Abstract
Hepatocyte growth factor/scatter factor (HGF/SF) induces cell scattering through the tyrosine kinase–type HGF/SF receptor c-Met. We have previously shown that Rho small G protein (Rho) is involved in the HGF/SF-induced scattering of Madin-Darby canine kidney (MDCK) cells by regulating at least the assembly and disassembly of stress fibers and focal adhesions, but it remains unknown how c-Met regulates Rho activity. We have found here a novel signaling pathway of c-Met consisting of SHP-2-Rho that regulates the assembly and disassembly of stress fibers and focal adhesions in MDCK cells. SHP-2 is a protein-tyrosine phosphatase that contains src homology-2 domains. Expression of a dominant negative mutant of SHP-2 (SHP-2-C/S) markedly increased the formation of stress fibers and focal adhesions in MDCK cells and inhibited their scattering. C3, a Clostridium botulinum ADP-ribosyltransferase, and Y-27632, a specific inhibitor for ROCK, reversed the stimulatory effect of SHP-2-C/S on stress fiber formation and the inhibitory effect on cell scattering. Vav2 is a GDP/GTP exchange protein for Rho. Expression of a dominant negative mutant of Vav2 blocked the stimulatory effect of SHP-2-C/S on stress fiber formation. Conversely, expression of mutants of Vav2 that increased stress fiber formation inhibited HGF/SF-induced cell scattering. These results indicate that SHP-2 physiologically modulates the activity of Rho to form stress fibers and focal adhesions and thereby regulates HGF/SF-induced cell scattering. In addition, Vav2 may be involved in the SHP-2-Rho pathway.
INTRODUCTION
Cell migration is a crucial process required during a variety of biological phenomena, including normal embryonic development, wound healing, inflammatory responses, and metastasis. Hepatocyte growth factor/scatter factor (HGF/SF) is known to induce cell migration in many types of cultured cells, including Madin-Darby canine kidney (MDCK) cells (Stoker and Gherardi, 1991). These stimulatory effects of HGF/SF are mediated through the HGF/SF receptor c-Met, which possesses tyrosine kinase activity (Bottaro et al., 1991; Naldini et al., 1991; Schlessinger, 1994). The tyrosine kinase activity of c-Met is essential for cellular responses to HGF/SF (Weidner et al., 1993; Zhu et al., 1994). Activation of c-Met tyrosine kinase induces the rapid autophosphorylation of c-Met itself as well as tyrosine phosphorylation of several cytoplasmic proteins (Rodrigues and Park, 1994; Nguyen et al., 1997).
Recent studies have shown that cyclical inactivation and activation of the Rho small G protein (Rho) are involved in HGF/SF-induced cell scattering. Expression of a dominant active mutant of Rho inhibits HGF/SF-induced cell scattering (Ridley et al., 1995; Imamura et al., 1998; Kamei et al., 1999), whereas C3, a Clostridium botulinum ADP-ribosyltransferase that inhibits Rho function, or Rho GDP dissociation inhibitor, which inhibits Rho activation, blocks HGF/SF-induced cell scattering (Takaishi et al., 1993, 1994). The mode of action of Rho in cell scattering remains to be clarified, but the Rho-regulated assembly and disassembly of stress fibers and focal adhesions have been suggested to be, at least in part, involved in cell scattering (Takaishi et al., 1994; Ridley et al., 1995; Imamura et al., 1998; Kamei et al., 1999; Ridley et al., 1999). In contrast to the downstream pathway of Rho, it is largely unknown how the activation of c-Met by HGF/SF leads to the regulation of Rho, which controls the assembly and disassembly of stress fibers and focal adhesions during HGF/SF-induced cell scattering.
To investigate the signaling mechanism from c-Met to Rho, we have now examined the effects of the expression of a dominant negative mutant of SHP-2, a protein tyrosine phosphatase, on the formation of stress fibers and focal adhesions and on cell scattering in response to HGF/SF in MDCK cells. Our data indicate that SHP-2 modulates Rho activity and thereby regulates HGF/SF-induced cell scattering. In addition, Vav2, a GDP/GTP exchange protein (GEP), may be involved in the SHP-2–mediated regulation of Rho activity. SHP-2 is a non-transmembrane protein tyrosine phosphatase that contains two src homology-2 (SH2) domains (Adachi et al., 1996; Matozaki and Kasuga, 1996; Neel and Tonks, 1997). SHP-2 binds through its SH2 domains to Gab-1, which undergoes tyrosine phosphorylation and subsequently binds Grb2 and the p85 subunit of phosphoinositide 3-kinase (PI 3-kinase) in response to HGF/SF (Holgado-Madruga et al., 1996; Weidner et al., 1996). Thus, SHP-2 is implicated in mediating the signals required for HGF/SF-induced cell scattering. Vav2 is a GEP for Rho that contains an SH2 domain flanking two src homology-3 domains (Schuebel et al., 1996, 1998). In addition, the catalytic activity of Vav2 has been shown to be enhanced by its tyrosine phosphorylation (Schuebel et al., 1996, 1998). The molecular mechanism by which SHP-2 regulates the Vav2 activity are also discussed.
MATERIALS AND METHODS
Materials and Chemicals
The myc-tagged mouse cDNA of a dominant negative mutant of SHP-2 with a mutation of amino acid 463 from Cys to Ser was provided by Dr. H. Ohnishi (Mitsubishi Kasei Institute of Life Science, Machida, Japan). The full-length mouse Vav2 cDNA was a gift from Dr. X. Bustelo (State University of New York, Stony Brook, NY). MDCK cells were supplied by Dr. W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany). Human recombinant HGF/SF was provided by Dr. T. Nakamura (Osaka University, Suita, Japan). The p160 Rho-associated coiled coil–forming protein kinase (p160ROCK) specific inhibitor, Y-27632, was provided by Yoshitomi Pharmaceutical Industries (Saitama, Japan). To generate a polyclonal antibody (Ab) to Vav2, female rabbits were injected with a GST fusion protein containing the C-terminal region (amino acids [aa] 768–868) of Vav2. A rabbit polyclonal Ab to SHP-2 was generated with a GST fusion protein containing the C-terminal region of human SHP-2 (Noguchi et al., 1994). An anti-Gab-1 rabbit polyclonal Ab to Gab-1 was obtained from Upstate Biotechnology (Lake Placid, NY). Hybridoma cells expressing the 9E10 anti-Myc mouse mAb were purchased from the American Type Culture Collection (Rockville, MD). Rhodamine-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR). An anti-vinculin mouse mAb (V115) was obtained from Sigma Chemical (St. Louis, MO). A HRP-conjugated mAb (PY20) to phosphotyrosine was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Second Abs for immunofluorescence microscopy were obtained from Chemicon International (Temecula, CA). Other materials and chemicals were obtained from commercial sources.
