Abstract
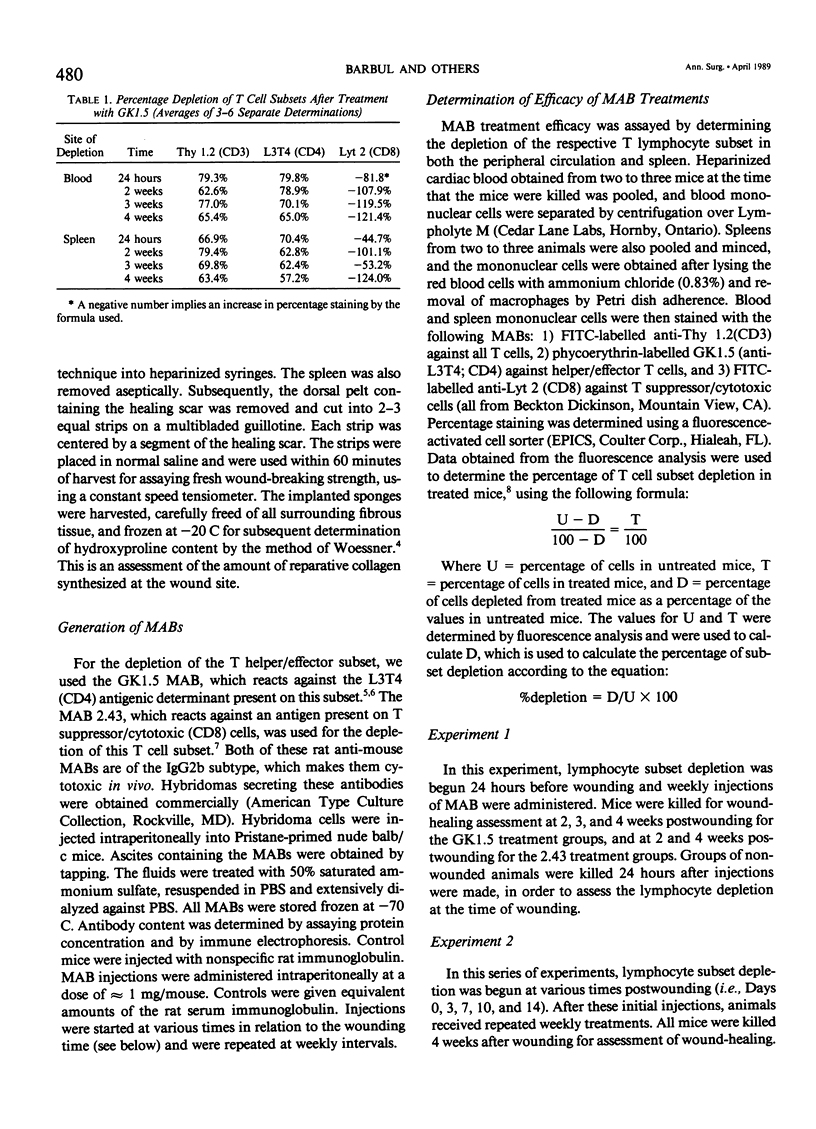
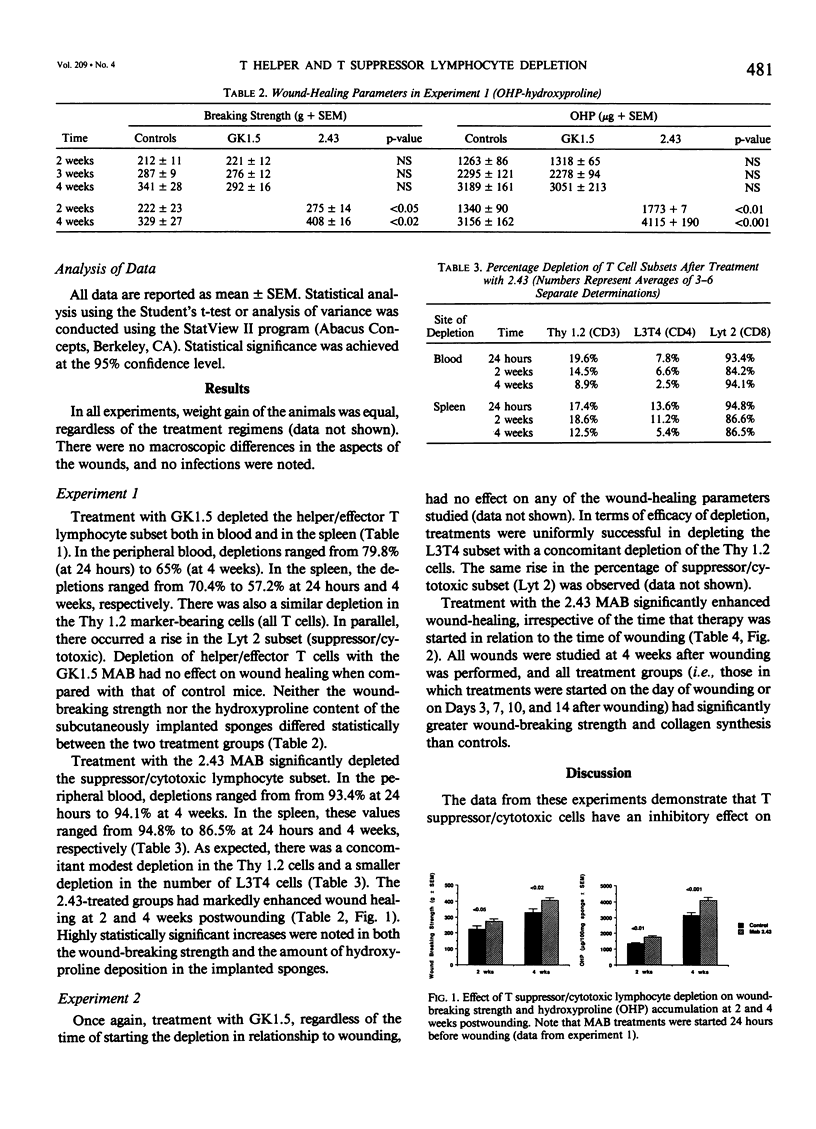
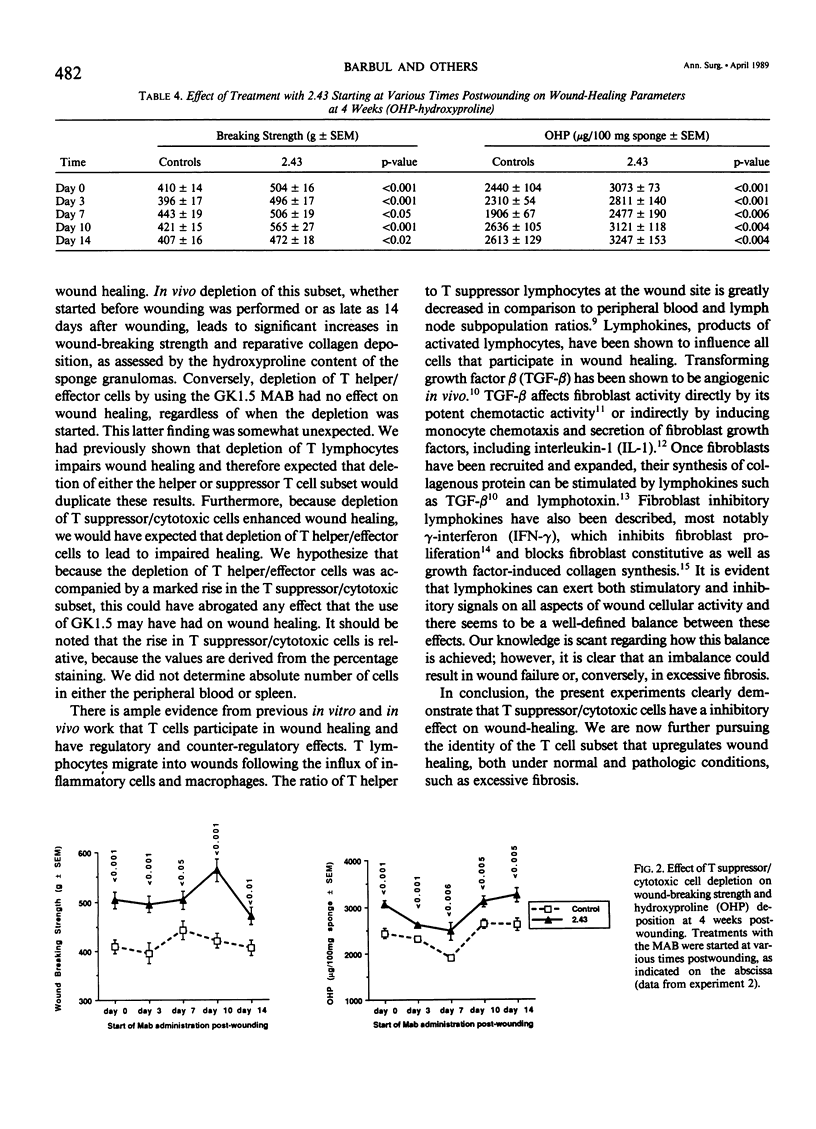
The role of T lymphocytes in wound healing is still not well-defined. Because it had been previously shown that in vivo depletion of T cells leads to impaired wound healing, the effect of depleting T cell subsets on subsequent fibroplasia was studied. T helper/effector cells were depleted by the use of the monoclonal antibody GK1.5, reactive against the L3T4 antigen (CD4). T suppressor/cytotoxic lymphocytes were depleted by using the 2.43 monoclonal antibody reactive against the Lyt 2 antigen (CD8). In the first experiment, Balb/c mice were treated with the antibodies starting at 24 hours before wounding was performed, and weekly thereafter. Depletion of the T helper/effector cells had no effect on wound-breaking strength or hydroxyproline deposition in sponge granulomas, whereas depletion of T suppressor/cytotoxic cells significantly enhanced both of these healing parameters. In a second experiment, T cell subset depletion was