Abstract
Background
Cardiac rehabilitation programs (CRP) represent comprehensive interventions that are typically limited to four months. Following completion of CRP, it appears that risk factors and lifestyle behaviours may deteriorate. The Extensive Lifestyle Management Intervention (ELMI) Following Cardiac Rehabilitation trial will investigate the benefits of a randomized intervention to prevent these adverse changes.
Methods
Patients with ischemic heart disease (IHD) were randomized following a standard CRP to the ELMI or to usual care. The ELMI program is a case-managed intervention aimed at individualizing risk factor and lifestyle management based on current treatment guidelines. The program consists of cardiac rehabilitation sessions, telephone follow-up and risk factor and lifestyle counselling sessions. Health professionals work with participants using behavioural counselling and communications with participants' family physicians. Usual care participants return to their family physicians' care, and come to the study clinic only to undergo annual outcomes assessment. The primary outcome is change in IHD global risk after four years. Secondary outcomes include combined cardiovascular events, health care utilization, lifestyle adherence, quality of life and risk factors.
Results
Over 28 months, 302 men and women were randomized. This represented 29% of the total population screened. The average age of study participants is 64 years, 18% are women, 53% have had a previous myocardial infarction, 73% have undergone previous revascularization and 20% have diabetes mellitus. Ischemic heart disease risk factors for the entire cohort improved significantly after subjects had gone through previous CRPs. Baseline risk factors, lifestyle behaviours and medications were similar between the groups.
Conclusions
This study population is representative of patients completing a standard CRP. Results of the ELMI trial will provide valuable information for the future design of CRPs.
Keywords: Cardiac rehabilitation, risk factor management, lifestyle management, compliance, ischemic heart disease, randomized trials
Background
Current cardiac rehabilitation programs (CRPs) employ pharmacological management, smoking cessation, nutrition, and exercise and behavioural counselling to effectively manage ischemic heart disease (IHD) risk factors and to promote favourable lifestyle changes. Previous research has demonstrated that CRPs can reduce morbidity and mortality as well as cost of care. [1-5] Studies such as the Stanford Coronary Risk Intervention Project (SCRIP) and the Lifestyle Heart Trial have demonstrated that long-term lifestyle and risk factor management results in regression of atherosclerosis and reduction in cardiovascular events.[3,4] Despite this finding, many North American programs are of only 3 to 4 months duration, due to budgetary and resource constraints, in addition to insurance coverage limitations. The Multi-fit trial tried to address this issue by conducting a post-MI nurse case-managed intervention consisting of counselling sessions and telephone follow-up that take place immediately following patients' cardiac events. The program, however, was not overwhelmingly successful in demonstrating long-tem comprehensive effectiveness.[6] Therefore, current CRPs face the daunting task of teaching life-long risk factor and lifestyle management within a short time frame.
Lifestyle adherence is difficult to achieve. Based on one report, less than one third of women were exercising the recommended three times per week within one year of completing a CRP.[7] Corresponding worsening of risk factors following completion of a CRP has also been described (body mass index [BMI], total cholesterol [TC], LDL-C and triglycerides [TG] deteriorated in the years following a CRP, with some risk factors reported to be worse than the pre-CRP values.[8,9]
At the time of the current study's development, no previous reports had investigated a CRP follow-up intervention. We therefore conducted our own pilot study to investigate lifestyle adherence and risk factors for six months following a CRP.[10] Thirty-six men and women were randomized to either a comprehensive lifestyle and risk factor intervention or to usual care. After a six-month intervention of six cardiac rehabilitation exercise sessions and two telephone follow-up calls, we reported significant decreases in TC and LDL-C in the intervention group only.
