Abstract
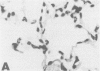
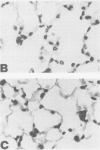
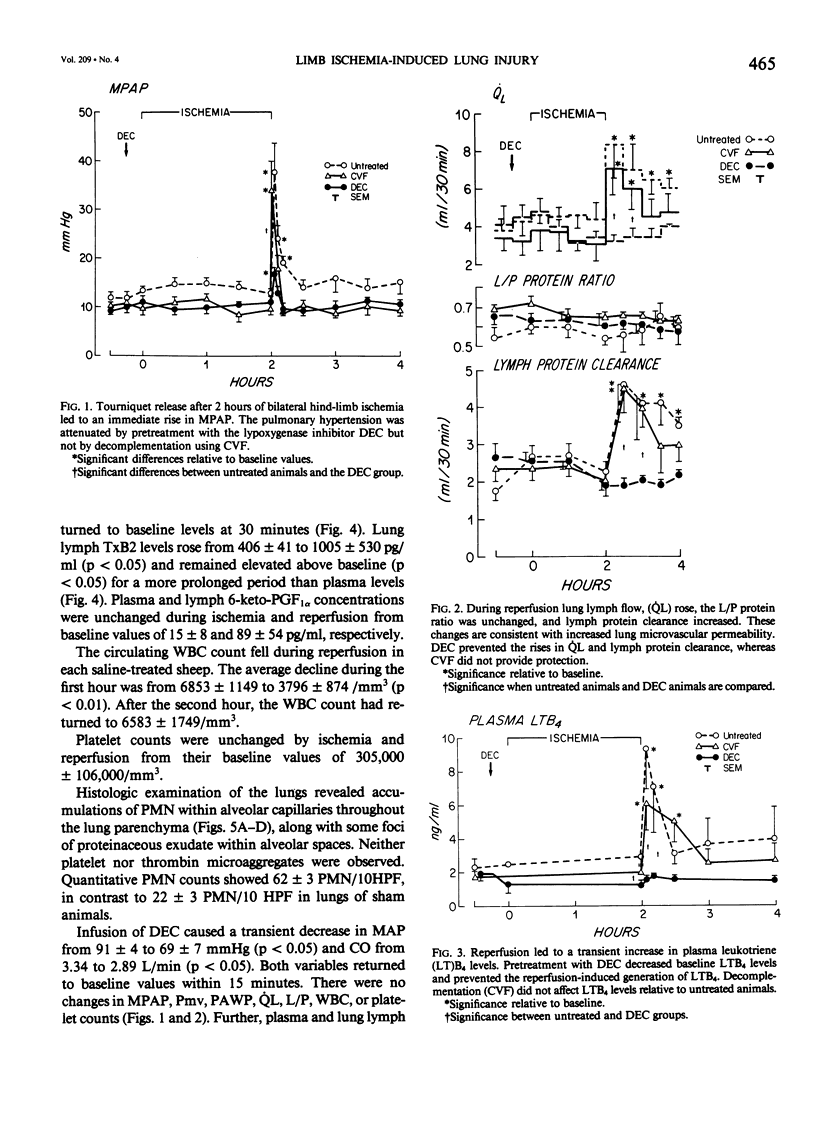
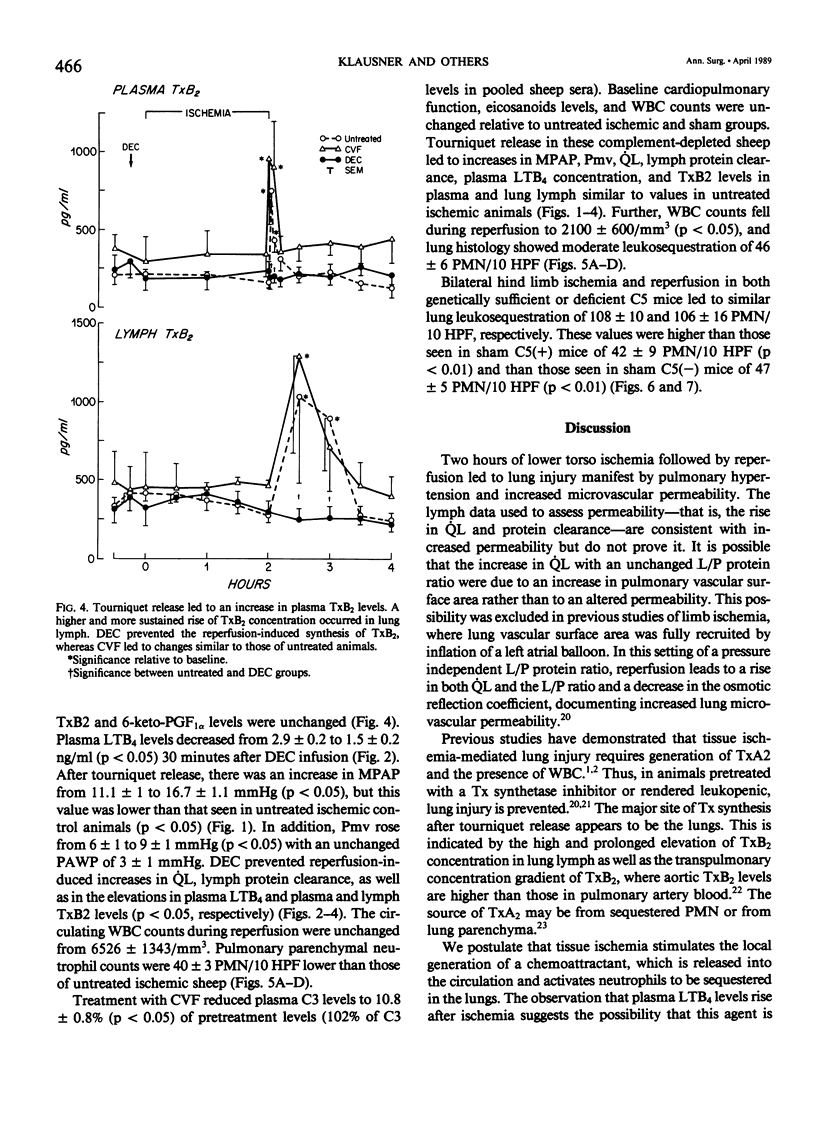
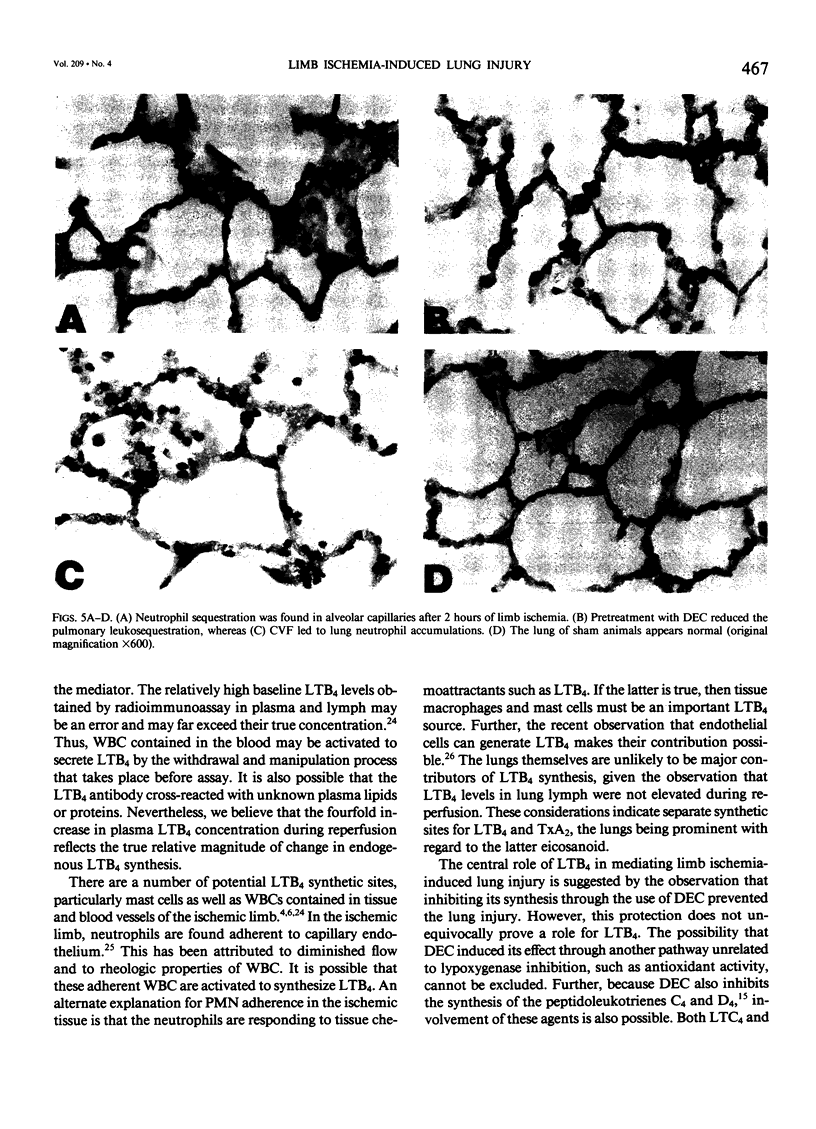
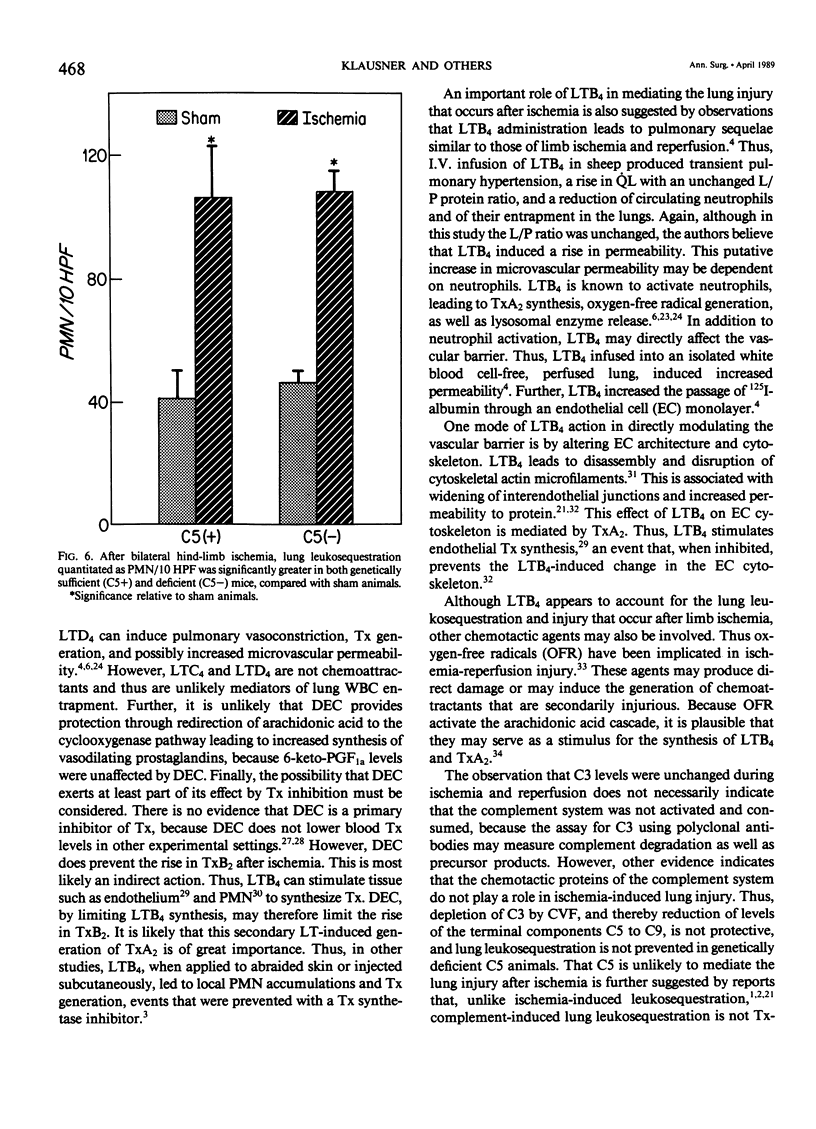
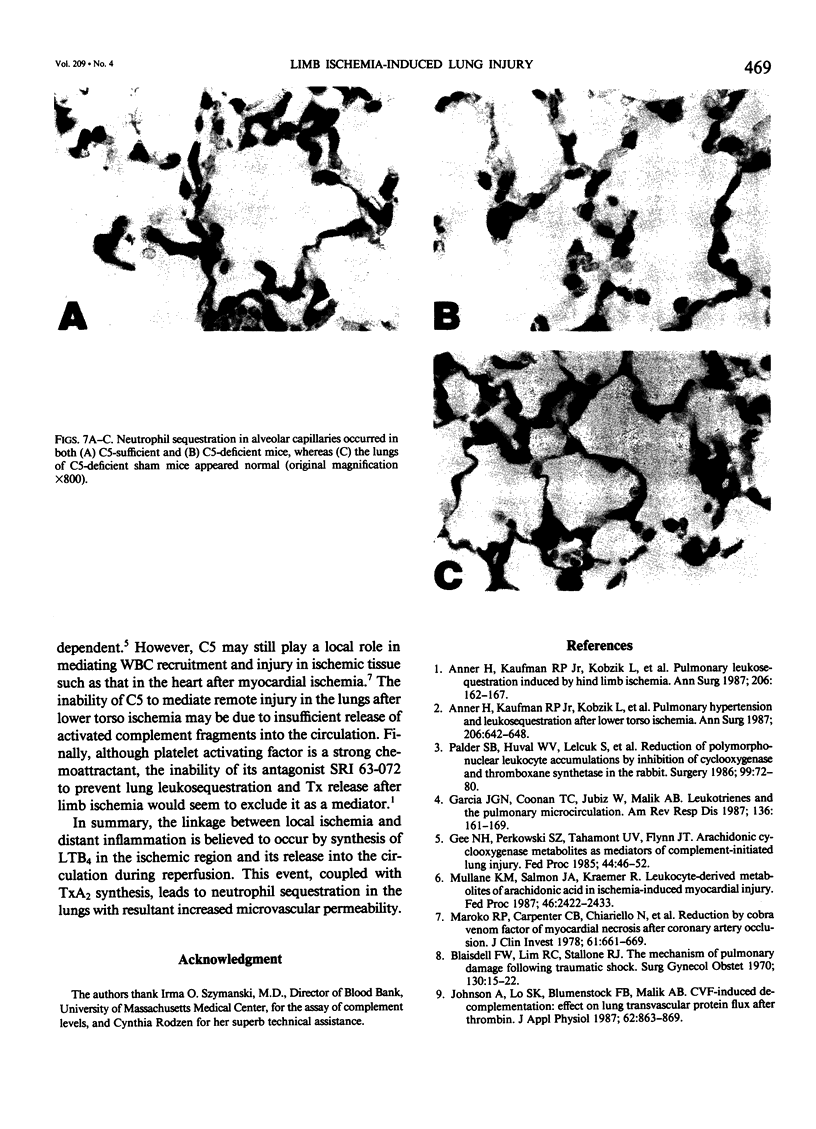
Reperfusion after limb ischemia leads to sequestration of polymorphonuclear leukocytes (PMN) in the lungs and to leukocyte- (WBC) and thromboxane- (Tx) dependent respiratory dysfunction. This study examines the intermediary role of the chemoattractants leukotriene (LT)B4 and complement (C) fragments. Anesthetized sheep with chronic lung lymph fistulae underwent 2 hours of tourniquet ischemia of both hind limbs. In untreated controls (n = 7), 1 minute after tourniquet release, mean pulmonary artery pressure (MPAP) rose from 13 to 38 mmHg (p less than 0.05) and returned to baseline within 30 minutes. Pulmonary artery wedge pressure was unchanged from 3.6 mmHg. There were increases in plasma LTB4 levels from 2.46 to 9.34 ng/ml (p less than 0.01), plasma TxB2 levels from 211 to 735 pg/ml (p less than 0.05), and lung lymph TxB2 from 400 to 1005 pg/ml (p less than 0.05). C3 levels were 96% of baseline values. Thirty minutes after reperfusion, lung lymph flow (QL) increased from 4.3 to 8.3 ml/30 minutes (p less than 0.05), lymph/plasma protein ratio was unchanged