Construction of Expression Plasmids of Vav2 Mutants
Expression vectors were constructed with the pCIneo-myc plasmid with the use of standard molecular biology methods. To construct the pCIneo-myc vector, the pCIneo plasmid was cut at the NheI and XhoI sites and ligated to a double-stranded oligonucleotide encoding the peptide sequence MEQKLISEEDL, which is the epitope of the 9E10 anti-Myc mAb. Various constructs shown in Figure 6 encode Vav2 proteins containing the following aa: pCIneo-myc-Vav2-wild-type (WT), aa 1–868; pCIneo-myc-Vav2-ΔDbl-homology (DH) plus pleckstrin homology (PH) domains (ΔDH+PH), aa 1–196 and 507–868; and pCIneo-myc-Vav2-ΔN-terminal region (ΔN), aa 184–868. The point mutations into wild-type Vav2 cDNA or the ΔDH+PH domain cDNA were generated by site-directed mutagenesis as described previously (Takada et al., 1998).
Figure 6.
Expression of wild-type Vav2 and its mutants. (A) Structural domains of Vav2 are schematically illustrated at the top, and wild-type Vav2 cDNA and six Vav2 mutants are represented by thick lines below. Numbers indicate amino acid residues of the N and C termini of each domain. LR, leucine-rich domain; AD, acid-rich domain; CR, cystein-rich domain; SH3, src homology-3; ×, positions of point mutations with amino acid numbers. (B) Various pCIneo-myc plasmids encoding wild-type Vav2 and each Vav2 mutant were transiently transfected into COS-7 cells. At 48 h after transfection, the cell lysates were prepared from each transfected cell and subjected to immunoblotting with the anti-Myc mAb.
Cell Culture and Microinjection
MDCK cells were maintained at 37°C in a humidified atmosphere of 10% CO2 and 90% air in DMEM containing 10% FCS (GIBCO-BRL, Gaithersburg, MD), 100 U/ml penicillin, and 100 μg/ml streptomycin. MDCK cells expressing a dominant negative mutant of SHP-2 (MDCK-SHP-2-C/S) were established as described (Takaishi et al., 1997; Imamura et al., 1998). Briefly, MDCK cells (1 × 105 cells per 35-mm grid dish) were transfected with 1 μg of pcDNA3-neo containing the mutant SHP-2 cDNA with the use of 4 μl of LipofectAMINE reagent and 6 μl of PLUS reagent (GIBCO-BRL). The cells were cultured in DMEM containing neomycin (900 mg/ml; Wako, Osaka, Japan) and 10% FCS, and colonies were isolated 10–14 d after transfection. Several cell lines expressing the mutant SHP-2 protein were identified by immunostaining of cells with the 9E10 anti-Myc mouse mAb. For transient transfection experiments, MDCK cells were seeded at a density of 1 × 105 cells per dish onto 35-mm grid dishes and transfected with 1 μg of pCIneo containing wild-type or various mutant Vav2 cDNAs with the use of LipofectAMINE and PLUS reagents (GIBCO-BRL) at 24 h after seeding.
For microinjection experiments, MDCK cells were seeded at a density of 1 × 105 cells per dish onto 35-mm grid dishes. At 48 h after seeding, C3 at a concentration of 40 μg/ml was comicroinjected along with a marker protein (rat immunoglobulin G) into the cytoplasm of the cells and then returned to the incubator for 1 h before HGF/SF stimulation or fixation (Imamura et al., 1998).
Immunofluorescence Microscopy
For immunofluorescence staining, cells were fixed in 3.7% paraformaldehyde in PBS for 20 min. The fixed cells were incubated with 50 mM NH4Cl in PBS for 10 min and permeabilized with PBS containing 0.2% Triton X-100 for 10 min. After being soaked in 10% FCS/PBS for 30 min, they were treated with the first Ab in 10% FCS/PBS for 1 h. The cells were then washed with PBS three times, followed by incubation with the second Ab in 10% FCS/PBS for 1 h. For the double staining, the second Abs, which did not cross-react with each other, were chosen. After the cells were washed with PBS three times, they were examined with the use of an LSM 410 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany) as described (Kodama et al., 1999).
Immunoprecipitation and Immunoblot Analysis
MDCK cells (on a 10-cm plate) were frozen in liquid nitrogen and then lysed on ice in 1 ml of ice-cold lysis buffer (20 mM Tris-HCl, pH 7.6, 140 mM NaCl, 2.6 mM CaCl2, 1 mM MgCl2, 1% [vol/vol] Nonidet P-40, 10% [vol/vol] glycerol) containing 1 mM PMSF, 10 μg/ml aprotinin, and 1 mM sodium vanadate. The lysates were centrifuged at 10,000 × g at 4°C for 15 min, and the resulting supernatants were subjected to immunoprecipitation and immunoblot analysis. Briefly, supernatants were incubated at 4°C with the anti-Gab-1 polyclonal Ab or anti-SHP-2 polyclonal Ab bound to protein G–Sepharose beads (2 μg of Ab per 20 μl of beads; Amersham-Pharmacia Biotech, Uppsala, Sweden) for 4 h, after which the beads were washed twice with 1 ml of WG buffer (50 mM HEPES-NaOH, pH 7.6, 150 mM NaCl, 0.1% [vol/vol] Triton X-100) and resuspended in SDS sample buffer. The GST fusion proteins containing either the SH2 domain of Vav2 (aa 663–757) or two SH2 domains of SHP-2 (Noguchi et al., 1996) were generated. Cell lysates, which were prepared from HGF/SF-stimulated or unstimulated MDCK cells, were incubated with these GST fusion proteins immobilized on glutathione–Sepharose beads (Amersham-Pharmacia Biotech) for 2 h. The beads were then washed twice with 1 ml of WG buffer and resuspended in SDS sample buffer. SDS-PAGE and immunoblot analysis with various antibodies were performed with the use of an ECL detection kit (Amersham-Pharmacia Biotech).