These findings provided the impetus for undertaking the current Extensive Lifestyle Management Intervention (ELMI), a four-year study of 302 men and women with IHD. We hypothesize that patients with IHD who have been randomized to the four-year ELMI program following a standard CRP will decrease their global risk for IHD compared to a similar patient cohort undergoing usual care. To assess global risk, we will use and individually analyze two independent global risk scores: the Framingham (FRA) and the Procam risk scores, which reflect modifiable risk factors such as total cholesterol (TC), HDL-cholesterol (HDL-C), blood pressure (BP) and smoking.[11,12]
Methods
Recruitment and randomization
Men and women from two identical, hospital-based CRPs were screened for the study inclusion and exclusion criteria (Table 1). Those patients meeting the study criteria were asked to provide informed consent at the time of their exit CRP assessment (approved by the University of British Columbia and the St. Paul's Hospital Ethics Committees). The study criteria were designed so that the majority of those individuals participating in cardiac rehabilitation would be eligible for the study. Patients with IHD and other cardiovascular co-morbidities (i.e., heart failure, atrial fibrillation, pacemaker, exercise-induced angina, etc.) were included in the study as long as the investigators believed that these co-morbidities would not interfere with full participation. Those patients whose physical conditioning was severely compromised were not discharged from the preceding CRP and, therefore, were not considered for the study. Consenting participants underwent a baseline lifestyle and risk factor assessment (exercise stress test, leisure time physical activity [LTPA], diet, quality of life, smoking status, blood pressure (BP), lipids, blood sugar, BMI, waist circumference (WC) and medication assessment).
Table 1.
Study inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
| • Patients with IHD who have completed a four month CRP. | • Inability to give informed consent. |
| • Men and women >18 years of age. | • Patients who have difficulty understanding the English language. |
| • No moving plans or extended trips. | • Participation in other research trials. |
| • Provide informed consent. | • Patients currently waiting for a surgical intervention. |
| • Patients with any other medical condition that would make survival for the duration of the study unlikely, interfere with optimal participation or produce a significant risk to the patient. |
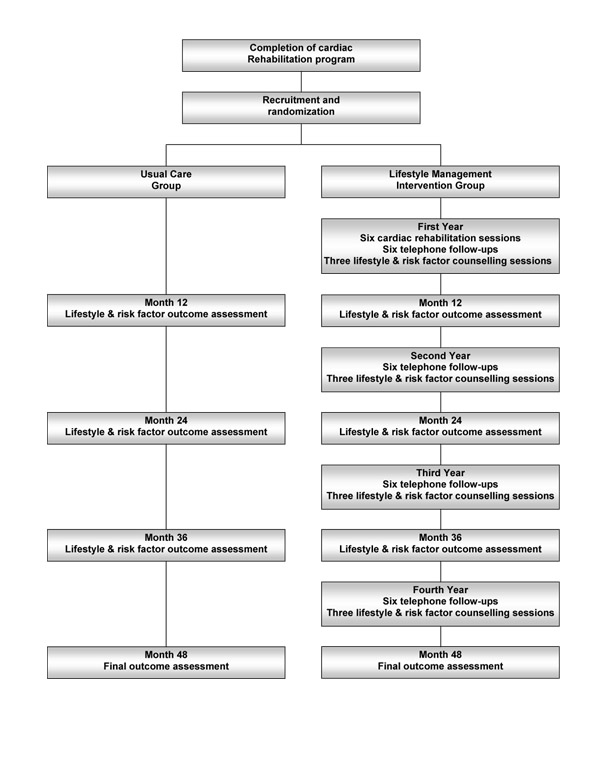
The randomization was balanced for age (<63 or ≥ 63 years), gender and adjustments in lipid-lowering medication (part of the initial CRP protocol) at the time of recruitment (prior to randomization). Random group assignment was conducted by a blinded research associate using computer-generated variable block randomization. Participants were randomized to either the ELMI or Usual Care (UC) groups, as depicted in Figure 1. Due to the nature of the study, participants were not blinded to their group assignment.
Figure 1.
Diagrammatic outline of the study.
Usual care group
Participants randomized to the UC group were informed that they would be contacted once per year to schedule their annual outcome assessment visits. No other contact is initiated with the UC participants throughout the study. After each outcome assessment, UC participants are informed of their results and of the desired targets for the specific risk factors. A copy of the lab results is sent to the UC participants' family physicians. Usual Care participants are instructed to direct any further questions to their family physician.
