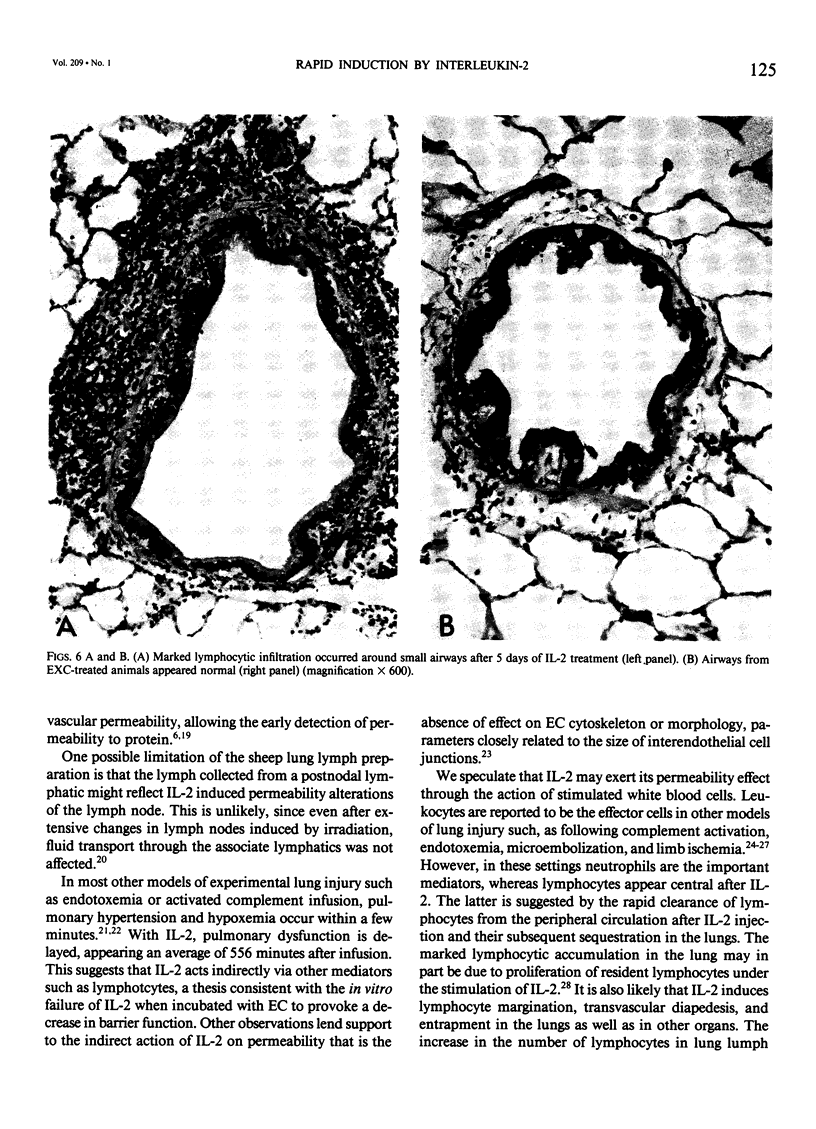
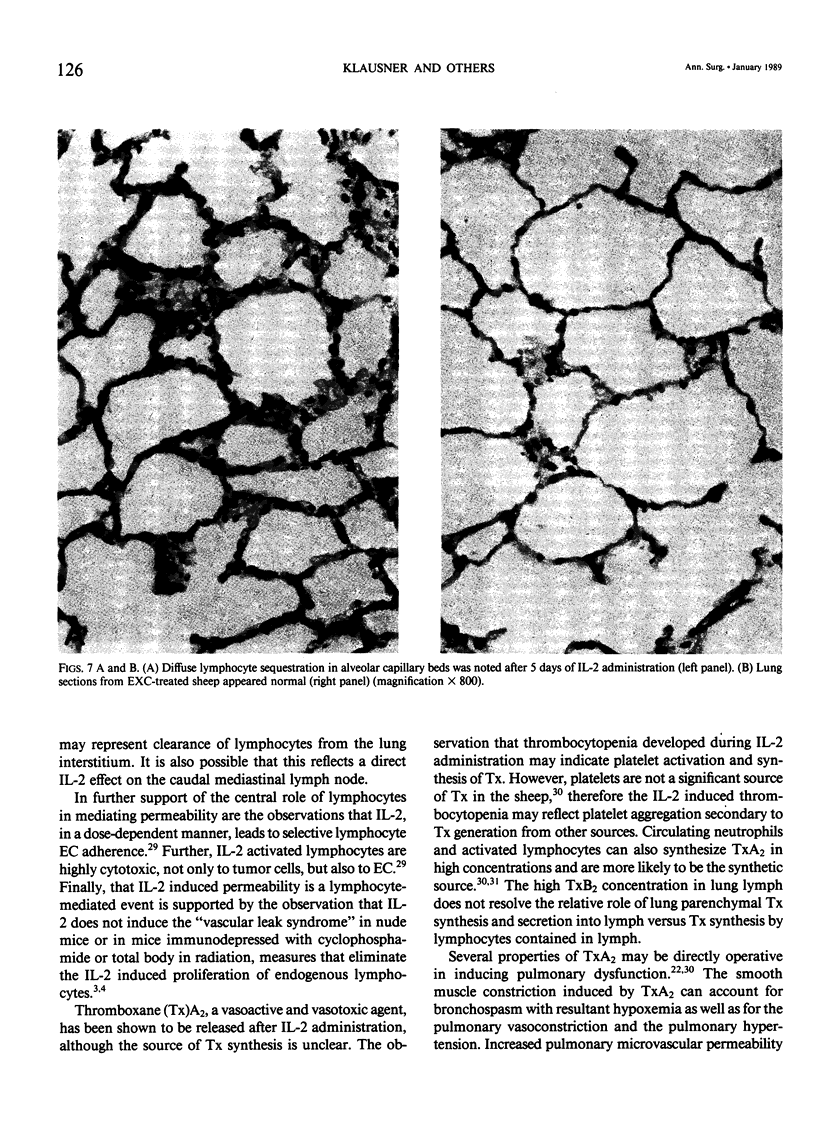
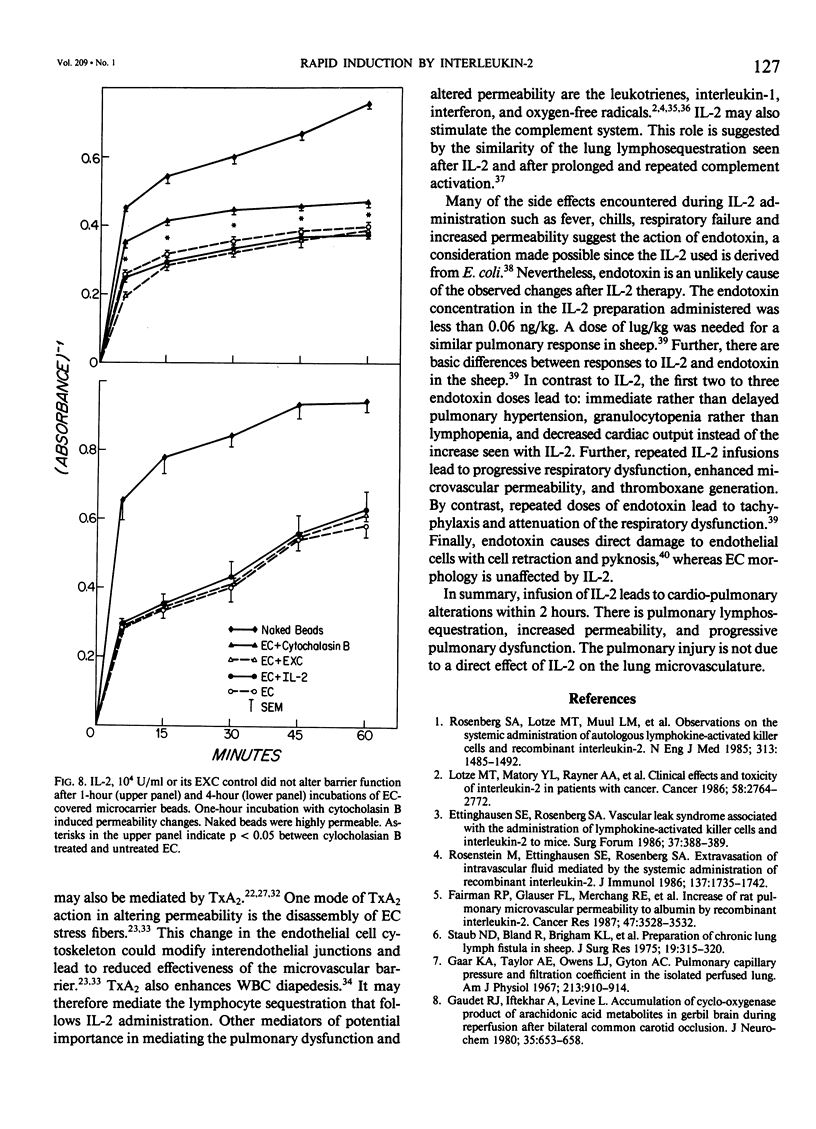
Abstract
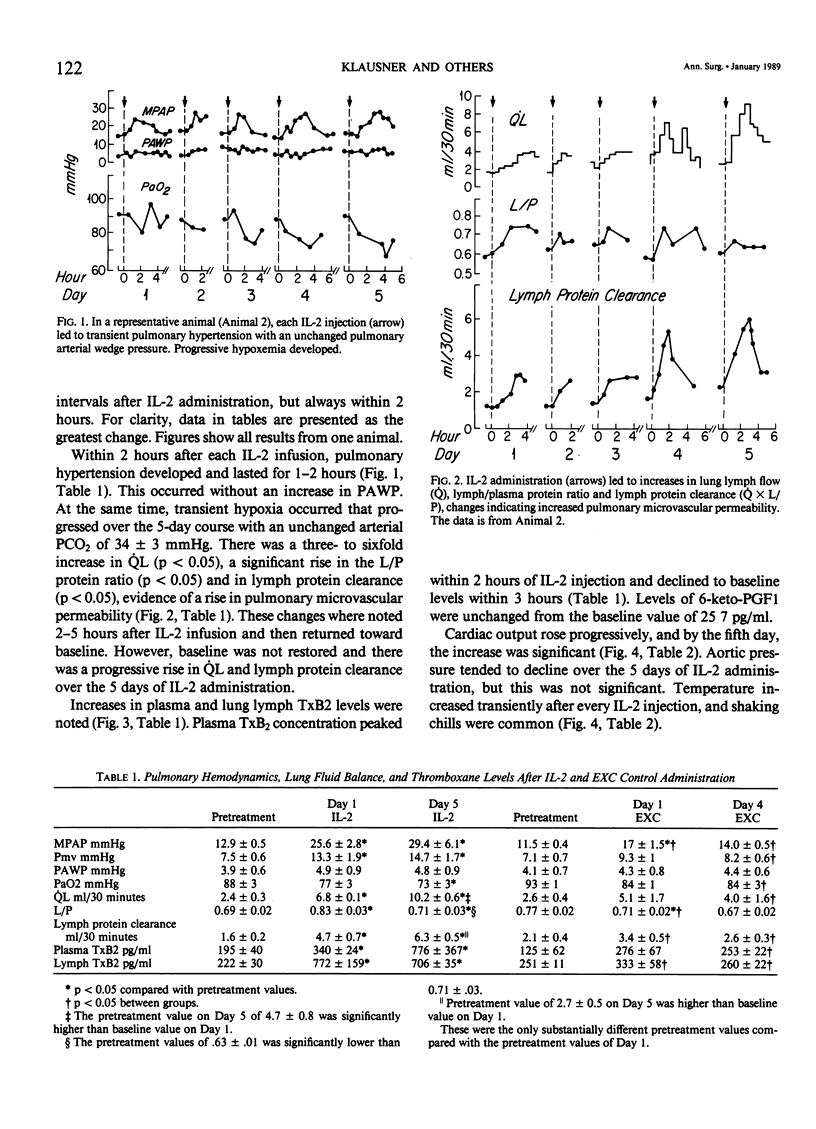
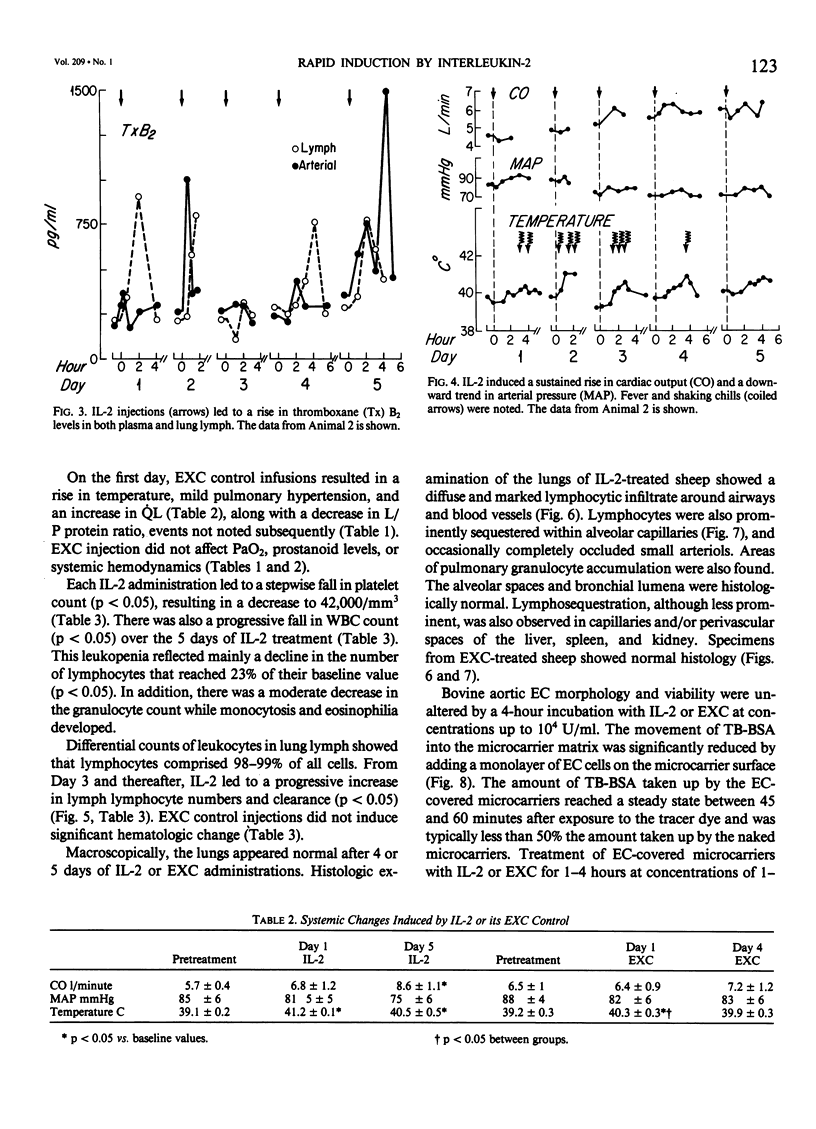
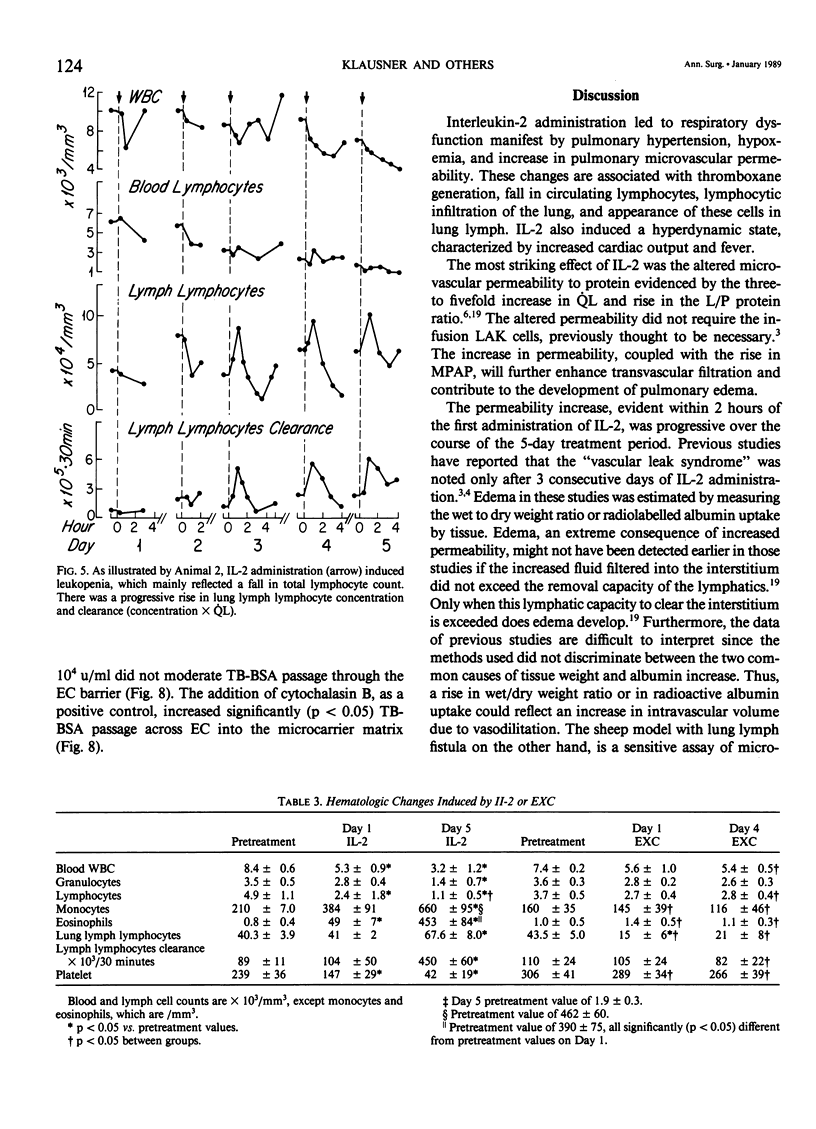
The clinical use of interleukin-2 (IL-2) is limited by severe cardiopulmonary dysfunction. This study examines the mechanism of respiratory failure related to IL-2, using sheep with chronic lung lymph fistulae. Awake animals were infused with an intravenous (I.V.) bolus of IL-2 10(5) U/kg (n = 5) or its excipient (EXC) control (n = 3), every 8 hours for 4 to 5 days. Cardiopulmonary function was monitored daily for at least one 8-hour period. Within 2 hours after each IL-2 administration, mean pulmonary arterial pressure (MPAP) rose. On Day 1, the mean rise was from 13 to 26 mmHg (p less than 0.05), and on Day 5, to 29 mmHg (p less than 0.05). MPAP returned to baseline levels after 2-3 hours. Pulmonary arterial wedge pressure was unchanged from 4 mmHg. There were transient falls in arterial oxygen tension, from 88 to 77 mmHg on Day 1 and to 73 mmHg (p less than 0.05) on Day 5. Lung