started on Days 0, 3, 7, 10, and 14 postwounding, and treatments were continued weekly thereafter. Once again, depletion of T helper/effector cells had no effect on wound healing, whereas depletion of T suppressor/cytotoxic cells markedly increased both wound-breaking strength and collagen synthesis. In conclusion, the data show that T suppressor/cytotoxic cells have a counter-regulatory role in wound healing, whereas the T cell subset responsible for up-regulating wound healing remains to be identified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Gamma interferon is the lymphokine and beta interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J Exp Med. 1985 Aug 1;162(2):516–527. doi: 10.1084/jem.162.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel R. S., Barbul A., Beschorner W. E., Wasserkrug H. L., Efron G. Lymphocyte participation in wound healing. Morphologic assessment using monoclonal antibodies. Ann Surg. 1987 Jul;206(1):25–29. doi: 10.1097/00000658-198707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Freundlich B., Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest. 1984 Sep;74(3):1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. M., Barbul A., Breslin R. J., Wasserkrug H. L., Efron G. Significance of T-lymphocytes in wound healing. Surgery. 1987 Aug;102(2):300–305. [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Dialynas D. P., Lancki D. W., Wall K. A., Lorber M. I., Loken M. R., Fitch F. W. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Wofsy D., Greenspan J. S., Ledbetter J. A. Treatment of autoimmune MRL/Ipr mice with monoclonal antibody to Thy-1.2: a single injection has sustained effects on lymphoproliferation and renal disease. J Immunol. 1983 Apr;130(4):1713–1718. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wahl S. M. Host immune factors regulating fibrosis. Ciba Found Symp. 1985;114:175–195. doi: 10.1002/9780470720950.ch12. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]
- Wilde D. B., Marrack P., Kappler J., Dialynas D. P., Fitch F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983 Nov;131(5):2178–2183. [PubMed] [Google Scholar]



