian K Shaw, Email: ishaw@nimr.mrc.ac.uk.
Michel Cot, Email: Michel.cot@tnn.ap-hop-paris.fr.
Anthony A Holder, Email: aholder@nimr.mrc.ac.uk.
Gérard Chaouat, Email: Gerardchaouatg@aol.com.
Philippe Deloron, Email: Philippe.Deloron@ird.fr.
References
- McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg. 1984;33:517–25. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- Brabin B. An assessment of low birthweight risk in primiparae as an indicator of malaria control in pregnancy. Int J Epidemiol. 1991;20:276–83. doi: 10.1093/ije/20.1.276. [DOI] [PubMed] [Google Scholar]
- Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- Beeson JG, Rogerson SJ, Cooke BM, Reeder JC, Chai W, Lawson AM, Molyneux ME, Brown GV. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffet PA, Gamain B, Scheidig C, Baruch D, Smith JD, Hernandez-Rivas R, Pouvelle B, Oishi S, Fujii N, Fusai T, et al. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: A receptor for human placental infection. Proc Natl Acad Sci USA. 1999;96:12743–8. doi: 10.1073/pnas.96.22.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder JC, Cowman AF, Davern KM, Beeson JG, Thompson JK, Rogerson SJ, Brown GV. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc Natl Acad Sci USA. 1999;96:5198–202. doi: 10.1073/pnas.96.9.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubert B, Fievet N, Tami G, Boudin C, Deloron P. Cytoadherence of Plasmodium falciparum-infected erythrocytes in the human placenta. Parasite Immunol. 2000;22:191–9. doi: 10.1046/j.1365-3024.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infect Immun. 1999;67:5367–71. doi: 10.1128/iai.67.10.5367-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, Hviid L. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–16. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- Meeusen EN, Bischof RJ, Lee CS. Comparative T-cell responses during pregnancy in large animals and humans. Am J Reprod Immunol. 2001;46:169–79. doi: 10.1111/j.8755-8920.2001.460208.x. [DOI] [PubMed] [Google Scholar]
- Riley EM, Schneider G, Sambou I, Greenwood BM. Suppression of cell-mediated immune responses to malaria antigens in pregnant Gambian women. Am J Trop Med Hyg. 1989;40:141–4. doi: 10.4269/ajtmh.1989.40.141. [DOI] [PubMed] [Google Scholar]
- Fievet N, Cot M, Chougnet C, Maubert B, Bickii J, Dubois B, Lehesran JY, Frobert Y, Migot F, Romain F, et al. Malaria and pregnancy in Cameroonian primigravidae – humoral and cellular immune responses to Plasmodium falciparum blood-stage antigens. Am J Trop Med Hyg. 1995;53:612–7. doi: 10.4269/ajtmh.1995.53.612. [DOI] [PubMed] [Google Scholar]
- Fievet N, Cot M, Ringwald P, Bickii J, Dubois B, Le Hesran JY, Migot F, Deloron P. Immune response to Plasmodium falciparum antigens in Cameroonian primigravidae: evolution after delivery and during second pregnancy. Clin Exp Immunol. 1997;107:462–7. doi: 10.1046/j.1365-2249.1997.d01-966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagne N, Rogier C, Sokhna CS, Tall A, Fontenille D, Roussilhon C, Spiegel A, Trape JF. Increased susceptibility to malaria during the early postpartum period. N Engl J Med. 2000;343:598–603. doi: 10.1056/NEJM200008313430901. [DOI] [PubMed] [Google Scholar]
- Burghaus PA, Holder AA. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol. 1994;64:165–9. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Gay F, Robert C, Pouvelle B, Peyrol S, Scherf A, Gysin J. Isolation and characterization of brain microvascular endothelial cells from Saimiri monkeys. An in vitro model for sequestration of Plasmodium falciparum-infected erythrocytes. J Immunol Methods. 1995;184:15–28. doi: 10.1016/0022-1759(95)00070-Q. [DOI] [PubMed] [Google Scholar]
- Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–7. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed FN, Bulmer JN, Dunn DT, Menendez C, Jawla MF, Jepson A, Jakobsen PH, Greenwood BM. Suppressed peripheral and placental blood lymphoproliferative responses in first pregnancies: relevance to malaria. Am J Trop Med Hyg. 1993;48:154–60. doi: 10.4269/ajtmh.1993.48.154. [DOI] [PubMed] [Google Scholar]
- Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Khattab A, Kun J, Deloron P, Kremsner PG, Klinkert MQ. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J Inf Dis. 2001;183:1165–9. doi: 10.1086/319288. [DOI] [PubMed] [Google Scholar]
- Staalsoe T, Giha HA, Dodoo D, Theander TG, Hviid L. Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry. 1999;35:329–36. doi: 10.1002/(SICI)1097-0320(19990401)35:4<329::AID-CYTO5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, Taylor DW, Deloron P, Hviid L. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Inf Dis. 2001;184:618–26. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- Moore JM, Nahlen BL, Lal AA, Udhayakumar V. Immunologic memory in the placenta: a lymphocyte recirculation hypothesis. Med Hypotheses. 2000;54:505–510. doi: 10.1054/mehy.1999.0888. [DOI] [PubMed] [Google Scholar]
- Greenwood AM, Menendez C, Alonso PL, Jaffar S, Langerock P, Lulat S, Todd J, M'Boge B, Francis N, Greenwood BM. Can malaria chemoprophylaxis be restricted to first pregnancies? Trans R Soc Trop Med Hyg. 1994;88:681–2. doi: 10.1016/0035-9203(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Bull PC, Lowe BS, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–9. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]