lymph flow (QL) rose from 2.4 to 6.8 ml/30 minutes (p less than 0.05) on Day 1, and from 4.7 to 10.2 ml/30 minutes (p less than 0.05) on Day 5, whereas the lymph/plasma protein ratio increased on Day 1 from 0.69 to 0.83 (p less than 0.05) and from 0.63 to 0.71 (p less than 0.05) on Day 5. This documents an increase in pulmonary microvascular permeability. Thromboxane (Tx)B2 levels increased transiently after each IL-2 injection in plasma from 195 to 340 pg/ml (p less than 0.05) and in lung lymph from 222 to 772 pg/ml (p less than 0.05) on Day 1, and to similar levels on Day 5. There was a progressive rise in cardiac output from 5.7 to 8.6 1/minute (p less than 0.05) during the 5 days of infusion. Systemic blood pressure did not change. Temperature rose from 39.1 to 41.2 C (p less than 0.05), and shaking chills were common. There was a progressive fall in leukocyte count, from 8.4 to 3.2 X 10(3)/mm3 (p less than 0.05) by Day 5, reflecting a 77% fall in lymphocytes. Lung lymph lymphocyte counts rose, and lymphocyte clearance increased.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boiadjieva S., Hallberg C., Högström M., Busch C. Methods in laboratory investigation. Exclusion of trypan blue from microcarriers by endothelial cells: an in vitro barrier function test. Lab Invest. 1984 Feb;50(2):239–246. [PubMed] [Google Scholar]
- Bottaro D., Shepro D., Peterson S., Hechtman H. B. Serotonin, norepinephrine, and histamine mediation of endothelial cell barrier function in vitro. J Cell Physiol. 1986 Aug;128(2):189–194. doi: 10.1002/jcp.1041280208. [DOI] [PubMed] [Google Scholar]
- Bowers T. K., Ozolins A. L., Ratliff N. B., Jacob H. S., Hammerschmidt D. E. Hyperacute pulmonary vasculitis in rabbits receiving prolonged infusions of activated complement. A possible model for triggering events in adult respiratory distress syndrome. Inflammation. 1983 Mar;7(1):1–13. doi: 10.1007/BF00918003. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Bowers R., Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979 Aug;45(2):292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V., Bender J. R., Bradley E. C. Interleukin 2-activated human lymphocytes exhibit enhanced adhesion to normal vascular endothelial cells and cause their lysis. J Immunol. 1987 Mar 15;138(6):1779–1785. [PubMed] [Google Scholar]