Extensive lifestyle management intervention group
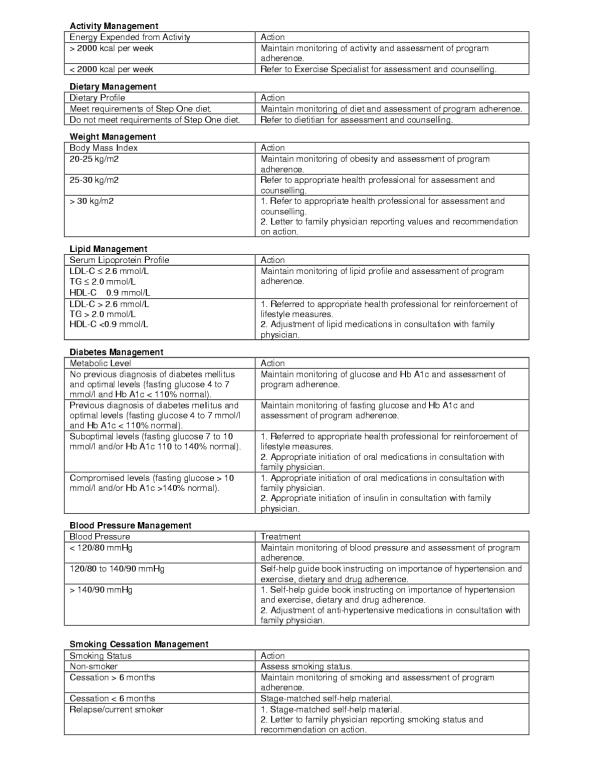
Participants randomized to the ELMI group were contacted by the case manager and scheduled for their first CRP exercise sessions. A copy of the Treatment Algorithms used in the intervention was mailed to their family physicians for information purposes only (Figure 2). The ELMI is a case-managed intervention designed so that each participant receives some form of contact every month during the first year of intervention and every other month in the following three years (Figure 1). This will result in greater exposure to health care services than usual care and will allow us to evaluate whether a new model of health services delivery yields additional health benefits compared to usual care practices. The first year of the ELMI consists of six CRP exercise sessions over the first three months, six telephone follow-ups and three lifestyle and risk factor counselling sessions. Thereafter, ELMI participants are scheduled for lifestyle and risk factor counselling sessions every six months, interspersed with telephone follow-ups at two-month intervals for three years. These modes of contact were chosen based on those used successfully in previous studies [3,4,6] as well as in our pilot study.[10]
Figure 2.
Lifestyle and risk factor treatment algorithms utilized during the ELMI lifestyle and risk factor counselling sessions.
The ELMI program employs the principles of the Transtheoretical Model of Change[13] and the Social Cognitive Theory.[14] During the CRP exercise sessions as well as during telephone follow-up calls and lifestyle and risk factor counselling sessions, each participant's stage of change is assessed prior to appropriate stage-based counselling. Participants are counselled on the positives of changing or maintaining a desired behaviour and are advised about the use of individual goal setting, based on readiness to change, personal experience and environment.
The six cardiac rehabilitation exercise sessions (once per week for four weeks and once per month for two months) are monitored by the case manager and by an exercise leader. Each session consists of a warm up, a medically prescribed target heart rate aerobic exercise, and a cool down period (approximately 75 minutes). During these sessions, participants are counselled and given guidance on how to establish a home-based exercise program. Each participant receives an ELMI Participant Manual at the onset of the exercise sessions. The manual contains a logbook for exercise, dietary and medication information to aid in lifestyle adherence and a timetable for scheduled intervention contacts.
Upon entry to the ELMI and at every lifestyle and risk factor counselling session (a total of ten), participants receive an ELMI Lifestyle and Risk Factor Report (Figure 3), designed to educate and empower ELMI participants to assume a greater role in their health care. The Report summarizes the participants' current lifestyle and risk factor profiles, their previous profile, current goals, and ideal risk factor and lifestyle targets. The Report is given to participants by the case manager, who explains it in detail and stresses the importance of managing each risk factor and lifestyle behaviour. On the reverse side of the Report, goals, recommendations and motivational comments are documented. A copy of the Report is forwarded to each participant's family physician, and, when applicable, to the cardiologist.
Figure 3.