RESULTS
Stimulation of Formation of Stress Fibers and Focal Adhesions and Inhibition of HGF/SF-induced Cell Scattering by a Dominant Negative Mutant of SHP-2
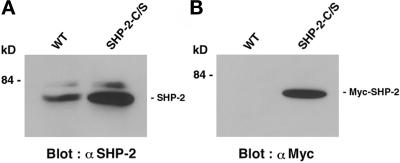
The myc-tagged mutant SHP-2 cDNA, in which Cys463 was altered to Ser, was transfected into MDCK cells, and several cell lines overexpressing a catalytically inactive mutant of SHP-2 (SHP-2-C/S) were established. The Cys463-to-Ser mutation has been shown to totally abolish the tyrosine phosphatase activity of SHP-2, and the mutant protein works as a dominant negative mutant of SHP-2 when it is expressed in a variety of cells (Noguchi et al., 1994; Matozaki and Kasuga, 1996; Neel and Tonks, 1997). We have obtained several independent cell lines that expressed SHP-2-C/S. MDCK-SHP-2-C/S (clone 14), which showed a high expression level of Myc-SHP-2-C/S, was chosen for further characterization. The extent of the expression of exogenous SHP-2 was assessed by immunoblotting of the cell lysates with the anti-SHP-2 Ab (Figure 1A). The amount of total SHP-2 in MDCK-SHP-2-C/S cells was approximately five times more than that in the parent MDCK cells. Exogenously expressed SHP-2 was also confirmed by immunoblotting with the anti-Myc mAb (Figure 1B). The other cell lines showed the same phenotype as that observed in clone 14 (our unpublished results).
Figure 1.
Expression of SHP-2 in wild-type MDCK cells and MDCK-SHP-2-C/S cells. Cell lysates were prepared from wild-type MDCK cells (WT) and MDCK-SHP-2-C/S cells (SHP-2-C/S) and subjected to immunoblot analysis with the polyclonal anti-SHP-2 Ab (α SHP-2) (A) or the anti-Myc mAb (α Myc) (B). The positions of SHP-2 and Myc-tagged SHP-2 are indicated by bars, and the molecular mass is indicated in kilodaltons (kD).
We next examined the effect of the expression of SHP-2-C/S on stress fibers by staining with rhodamine-conjugated phalloidin. Confocal microscopic images showed weak formation of stress fibers at the basal level in wild-type MDCK cells (Figure 2A). In contrast, prominent stress fibers were observed in MDCK-SHP-2-C/S cells at the basal level (Figure 2B). The staining of vinculin, which localizes at focal adhesions (Burridge and Mangeat, 1984), was markedly increased in MDCK-SHP-2-C/S cells compared with that observed in wild-type cells (Figure 2, C and D). These results indicate that the expression of a dominant negative mutant of SHP-2 induces marked alterations of stress fibers and focal adhesions.
Figure 2.
Stimulatory effects of the expression of a dominant negative mutant of SHP-2 on the formation of stress fibers and focal adhesions in MDCK cells. Wild-type MDCK cells (WT) (A and C) and MDCK-SHP-2-C/S cells (SHP-2-C/S) (B and D) were stained with rhodamine-conjugated phalloidin (A and B) or the anti-vinculin mAb (C and D) and then analyzed by confocal microscopy at the basal level. The results shown are representative of three independent experiments. Bar, 10 μm.
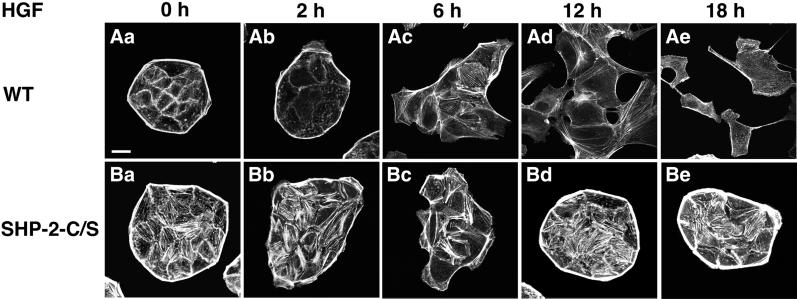
We then examined the effect of a dominant negative mutant of SHP-2 on the HGF/SF-induced cell scattering of MDCK cells. As described previously (Imamura et al., 1998; Kodama et al., 1999), stimulation of wild-type MDCK cells with HGF/SF caused spreading of the cells without dissociation of the cells during the first 2–6 h (Figure 3, Aa–Ac). Between 6 and 18 h, the cell–cell contacts were disrupted and the cells were scattered (Figure 3, Ac–Ae). Stress fibers decreased within 2 h, increased in a part of the cells between 4 and 6 h, and mostly disappeared at 18 h (Figure 3, Aa–Ae). In contrast, scattering of MDCK-SHP-2-C/S cells in response to HGF/SF was markedly reduced (Figure 3, Ba–Be). Cell spreading and membrane ruffling in response to HGF/SF occurred similarly in MDCK-SHP-2-C/S cells and wild-type cells during the first 2–6 h. However, the transient decrease of stress fibers within the first 2 h was markedly impaired, and stress fibers were still apparent even at 18 h after HGF/SF stimulation (Figure 3, Bb and Be). These results indicate that SHP-2 is involved in HGF/SF-induced cell scattering.
Figure 3.