- Demling R. H., Lalonde C. C., Jin L. J., Albes J., Fiori N. The pulmonary and systemic response to recurrent endotoxemia in the adult sheep. Surgery. 1986 Nov;100(5):876–883. [PubMed] [Google Scholar]
- Edidin M. A rapid, quantitative fluorescence assay for cell damage by cytotoxic antibodies. J Immunol. 1970 May;104(5):1303–1306. [PubMed] [Google Scholar]
- Ettinghausen S. E., Lipford E. H., 3rd, Mulé J. J., Rosenberg S. A. Systemic administration of recombinant interleukin 2 stimulates in vivo lymphoid cell proliferation in tissues. J Immunol. 1985 Aug;135(2):1488–1497. [PubMed] [Google Scholar]
- Fairman R. P., Glauser F. L., Merchant R. E., Bechard D., Fowler A. A. Increase of rat pulmonary microvascular permeability to albumin by recombinant interleukin-2. Cancer Res. 1987 Jul 1;47(13):3528–3532. [PubMed] [Google Scholar]
- Gaar K. A., Jr, Taylor A. E., Owens L. J., Guyton A. C. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am J Physiol. 1967 Oct;213(4):910–914. doi: 10.1152/ajplegacy.1967.213.4.910. [DOI] [PubMed] [Google Scholar]