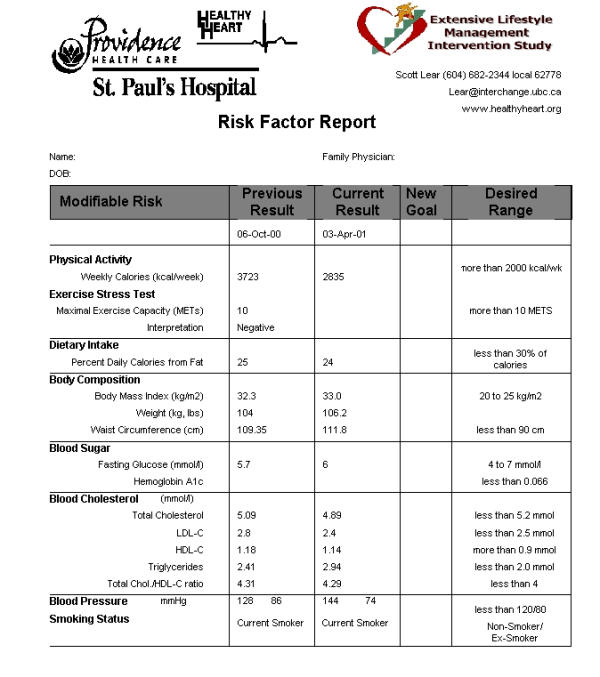
Lifestyle and Risk Factor Report developed at baseline and during the ten lifestyle and risk factor counselling sessions.
The telephone follow-up calls adhere to a formatted outline to 1) identify any new symptoms or change in symptoms, 2) follow-up on goal progress and 3) assess and counsel on exercise, diet, medications, smoking cessation and diabetes management, where applicable. This format is similar to that used successfully in our previous pilot study.[10] The case manager conducting the telephone follow-up reviews the last contact with the participant (either a previous follow-up call or an assessment and counselling visit) and the Lifestyle and Risk Factor Report. At this time, any previously set goals are discussed, and new goals are developed, if required. If the participant is unavailable, a message is left. Up to three unanswered messages are made before marking the telephone follow-up as a missed event. For those participants who do not have a means of receiving a message, the call is not counted as part of the possible three calls. Returned messages to the case manager are also not counted in the three possible calls.
The ELMI lifestyle and risk factor counselling sessions are conducted by the case manager a total of nine times per participant over the four-year intervention. Prior to these sessions, participants are asked to complete the LTPA and the 3-Day Food Record questionnaires and to undergo a fasting blood draw (lipid profile, glucose and HbA1c if diabetic). The sessions consist of assessment of BP, weight, WC, symptoms, medications and medication compliance. Month 6, 12, 24, 36 and 48 assessments also include a graded exercise stress test. The case manager reviews the new Lifestyle and Risk Factor Report, provides lifestyle and risk factor counselling as appropriate, and implements the ELMI Treatment Algorithms (Figure 2). Additional counselling from a dietitian, exercise specialist or smoking cessation nurse is determined from the Treatment Algorithms.
The Treatment Algorithms for the ELMI, derived from current guidelines and expert consensus [15-19], are aimed at bridging the gap between current guidelines and current practices.[20] For weight, diabetes, lipid and BP management, the last category of the Treatment Algorithms dictates that the family physician be contacted by letter regarding recommendations for intervention. These recommendations are authorized following consultation between the case manager and the program cardiologist (the cardiologist does not meet with the participant). Once the program cardiologist has been consulted and the letter of recommendation mailed, participants are asked to contact their family physicians. This process allows the ELMI to work within the framework of the health care system, recognizing the family physician as being the primary care provider. A copy of the letter is kept in the participant's chart and reviewed during the next participant contact.
Staff training
The case manager and health care professionals taking part in the ELMI (dieticians, exercise specialists, nurses, etc.) were from the St. Paul's Hospital Healthy Heart Program CRP. All have had several years' previous experience. In addition, all staff had previously undergone formal training in counselling techniques and the stages of change. A trained research coordinator is responsible for conducting the annual outcomes assessment for all participants.