Effects of the expression of a dominant negative mutant of SHP-2 on the HGF/SF-induced cell scattering and formation of stress fibers in MDCK cells. Wild-type MDCK cells (WT) (Aa–Ae) and MDCK-SHP-2-C/S cells (SHP-2-C/S) (Ba–Be) were cultured with 10 ng/ml HGF/SF for the indicated periods. These cells were stained with rhodamine-conjugated phalloidin and then analyzed by confocal microscopy at the basal level. The results shown are representative of three independent experiments. Bar, 10 μm.
Involvement of Rho and ROCK in the SHP-2-C/S Effects
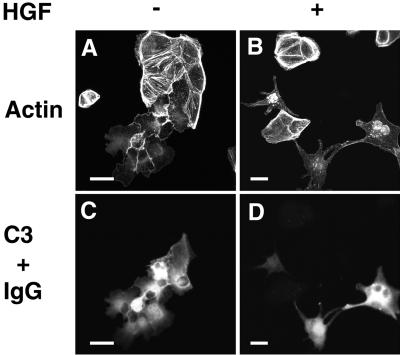
The increased formation of both stress fibers and focal adhesions has been observed in MDCK cells expressing a dominant active mutant of RhoA (Ridley et al., 1995; Takaishi et al., 1997; Imamura et al., 1998). Furthermore, it has been shown that HGF/SF-induced cell scattering is markedly inhibited in MDCK cells expressing a dominant active mutant of RhoA (Ridley et al., 1995; Kamei et al., 1999). The phenotype of the actin cytoskeleton induced by SHP-2-C/S resembled that of the cells expressing a dominant active mutant of RhoA. In addition, HGF/SF-induced cell scattering was markedly blocked by the expression of SHP-2-C/S. Therefore, it is possible that the expression of SHP-2-C/S somehow enhances the activity of Rho, thereby stimulating the formation of stress fibers and focal adhesions and finally inhibiting HGF/SF-induced cell scattering. To test this possibility, we next examined the effect of microinjection of C3, a Clostridium botulinum ADP-ribosyltransferase, into wild-type MDCK cells and MDCK-SHP-2-C/S cells. C3 has been shown to induce the ADP-ribosylation of Rho and subsequently to prevent this protein from interacting with its downstream targets, resulting in the inactivation of the protein (Kikuchi et al., 1988; Narumiya et al., 1988). We have previously shown that microinjection of C3, at a concentration of 40 μg/ml, into wild-type MDCK cells induces the disappearance of stress fibers and the subsequent disruption of cell–cell adhesion sites (Takaishi et al., 1997). We confirmed these observations in wild-type MDCK cells (our unpublished results). Microinjection of C3 at a similar concentration has been shown to induce the disappearance of stress fibers but does not affect the movement of primary embryo fibroblasts in an in vitro wound healing assay, whereas at a much higher concentration, C3 significantly inhibits wound closure (Nobes and Hall, 1999). Therefore, we chose the moderate concentration of 40 μg/ml for C3. Microinjection of C3 into unstimulated MDCK-SHP-2-C/S cells also induced the disappearance of stress fibers (Figure 4, A and C) and made these cells able to scatter in response to HGF/SF (Figure 4, B and D).
Figure 4.
Reversal by C3 of the inhibition of HGF/SF-induced cell scattering in MDCK-SHP-2-C/S cells. MDCK-SHP-2-C/S cells were microinjected with 40 μg/ml C3 plus 5 mg/ml rat immunoglobulin G (IgG). At 30 min after microinjection, the cells were stimulated with no HGF/SF (A and C) or 10 ng/ml HGF/SF (B and D). At 18 h after HGF/SF stimulation, the cells were fixed, stained with rhodamine-conjugated phalloidin (A and B), and analyzed by confocal microscopy. The microinjected cells are shown by the staining of microinjected rat immunoglobulin G (C and D). Confocal images are shown at the basal level. The results shown are representative of three independent experiments. Bars, 10 μm.
We then examined the effect of Y-27632, a p160ROCK inhibitor (Uehata et al., 1997; Hirose et al., 1998), on the scattering of wild-type MDCK cells and MDCK-SHP-2-C/S cells in the absence or presence of HGF/SF. p160ROCK, also named ROKα/Rho-kinase, is a recently identified serine/threonine kinase (Leung et al., 1995; Ishizaki et al., 1996; Matsui et al., 1996) that has been suggested to mediate the Rho-dependent formation of both stress fibers and focal adhesions in cultured fibroblasts and epithelial cells (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997; Nakano et al., 1999). After MDCK cells were treated with 10 μM Y-27632 for 18.5 h, the diameter of both wild-type cells and MDCK-SHP-2-C/S cells became smaller than that observed in the absence of Y-27632 (Figure 5, Aa, Ab, Ba, and Bb). The formation of stress fibers in both wild-type MDCK cells and MDCK-SHP-2-C/S cells was markedly reduced in the presence of Y-27632 (Figure 5, Aa, Ab, Ba, and Bb). Pretreatment of wild-type cells with Y-27632 did not affect the cell scattering induced by HGF/SF (Figure 5, Ac and Ad). However, Y-27632 reversed the inhibition of HGF/SF-induced cell scattering in MDCK-SHP-2-C/S cells (Figure 5, Bc and Bd). These data suggest that the enhanced activity of Rho results in the inhibition of HGF/SF-induced cell scattering in MDCK-SHP-2-C/S cells.
Figure 5.
Reversal by Y-27632 of the inhibition of HGF/SF-induced cell scattering in MDCK-SHP-2-C/S cells. Wild-type MDCK cells (WT) (Aa–Ad) and MDCK-SHP-2-C/S cells (SHP-2-C/S) (Ba–Bd) were incubated with no Y-27632 (Aa, Ac, Ba, and Bc) or 10 μM Y-27632 (Ab, Ad, Bb, and Bd). At 30 min after the addition of Y-27632, the cells were stimulated with no HGF/SF (Aa, Ab, Ba, and Bb) or 10 ng/ml HGF/SF (Ac, Ad, Bc, and Bd). At 18 h after HGF/SF stimulation, the cells were fixed, stained with rhodamine-conjugated phalloidin, and analyzed by confocal microscopy. Confocal images are shown at the basal level. The results shown are representative of three independent experiments. Bar, 10 μm.