- Gaudet R. J., Alam I., Levine L. Accumulation of cyclooxygenase products of arachidonic acid metabolism in gerbil brain during reperfusion after bilateral common carotid artery occlusion. J Neurochem. 1980 Sep;35(3):653–658. doi: 10.1111/j.1471-4159.1980.tb03704.x. [DOI] [PubMed] [Google Scholar]
- Geczy C. L. The role of lymphokines in delayed-type hypersensitivity reactions. Springer Semin Immunopathol. 1984;7(4):321–346. doi: 10.1007/BF00201965. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goetzl E. J. Selective feed-back inhibition of the 5-lipoxygenation of arachidonic acid in human T-lymphocytes. Biochem Biophys Res Commun. 1981 Jul 30;101(2):344–350. doi: 10.1016/0006-291x(81)91266-3. [DOI] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Malik A. B. Effect of granulocytopenia on extravascular lung water content after microembolization. Am Rev Respir Dis. 1980 Oct;122(4):561–566. doi: 10.1164/arrd.1980.122.4.561. [DOI] [PubMed] [Google Scholar]
- Jovanovic D. The influence of radiation on blood vessels and circulation. Chapter VI. Lymphatics. Curr Top Radiat Res Q. 1974 Jun;10(1):85–97. [PubMed] [Google Scholar]