Outcomes
The primary outcome is the absolute change in global risk for IHD from baseline to year four between the Usual Care and ELMI groups. Global IHD risk assessment was selected because it is considered to be superior to any single lifestyle behaviour or IHD risk factor, given that it reflects the multi-factorial nature of IHD.[21,22] Global risk is determined by using two independent global risk scores: 1) the Framingham (FRA) risk score (a sum of categorical points based on age, gender, TC, HDL-C, BP, presence of diabetes and smoking status)[11] and 2) the Procam risk score (a regression equation based on age, gender, TC, HDL-C, BP, presence of diabetes, smoking status, previous family history and presence of angina).[12] We have chosen to use two risk scores, since no one single score was felt to be superior to another for secondary prevention; however, these two scores will be analyzed and interpreted separately.
While the FRA and Procam risk scores were developed to predict future risk of disease, their use as an outcome measure does not rely on the accuracy to predict events but as an instrument for assessing change in global risk. Prior to their use in this study, minor modifications were made to the risk scores, to ease interpretation of changes in risk. These modifications will not affect how each individual risk factor contributes to the final score (i.e., age will change equally for all participants; therefore, this has been held constant). For the FRA risk score, age is held constant, results of variables falling above or below the highest or lowest point categories for each variable are given the score of the closest category, ex-smokers <12 months are treated as non-smokers, and those without previously diagnosed diabetes but with glucose >7.8 mmol/L are scored as diabetics. (This latter cut point was used to define individuals with diabetes in the original Framingham cohort.) For the Procam risk score, age is held constant, family history is recorded at baseline and carried through to follow-up unchanged, and participants without previously diagnosed diabetes but with glucose >6.7 mmol/L were scored as diabetics. (This latter value was used to define individuals with diabetes in the original Procam cohort.)
A number of secondary outcomes will be captured to reflect the multi-factorial nature of the intervention. These include combined cardiovascular events (myocardial infarction, revascularization procedures, hospital admissions of a cardiovascular nature), health care utilization, cost comparison analysis, lifestyle adherence (physical activity, exercise capacity, diet composition, smoking status), quality of life measures (stress, illness intrusiveness, self-efficacy), anthropometric measures (body mass index, waist circumference), IHD risk factors (lipids, fasting glucose, blood pressure) and percent participants treated to target.
Assessment methods
Cardiovascular events and health care utilization, identified through documentation of medical records, will be linked to the provincial Ministry of Health database. Lifestyle variables are captured through questionnaires, which will be distributed to participants via mail three to four weeks prior to their next scheduled outcomes assessment. Participants will be asked to complete the questionnaires at home and to give them to the research coordinator, who will review them to ensure that they have been fully completed.
Physical activity adherence is determined by the 4-week modified Minnesota LTPA questionnaire.[23] Exercise capacity, assessed by a symptom-limited treadmill exercise stress test with continuous 12-lead ECG monitoring, is reported as the maximal metabolic equivalents (METs) attained during the test. This method allows for the capture of ischemia and silent ischemia, based on ECG changes. Dietary adherence will be determined from a 3-Day Food Record [24] and will be analyzed using Nutritionist IV Diet Analysis software by First Data Bank. Average percent daily kilocalories (kcal) are recorded for protein, carbohydrates, total fat, saturated fat and unsaturated fat. Quality of life is assessed by the Perceived Stress Scale [25] and the Illness Intrusive Rating[26], both of which use Likert scoring. Self-efficacy is reported as both a general score and an exercise-specific self-efficacy score that is based on Likert scoring. The general self-efficacy questionnaire assesses an individual's perception of his/her ability to successfully achieve various health-related behaviours (i.e., diet, exercise, medications, etc.). The exercise-specific self-efficacy questionnaire assesses an individual's perception of his/her ability to successfully participate in structured exercise in the presence of various potential barriers such as inclement weather, social occasions, family, etc.