from 0.6, and the lymph protein clearance increased from 2.6 to 4.6 ml/30 minutes (p less than 0.05), data consistent with increased microvascular permeability. WBC count fell within the first hour from 6853 to 3793/mm3 (p less than 0.01). Lung histology showed leukosequestration, 62 PMN/10 high-power fields (HPF) and proteinaceous exudates. In contrast to this untreated ischemic group, animals treated with the lypoxygenase inhibitor diethylcarbamazine (n = 5) demonstrated a blunted reperfusion-induced rise in MPAP to 17 mmHg (p less than 0.05). There were no increases in LTB4, TxB2, QL or lymph protein clearance (p less than 0.05). WBC count was unchanged and lung leukosequestration was reduced to 40 PMN/10 HPF (p less than 0.05). Decomplementation with cobra venom factor (n = 4) resulted in plasma C3 levels, 10% of baseline, but tourniquet release still led to pulmonary hypertension, elevated LTB4, TxB2 levels, and a decline in WBC count similar to that of untreated ischemic control animals. Histology showed 46 PMN/10 HPF sequestered in the lungs. Further, bilateral hind limb ischemia in either genetically sufficient (n = 10) or deficient (n = 10) C5 mice led to significant lung leukosequestration of 108 and 106 PMN/10 HPF, respectively, compared with 42 and 47 PMN/10 HPF in sham C5(+) and C5 (-) mice (n = 20) (p less than 0.01). These