Involvement of Vav2 in SHP-2–regulated Rho Activity
Because C3 reversed stress fiber formation and the inhibition of HGF/SF-induced cell scattering in MDCK-SHP-2-C/S cells, the expression of SHP-2-C/S may regulate an upstream element necessary for Rho activation. The activity of Rho is positively regulated by a GEP through conversion of the GDP-bound form to the GTP-bound form (Hall, 1994, 1998; Takai et al., 1995). Among several GEPs for Rho, we focused on Vav2, a ubiquitously expressed GEP for Rho, because this GEP has one SH2 domain flanking two src homology-3 domains, and its catalytic activity is enhanced by its tyrosine phosphorylation (Schuebel et al., 1996, 1998). We have confirmed that Vav2 is expressed in the MDCK cells used here by immunoblotting with the polyclonal anti-Vav2 Ab (our unpublished results). We then examined whether Vav2 is involved in the enhanced activity of Rho in MDCK-SHP-2-C/S cells. To this end, we constructed expression vectors containing wild-type and various mutant Vav2 cDNAs, all of which were tagged with the Myc epitope, and confirmed their expressions by transient transfection into COS-7 cells (Figure 6).
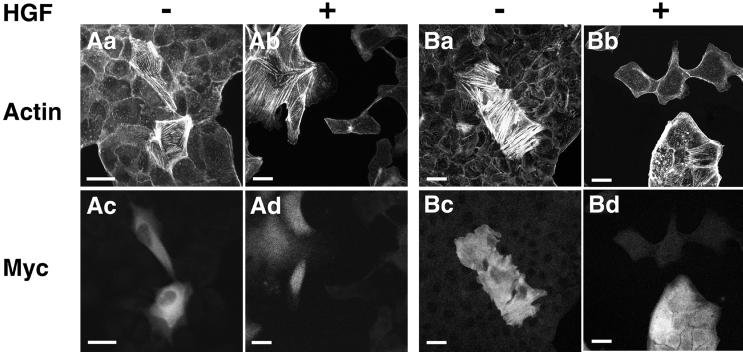
After we transfected MDCK cells with these plasmid vectors, we determined stress fiber formation by monitoring the cells with rhodamine-conjugated phalloidin. Expressions of exogenous Vav2 proteins were ensured by immunostaining with the anti-Myc mAb. Expression of a Vav2 mutant lacking both DH and PH domains (ΔDH+PH) markedly reduced the increased stress fiber formation in MDCK-SHP-2-C/S cells (Figure 7, Aa and Ab). It has been shown that the Leu213-to-Gln mutation of Vav1 abolishes its catalytic activity for Rac activation and that this mutant of Vav1 works in a dominant negative manner (Crespo et al., 1997; Ma et al., 1998). By analogy, we expressed a Vav2 mutant with a mutation of Leu212 to Gln (L212Q). Expression of the L212Q mutant markedly reduced the increased stress fiber formation in MDCK-SHP-2-C/S cells (Figure 7, Ac and Ad).
Figure 7.
Effects of dominant negative mutants of Vav2 on the stress fiber formation in MDCK-SHP-2-C/S cells and wild-type MDCK cells. MDCK-SHP-2-C/S cells were transfected with pCIneo-myc-Vav2-ΔDH+PH (ΔDH+PH) (Aa and Ab), pCIneo-myc-Vav2-L212Q (L212Q) (Ac and Ad), pCIneo-myc-Vav2-ΔDH+PH/R688K (ΔDH+PH/R688K) (Ba and Bb), pCIneo-myc-Vav2-L212Q/R688K (L212Q/R688K) (Bc and Bd), or pCIneo-myc-Vav2-wild-type (WT) (Ca and Cb). Wild-type MDCK cells were also transfected with pCIneo-myc-Vav2-ΔDH+PH (ΔDH+PH) (Cc and Cd). At 48 h after transfection, the cells were fixed, double stained with rhodamine-conjugated phalloidin (Actin) or the anti-Myc mAb (Myc), and analyzed by confocal microscopy. Confocal images are shown at the basal level. The results shown are representative of three independent experiments. Bar, 10 μm.
A specific Arg residue in the SH2 domain of Src, Abl, or Lck stabilizes the negatively charged phosphotyrosine group of bound phosphotyrosine ligand (Waksman et al., 1992; Eck et al., 1993), whereas an Arg-to-Lys mutation at this conserved residue leads to significantly weaker binding of phosphotyrosine peptides (Mayer et al., 1992). Thus, the Arg-to-Lys mutation was made in ΔDH+PH (ΔDH+PH/R688K) and L212Q (L212Q/R688K) at Arg688. Both ΔDH+PH/R688K and L212Q/R688K failed to suppress the increased stress fiber formation in MDCK-SHP-2-C/S cells (Figure 7, Ba–Bd). In contrast, expression of wild-type Vav2 did not affect the increased stress fiber formation in MDCK-SHP-2-C/S cells (Figure 7, Ca and Cb). Expression of wild-type Vav2 in wild-type MDCK cells had no significant effect on stress fiber formation (our unpublished results). Expression of ΔDH+PH reduced the stress fiber formation in wild-type MDCK cells (Figure 7, Cc and Cd). These data suggest that Vav2, at least in part, participates in the increased stress fiber formation in MDCK-SHP-2-C/S cells through its SH2 domain–dependent manner.