Body mass index is calculated from weight in kg divided by height in m squared. Waist circumference is measured directly over the skin (to the nearest 0.1 cm) at the point of maximal narrowing of the trunk as viewed from the anterior position, with the participant standing upright following a normal expiration.[27] A manual sphygmomanometer is used to determine blood pressure (mmHg), which is recorded as the average of two measures taken two minutes apart after five minutes of seated rest. Smoking status is determined by self-report. Serum TC, HDL-C, TG and glucose are assessed using standard methodology.[28] LDL-C is calculated using the Friedwald Equation (LDL-C = TC - HDL-C - TG/2.22).[29] The presence of angina is determined through patient interview, based on standard criteria.[30]
Statistical power considerations
At the time of the study's design, we were unable to project risk score variability (i.e., standard deviation) in this cohort over the selected follow-up period, since no data were available. We anticipated that 10% of participants would be lost to follow-up (consistent with follow-up frequencies in other long-term trials [3,31]), leaving 90% of participants available for assessment of the primary outcome. Reasons for being lost to follow-up include death, leaving the geographical area without a forwarding address and refusal to participate (either through direct contact or by not responding to repeated contact attempts). Importantly, lack of compliance is not a reason for withdrawal from the study. With a sample size of 135 people in each group, we will be able to detect 0.342 of a standard deviation of the change between the two groups at a power of 80% (α = 0.05, two sided, 0.363 of a standard deviation at a power of 90%). Based on data published from the SCRIP study, we will be able to detect differences in the mean change of three main contributors to the primary outcome and individual secondary outcomes as follows: TC of 0.28 mmol/L, HDL-C of 0.08 mmol/L, and systolic BP of 3.8 mmHg.[3] Therefore, after increasing the number randomized to account for the 10% lost to follow-up, a sample size of 300 participants was determined a priori, resulting in 150 participants being randomly assigned to each study group.
Data on 'lost to follow-up' participants will be analyzed to elucidate any relevant characteristics that may differ from the remainder of the study group. Every effort will be made to obtain final data on the primary outcome regardless of the extent of study participation to that point. All data for the primary and secondary outcomes will be analyzed using an intent-to-treat analysis such that participants will remain in their randomly assigned groups at final analysis, regardless of actual participation within that group.
Statistical analyses
Baseline characteristics of the ELMI and UC groups (age, gender, presence of concurrent disease, diagnosis, lifestyle behaviours and IHD risk factors and changes to these factors as a result of the prior CRP) were compared using Pearson Chi-square tests for categorical factors and independent samples t-test for continuous factors. Changes in IHD risk factors and lifestyle behaviours as a result of the initial CRP were analyzed for the entire cohort using a paired samples t-test. Change in smoking status as a result of the CRP was assessed by the McNemar Chi-square test.
Differences in the primary outcome between the ELMI and UC groups will be compared by an independent samples t-test. The two global risk scores will be analyzed and reported separately. The secondary outcome of combined cardiovascular events will be described using a Kaplan-Meier survival plot. Other secondary outcomes that are continuous variables (health care utilization, IHD lifestyle and risk factors) will be assessed by independent samples t-tests. Changes in categorical factors (smoking status and treat to target) will be assessed by the McNemar Chi-square test. Fulfillment of the ELMI program and use of medications will be presented as percentages and qualitative data. While not powered to draw conclusions for sub-group analyses, women and participants with diabetes will be analyzed for the purpose of hypothesis generation using non-parametric tests: the Mann-Whitney U test for independent samples and the Wilcoxon test for paired samples.
Data are reported as means ± standard deviations. All statistical analyses were performed using the SPSS 10.0.07 statistical package for Microsoft Windows. The significance level for all tests was set at 0.05 and all t-tests are two-tailed.
Results
Between January of 1998 and May of 2000, a total of 302 men and women who met the study criteria were recruited and randomized. Table 2 outlines the results of recruitment from both CRP sites. Approximately 60% of those patients exiting the CRP met the study inclusion/exclusion criteria. Reasons for eligible men and women refusing to provide consent included: not interested, conflict in schedule with proposed intervention (i.e., occupation), possible moving plans/uncertain of future plans, time in the CRP significantly extended due to medical reasons, and failure to be in contact after receiving the consent form. Of those eligible, approximately 49% provided informed consent and were randomized. Therefore, this cohort represents 29% of the entire population of patients who completed the two CRPs during the period of active recruitment. Those patients who were eligible but refused consent (n = 320) were no different from those that consented with respect to age, 63.5 ± 10.4 years (p > 0.05) and gender, 76% male (p > 0.05).