- Krausz M. M., Utsunomiya T., Dunham B., Valeri C. R., Shepro D., Hechtman H. B. Inhibition of permeability edema with imidazole. Surgery. 1982 Aug;92(2):299–308. [PubMed] [Google Scholar]
- Levine L., Alam I., Langone J. J. The use of immobilized ligands and [125I]protein a for immunoassays of thromboxane B2, prostaglandin D2, 13,14-dihydro-prostaglandin E2, 5,6-dihydro-prostaglandin I2, 6-keto-prostaglandin F1 alpha, 15-hydroxy-9 alpha, 11 alpha(epoxymethano)prosta-5,13-dienoic acid and 15-hydroxy-11 alpha,9 alpha(epoxymethano)prosta-5,13-dienoic acid. Prostaglandins Med. 1979 Mar;2(3):177–189. doi: 10.1016/0161-4630(79)90035-1. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Rayner A. A., Ettinghausen S. E., Vetto J. T., Seipp C. A., Rosenberg S. A. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986 Dec 15;58(12):2764–2772. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Ali M., Morgan E., Townsend E. R., Cooper J. D. Thromboxane synthesis by sources other than platelets in association with complement-induced pulmonary leukostasis and pulmonary hypertension in sheep. Circ Res. 1983 Jan;52(1):1–6. doi: 10.1161/01.res.52.1.1. [DOI] [PubMed] [Google Scholar]