results suggest that the lung leukosequestration and increased microvascular permeability after lower torso ischemia are mediated by the chemotactic agent LTB4, but not by the complement system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anner H., Kaufman R. P., Jr, Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Pulmonary hypertension and leukosequestration after lower torso ischemia. Ann Surg. 1987 Nov;206(5):642–648. doi: 10.1097/00000658-198711000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anner H., Kaufman R. P., Jr, Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Pulmonary leukosequestration induced by hind limb ischemia. Ann Surg. 1987 Aug;206(2):162–167. doi: 10.1097/00000658-198708000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell F. W., Stallone R. J. The mechanism of pulmonary damage following traumatic shock. Surg Gynecol Obstet. 1970 Jan;130(1):15–22. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Dunham B., Shepro D., Hechtman H. B. Leukotriene induction of TxB2 in cultured bovine aortic endothelial cells. Inflammation. 1984 Sep;8(3):313–321. doi: 10.1007/BF00916419. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Center D. M., Rounds S. Bovine and human endothelial cell production of neutrophil chemoattractant activity in response to components of the angiotensin system. Circ Res. 1985 Dec;57(6):898–902. doi: 10.1161/01.res.57.6.898. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W. Leukotrienes: their formation and role as inflammatory mediators. Fed Proc. 1985 Jan;44(1 Pt 1):25–29. [PubMed] [Google Scholar]
- Gaar K. A., Jr, Taylor A. E., Owens L. J., Guyton A. C. Pulmonary capillary pressure and filtration coefficient in the isolated perfused lung. Am J Physiol. 1967 Oct;213(4):910–914. doi: 10.1152/ajplegacy.1967.213.4.910. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Noonan T. C., Jubiz W., Malik A. B. Leukotrienes and the pulmonary microcirculation. Am Rev Respir Dis. 1987 Jul;136(1):161–169. doi: 10.1164/ajrccm/136.1.161. [DOI] [PubMed] [Google Scholar]
- Gaudet R. J., Alam I., Levine L. Accumulation of cyclooxygenase products of arachidonic acid metabolism in gerbil brain during reperfusion after bilateral common carotid artery occlusion. J Neurochem. 1980 Sep;35(3):653–658. doi: 10.1111/j.1471-4159.1980.tb03704.x. [DOI] [PubMed] [Google Scholar]
- Gee M. H., Perkowski S. Z., Tahamont M. V., Flynn J. T. Arachidonate cyclooxygenase metabolites as mediators of complement-initiated lung injury. Fed Proc. 1985 Jan;44(1 Pt 1):46–52. [PubMed] [Google Scholar]