During the course of construction of various Vav2 mutants, we found that expression of a Vav2 mutant with a mutation of amino acid Tyr172 to Phe (Y172F) induced prominent stress fiber formation in wild-type MDCK cells (Figure 8, Aa and Ac). It was demonstrated recently that Vav2 is a GEP for Rac and Cdc42 as well as Rho (Abe et al., 2000). In fact, the expression of the Y172F mutant also induced lamellipodia and ruffling formation (our unpublished results). Thus, we next examined the effect of this Y172F mutant of Vav2 on HGF/SF-induced cell scattering. Expression of the Y172F Vav2 mutant markedly reduced the scattering of wild-type MDCK cells in response to HGF/SF (Figure 8, Ab and Ad). The N-terminal–truncated mutant of Vav2 (ΔN) has been shown to induce stress fiber formation (Schuebel et al., 1998). Expression of the Vav2 ΔN mutant similarly induced stress fiber formation in wild-type MDCK cells (Figure 8, Ba and Bc) and markedly reduced their scattering in response to HGF/SF (Figure 8, Bb and Bd).
Figure 8.
Inhibitory effect of Y172F or the ΔN mutant of Vav2 on HGF/SF-induced cell scattering of MDCK cells. Wild-type MDCK cells were microinjected with pCIneo-myc-Vav2-Y172F (Y172F) (Aa–Ad) or pCIneo-myc-Vav2-ΔN-terminal region (ΔN) (Ba–Bd). At 24 h after microinjection, the cells were stimulated with no HGF/SF (Aa, Ac, Ba, and Bc) or 10 ng/ml HGF/SF (Ab, Ad, Bb, and Bd). At 18 h after HGF/SF stimulation, the cells were double stained with rhodamine-conjugated phalloidin (Actin; Aa, Ab, Ba, and Bb) or the anti-Myc mAb (Ac, Ad, Bc, and Bd) and analyzed by confocal microscopy. Confocal images are shown at the basal level. The results shown are representative of three independent experiments. Bars, 10 μm.
Complex Formation between Gab-1 and the SH2 Domain of Vav2
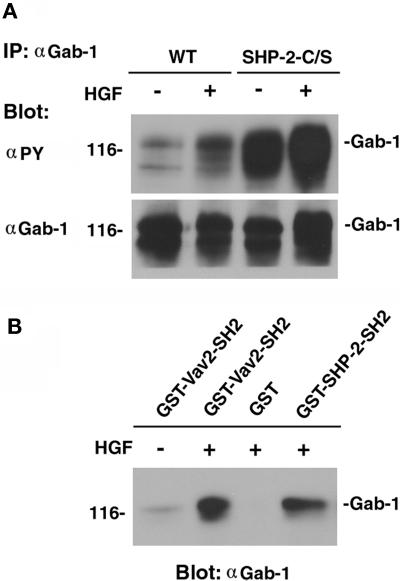
We found that the extent of tyrosine phosphorylation of Gab-1 markedly increased in MDCK-SHP-2-C/S cells compared with that of wild-type MDCK cells and that it further increased when MDCK-SHP-2-C/S cells were stimulated with HGF/SF (Figure 9A). Thus, the binding of Vav2 to tyrosine-phosphorylated Gab-1 could increase in MDCK-SHP-2-C/S cells. The GST-SH2 domain of the Vav2 fusion protein, as well as the GST-SH2 domains of SHP-2, bound to Gab-1 when Gab-1 was tyrosine phosphorylated by HGF/SF (Figure 9B). These results suggest that Vav2 may bind to tyrosine-phosphorylated Gab-1 through its SH2 domain. However, when Vav2 or Gab-1 was immunoprecipitated with its Ab, we did not succeed in detecting the complex formation of Vav2 with Gab-1 in either wild-type cells or MDCK-SHP-2-C/S cells (our unpublished results).
Figure 9.
Tyrosine phosphorylation of Gab-1 and the binding of the SH2 domain of Vav2 to Gab-1. (A) Wild-type cells and MDCK-SHP-2-C/S cells were stimulated with 20 ng/ml HGF/SF for 5 min. Cell lysates were then prepared from HGF/SF-stimulated and unstimulated wild-type MDCK cells and SHP-2-C/S cells and subjected to immunoprecipitation (IP) with the anti-Gab-1 polyclonal Ab (αGab-1). The immunoprecipitates were fractionated by SDS-PAGE and subjected to immunoblot analysis with the anti-phosphotyrosine mAb (αPY). The same filter was then reprobed with the anti-Gab-1 polyclonal Ab. (B) Cell lysates prepared from HGF/SF-stimulated or unstimulated MDCK cells were also incubated with the GST-SH2 domain of Vav2 (GST-Vav2-SH2), the GST-SH2 domains of SHP-2 fusion proteins (GST-SHP-2-SH2), or GST alone (GST) and immobilized on glutathione–Sepharose beads for 2 h. The beads were then subjected to immunoblotting with the anti-Gab-1 polyclonal Ab (αGab-1). The positions of Gab-1 are indicated by bars, and the molecular mass is indicated in kilodaltons on the left.
DISCUSSION
In the present study, we have demonstrated that expression of SHP-2-C/S markedly inhibits the HGF/SF-induced scattering of MDCK cells, indicating that SHP-2 positively regulates the HGF/SF-induced scattering of MDCK cells. SHP-2 mediates the activation of the Ras-MAPK pathway in response to various growth factors (Noguchi et al., 1994; Bennett et al., 1996; Saxton et al., 1997). More recently, migration on fibronectin of fibroblasts derived from Shp-2 knockout mice has been shown to be impaired in comparison with wild-type cells (Yu et al., 1998). In addition, SHP-2 has also been shown to be required for migration of human breast adenocarcinoma MCF-7 cells induced by insulin-like growth factor-1 or that of mouse fibroblasts induced by PDGF (Mañes et al., 1999; Qi et al., 1999). However, these authors have not clarified the mechanism by which SHP-2 regulates cell migration.