- Meyrick B. O. Endotoxin-mediated pulmonary endothelial cell injury. Fed Proc. 1986 Jan;45(1):19–24. [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Siegel J. H., Cerra F. B., Coleman B., Giovannini I., Shetye M., Border J. R., McMenamy R. H. Physiological and metabolic correlations in human sepsis. Invited commentary. Surgery. 1979 Aug;86(2):163–193. [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Tahamont M. V., Gee M. H., Flynn J. T. Aggregation and thromboxane synthesis and release in isolated sheep neutrophils and lymphocytes in response to complement stimulation. Prostaglandins Leukot Med. 1984 Nov;16(2):181–190. doi: 10.1016/0262-1746(84)90070-2. [DOI] [PubMed] [Google Scholar]
- Welles S. L., Shepro D., Hechtman H. B. Eicosanoid modulation of stress fibers in cultured bovine aortic endothelial cells. Inflammation. 1985 Dec;9(4):439–450. doi: 10.1007/BF00916343. [DOI] [PubMed] [Google Scholar]
- Welles S. L., Shepro D., Hechtman H. B. Vasoactive amines modulate actin cables (stress fibers) and surface area in cultured bovine endothelium. J Cell Physiol. 1985 Jun;123(3):337–342. doi: 10.1002/jcp.1041230307. [DOI] [PubMed] [Google Scholar]