- Huval W. V., Lelcuk S., Allen P. D., Mannick J. A., Shepro D., Hechtman H. B. Determinants of cardiovascular stability during abdominal aortic aneurysmectomy (AAA). Ann Surg. 1984 Feb;199(2):216–222. doi: 10.1097/00000658-198402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Lo S. K., Blumenstock F. B., Malik A. B. CVF-induced decomplementation: effect on lung transvascular protein flux after thrombin. J Appl Physiol (1985) 1987 Mar;62(3):863–869. doi: 10.1152/jappl.1987.62.3.863. [DOI] [PubMed] [Google Scholar]
- Lelcuk S., Threlfall L., Valeri C. R., Shepro D., Hechtman H. B. Nicotine stimulates pulmonary parenchymal thromboxane synthesis. Surgery. 1986 Nov;100(5):836–840. [PubMed] [Google Scholar]
- Maroko P. R., Carpenter C. B., Chiariello M., Fishbein M. C., Radvany P., Knostman J. D., Hale S. L. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978 Mar;61(3):661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews W. R., Murphy R. C. Inhibition of leukotriene biosynthesis in mastocytoma cells by diethylcarbamazine. Biochem Pharmacol. 1982 Jun 1;31(11):2129–2132. doi: 10.1016/0006-2952(82)90435-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Salmon J. A., Kraemer R. Leukocyte-derived metabolites of arachidonic acid in ischemia-induced myocardial injury. Fed Proc. 1987 May 15;46(7):2422–2433. [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palder S. B., Huval W., Lelcuk S., Alexander F., Shepro D., Mannick J. A., Hechtman H. B. Reduction of polymorphonuclear leukocyte accumulations by inhibition of cyclooxygenase and thromboxane syntase in the rabbit. Surgery. 1986 Jan;99(1):72–81. [PubMed] [Google Scholar]