Expression of SHP-2-C/S resulted in the increased formation of both stress fibers and focal adhesions in MDCK cells. Treatment of SHP-2-C/S cells with C3 or Y-27632 suppressed the formation of stress fibers and reversed the inhibition of cell scattering in response to HGF/SF. These data indicate that expression of SHP-2-C/S leads to increased activity of Rho, which causes increased formation of stress fibers and focal adhesions. The increased formation of stress fibers and focal adhesions may finally inhibit the scattering of MDCK cells in response to HGF/SF. It is also possible that the effects of the expression of SHP-2-C/S are mediated by tyrosine phosphorylation of other proteins that are involved in stabilizing stress fibers but not by the activation of Rho. However, the expression of ΔDH+PH and L212Q Vav2 mutants markedly inhibited the stress fiber formation in MDCK-SHP-2-C/S cells, whereas the expression of wild-type Vav2 did not. Furthermore, the expression of Vav2 mutants that increased stress fiber formation inhibited HGF/SF-induced cell scattering. Thus, these data suggest that Vav2 may be involved, at least in part, in the enhanced formation of stress fibers and focal adhesions in MDCK-SHP-2-C/S cells. In addition, these data further support the notion that Rho activity could be enhanced in MDCK-SHP-2-C/S cells.
We attempted to quantitate the GTP-bound form of Rho in wild-type MDCK cells or MDCK-SHP-2-C/S cells with the use of an affinity precipitation of the GTP-bound form of Rho essentially as described by Ren et al. (1999). However, we did not observe a significant increase in the amount of Rho associated with GST–Rho-binding domain in MDCK-SHP-2-C/S compared with that observed in wild-type MDCK cells (our unpublished results). We have previously shown that in yeast, <10% of Rho is physiologically activated (Yamochi et al., 1994). Moreover, evidence is accumulating that Rho is activated or inactivated in time- and site-specific manners, thereby regulating reorganization of the actin cytoskeleton (Sasaki and Takai, 1998). This subtle and temporal change of Rho activation could not be detected by the pull-down assay used here.
At present, we have not yet uncovered the molecular mechanism by which expression of SHP-2-C/S up-regulates Vav2 activity. The catalytic activity of Vav2 as a Rho GEP has been shown to be markedly enhanced by its tyrosine phosphorylation through a Src family kinase such as Lck (Schuebel et al., 1998). However, tyrosine phosphorylation of Vav2 was not apparent in wild-type MDCK cells, nor it was enhanced in MDCK-SHP-2-C/S cells (our unpublished results). Thus, it is unlikely that SHP-2 controls the activity of Vav2 through the regulation of tyrosine phosphorylation or dephosphorylation of Vav2.
It is also possible that expression of SHP-2-C/S results in the suppression of GTPase-activating protein (GAP) activity of Rho, thereby enhancing the activity of Rho. Tyrosine phosphorylation of p190 RhoGAP by a Src family kinase has been suggested to increase its catalytic activity (Fincham et al., 1999). However, expression of SHP-2-C/S did not affect the extent of tyrosine phosphorylation of p190 RhoGAP (our unpublished results), suggesting that SHP-2 does not regulate the Rho activity through tyrosine phosphorylation or dephosphorylation of p190 RhoGAP. Further study is clearly required to determine whether SHP-2 regulates the Rho activity through regulation of other Rho GAPs (Figure 10).
Figure 10.
A signaling pathway of c-Met for cell scattering.
In contrast, expression of a dominant negative mutant of SHP-2 has been shown to induce the hyperphosphorylation of several proteins, including insulin receptor substrate-1 and SHPS-1/BIT/SIRP (Noguchi et al., 1994; Fujioka et al., 1996; Noguchi et al., 1996; Ohnishi et al., 1996; Kharitonenkov et al., 1997). We have found that the extent of tyrosine phosphorylation of Gab-1 and its binding to Grb2 or the p85 subunit of PI 3-kinase increases in MDCK-SHP-2-C/S cells (our unpublished results). It has been suggested that the catalytic activities of SH2 domain–containing enzymes, such as SHP-2 and p85/p110 PI 3-kinase, are enhanced by the occupancy of their SH2 domains with phosphotyrosine residues (Backer et al., 1992; Sugimoto et al., 1994; Rordorf-Nikolic et al., 1995). Moreover, the SH2 domains of these enzymes mediate the translocation of these enzymes from the cytosol to sites near their targets. By this analogy, it is likely that Vav2 normally binds through its SH2 domain to a docking protein whose tyrosine phosphorylation is down-regulated by SHP-2, thereby being activated. One candidate for such a docking protein is Gab-1, because its tyrosine phosphorylation markedly increased in MDCK-SHP-2-C/S cells as described above. In addition, the GST-SH2 domains of Vav2 bind to tyrosine-phosphorylated Gab-1 in vitro. Thus, Vav2 may bind to Gab-1 through its SH2 domain, and its binding to Gab-1 may increase in MDCK-SHP-2-C/S cells, although we have not yet succeeded in detecting the complex formation of Gab-1 with Vav2 in either wild-type MDCK cells or MDCK-SHP-2-C/S cells. It is also possible that Vav2 could bind to another, yet unidentified, protein whose phosphorylation is regulated by SHP-2. In MDCK-SHP-2-C/S cells, hyperphosphorylation of the putative docking protein for Vav2 may increase the activity of Vav2, leading to the enhanced formation of stress fibers and focal adhesions. Expression of a dominant negative mutant of Vav2 in MDCK-SHP-2-C/S cells may competitively block the binding of endogenous Vav2 to the putative docking protein through the SH2 domain and thereby suppress the formation of stress fibers and focal adhesions. In fact, we have shown that the inhibitory effects of dominant negative mutants of Vav2 require their SH2 domains.
Our present observations, therefore, suggest the following model. In unstimulated MDCK cells, SHP-2 may modulate the activity of Rho through the regulation of the GEP activity of Vav2 for Rho. Upon HGF/SF stimulation, the catalytic activity of SHP-2 may be up-regulated through its SH2 domain–mediated binding to a tyrosine-phosphorylated docking protein such as Gab-1 and could inhibit the Rho activity (Figure 10). This could reflect the early decrease in the formation of stress fibers and focal adhesions in HGF/SF-stimulated MDCK cells. Identification of the putative Vav2-binding protein whose tyrosine phosphorylation is down-regulated by SHP-2 appears to be the next crucial step.