- Puustinen T., Uotila P. Thromboxane formation in human polymorphonuclear leukocytes is inhibited by prednisolone and stimulated by leukotrienes B4, C4, D4 and histamine. Prostaglandins Leukot Med. 1984 May;14(2):161–167. doi: 10.1016/0262-1746(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Simmons P. M., Palmer R. M. A radioimmunoassay for leukotriene B4. Prostaglandins. 1982 Aug;24(2):225–235. doi: 10.1016/0090-6980(82)90148-4. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc. 1987 May 15;46(7):2397–2401. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Morris H. G., Schroeder W. R., Repine J. E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J Clin Invest. 1984 Aug;74(2):608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Welles S. L., Shepro D., Hechtman H. B. Eicosanoid modulation of stress fibers in cultured bovine aortic endothelial cells. Inflammation. 1985 Dec;9(4):439–450. doi: 10.1007/BF00916343. [DOI] [PubMed] [Google Scholar]
- Zadoff A. D., Kobayashi T., Brigham K. L., Newman J. H. Diethylcarbamazine on pulmonary vascular response to endotoxin in awake sheep. J Appl Physiol (1985) 1986 Apr;60(4):1380–1385. doi: 10.1152/jappl.1986.60.4.1380. [DOI] [PubMed] [Google Scholar]