ACKNOWLEDGMENTS
We thank Dr. W. Birchmeier for providing MDCK cells, Dr. H. Ohnishi for the cDNA of a mutant SHP-2, Dr. X. Bustelo for the mouse Vav2 cDNA, and Dr. T. Nakamura for HGF/SF. This investigation was supported by Grants-in-Aid for Scientific Research and for Cancer Research from the Ministry of Education, Science, Sports, and Culture, Japan (1998 and 1999).
Abbreviations used:
- aa
amino acid
- Ab
antibody
- DH
Dbl homology
- GAP
GTPase-activating protein
- GEP
GDP/GTP exchange protein
- HGF/SF
hepatocyte growth factor/scatter factor
- MDCK
Madin-Darby canine kidney
- PH
pleckstrin homology
- PI 3-kinase
phosphoinositide 3-kinase
- p160ROCK
Rho-associated coiled coil–forming protein kinase
- Rho
Rho small G protein
- SH2
src homology-2
REFERENCES
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- Adachi M, et al. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. doi: 10.1016/s0092-8674(00)81077-6. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Backer JM, et al. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AM, Hausdorff SF, O'Reilly AM, Freeman RM, Neel BG. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308:744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- Eck MJ, Shoelson SE, Harrison SC. Recognition of a high-affinity phosphotyrosyl peptide by the Src homology-2 domain of p56lck. Nature. 1993;362:87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112:947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada H, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Imamura H, Takaishi K, Nakano K, Kodama A, Oishi H, Shiozaki H, Monden M, Sasaki T, Takai Y. Rho and Rab small G proteins coordinately reorganize stress fibers and focal adhesions in MDCK cells. Mol Biol Cell. 1998;9:2561–2575. doi: 10.1091/mbc.9.9.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng Y-F, Nakano K, Takaishi K, Takai Y. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells: regulation by Rho, Rac, and Rab small G proteins. Oncogene. 1999;18:6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signaling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto K, Fujita T, Takai Y. ADP-ribosylation of the bovine brain rho protein by botulinum toxin type C1. J Biol Chem. 1988;263:16303–16308. [PubMed] [Google Scholar]
- Kodama A, Takaishi K, Nakano K, Nishioka H, Takai Y. Involvement of Cdc42 small G protein in cell-cell adhesion, migration, and morphology of MDCK cells. Oncogene. 1999;18:3996–4006. doi: 10.1038/sj.onc.1202773. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Ma AD, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase γ, a rac guanosine exchange factor, and rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañes S, Mira E, Gómez-Mouton C, Zhao ZJ, Lacalle RA, Martínez AC. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T, Kasuga M. Roles of protein tyrosine phosphatases in growth factor signaling. Cell Signal. 1996;8:13–19. doi: 10.1016/0898-6568(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ, Jackson PK, Van Etten RA, Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992;12:609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Takaishi K, Kodama A, Mammoto A, Shiozaki H, Monden M, Takai Y. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:2481–2491. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Narsimhan RP, Gaudino G, Zarnegar R, Michalopoulos GK, Comoglio PM. Hepatocyte growth factor (HGF) stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene. 1991;6:501–504. [PubMed] [Google Scholar]
- Narumiya S, Sekine A, Fujiwara M. Substrate for botulinum ADP-ribosyltransferase, Gb, has an amino acid sequence homologous to a putative rho gene product. J Biol Chem. 1988;263:17255–17257. [PubMed] [Google Scholar]
- Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Holgado-Madruga M, Maroun C, Fixman ED, Kamikura D, Fournier T, Charest A, Tremblay ML, Wong AJ, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Matozaki T, Fujioka Y, Yamao T, Tsuda M, Takada T, Kasuga M. Characterization of a 115-kDa protein that binds to SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in Chinese hamster ovary cells. J Biol Chem. 1996;271:27652–27658. doi: 10.1074/jbc.271.44.27652. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology-2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Kubota M, Ohtake A, Sato K, Sano S. Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J Biol Chem. 1996;271:25569–25574. doi: 10.1074/jbc.271.41.25569. [DOI] [PubMed] [Google Scholar]
- Qi JH, Ito N, Claesson-Welsh L. Tyrosine phosphatase SHP-2 is involved in regulation of platelet-derived growth factor-induced migration. J Biol Chem. 1999;274:14455–14463. doi: 10.1074/jbc.274.20.14455. [DOI] [PubMed] [Google Scholar]
- Ren X-D, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Allen WE, Peppelenbosch M, Jones GE. Rho family proteins and cell migration. Biochem Soc Symp. 1999;65:111–123. [PubMed] [Google Scholar]
- Rodrigues GA, Park M. Autophosphorylation modulates the kinase activity and oncogenic potential of the Met receptor tyrosine kinase. Oncogene. 1994;9:2019–2027. [PubMed] [Google Scholar]
- Rordorf-Nikolic T, Van Horn DJ, Chen D, White MF, Backer JM. Regulation of phosphatidylinositol 3′-kinase by tyrosyl phosphoproteins: full activation requires occupancy of both SH2 domains in the 85-kDa regulatory subunit. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and special determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998;245:641–645. doi: 10.1006/bbrc.1998.8253. [DOI] [PubMed] [Google Scholar]
- Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Schuebel KE, Bustelo XR, Nielsen DA, Song BJ, Barbacid M, Goldman D, Lee IJ. Isolation and characterization of murine vav2, a member of the vav family of proto-oncogenes. Oncogene. 1996;13:363–371. [PubMed] [Google Scholar]
- Schuebel KE, Movilla N, Rosa JL, Bustelo XR. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991;1072:81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Sugimoto S, Wandless TJ, Shoelson SE, Neel BG, Walsh CT. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine-containing peptide derived from insulin receptor substrate-1. J Biol Chem. 1994;269:13614–13622. [PubMed] [Google Scholar]
- Takada T, et al. Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J Biol Chem. 1998;273:9234–9242. doi: 10.1074/jbc.273.15.9234. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Waksman G, et al. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992;358:646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W, Tanaka K, Nonaka H, Maeda A, Musha T, Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J Cell Biol. 1994;125:1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- Zhu H, Naujokas MA, Fixman ED, Torossian K, Park M. Tyrosine 1356 in the carboxyl-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]