Table 2.
Results of recruitment from January, 1998 to May, 2000.
| CRP Site 1 | CRP Site 2 | |
| Patients screened | 751 | 295 |
| Eligible | 356 (47%) | 266 (90%) |
| Refused consent | 145 (19%) | 175 (59%) |
| Randomized | 211 (28%) | 91 (31%) |
Table 3 summarizes the IHD risk factors of the entire cohort before and after participation in the standard 16-week CRP, which preceded enrollment into this study. All parameters improved significantly, except for HDL-C and the proportion of smokers.
Table 3.
Changes to IHD risk factors from entire cohort as a result of CRP participation (n = 302).
| Before CRP | After CRP | |
| Total cholesterol (mmol/L) | 4.71 ± 0.95 | 4.52 ± 0.87** |
| LDL-C (mmol/L) | 2.75 ± 0.80 | 2.59 ± 0.72** |
| HDL-C (mmol/L) | 1.13 ± 0.32 | 1.14 ± 0.30 |
| Triglycerides (mmol/L) | 1.83 ± 0.98 | 1.71 ± 0.89* |
| TC/HDL-C | 4.40 ± 1.25 | 4.15 ± 1.12** |
| Blood Pressure (mmHg) | 131/76 ± 22/11 | 127/72 ± 21/10** |
| Smokers (%) | 16 (5%) | 12 (4%) |
| Exercise Capacity (METs) | 8.4 ± 2.6 | 10.0 ± 2.5** |
| BMI (kg/m2) | 28.0 ± 4.0 | 27.6 ± 4.0** |
* p < 0.01 compared to Before CRP (paired samples t-test). ** p < 0.001 compared to Before CRP (paired samples t-test).
Of the 302 participants randomized, 151 were assigned to the ELMI group and 151 were assigned to the UC group. Tables 4 through 6 describe the baseline characteristics of the two groups. The proportion of men and women was equal between the two groups, as were the sites of recruitment, proportion of participants with diabetes and those with previous myocardial infarction (MI). Participants randomized to the ELMI group had more percutaneous transluminal coronary angioplasty (PTCA) procedures, less coronary artery bypass graft (CABG) procedures and less frequency of IHD family history than those in the UC group. Age was similar between the two groups. Global risk scores and all metabolic IHD risk factors were similar between the ELMI and UC groups. Other IHD risk factors and lifestyle parameters were also similar between the two groups, except for BMI and WC, which were significantly lower in the UC group (Table 6). Changes in IHD risk factors as a result of previous CRP participation were no different between the two groups (data not shown).
Table 4.
Participant demographics: comparisons between ELMI and UC groups (totals with percentages).
| ELMI (n = 151) | UC (n = 151) | |
| Men | 125 (83%) | 124 (82%) |
| Age (years) | 64.8 ± 8.8 | 63.4 ± 10.2 |
| St. Paul's Hospital CRP | 108 (72%) | 103 (68%) |
| Family History | 43 (28%) | 58 (38%)* |
| IHD presentation | ||
| MI | 83 (55%) | 77 (51%) |
| CABG | 46 (30%) | 62 (41%)* |
| PTCA | 66 (44%) | 47 (31%)* |
| Other IHD indicators | 24 (16%) | 28 (19%) |
| Angina | 43 (28%) | 35 (23%) |
| Diabetes | 26 (17%) | 34 (23%) |
| Post-menopausal | 14 (54%) | 17 (63%) |
* p < 0.05 compared to ELMI group (independent samples t-test).
Table 6.
Baseline comparison of IHD global risk scores between ELMI and UC groups.
| ELMI (n = 151) | UC (n = 151) | |
| FRA Risk Score | 6.6 ± 3.1 | 6.5 ± 3.2 |
| PROCAM Risk Score (% incidence) | 20.0 ± 19.7 | 17.8 ± 18.5 |
| Total cholesterol (mmol/L) | 4.46 ± 0.87 | 4.59 ± 0.93 |
| LDL-C (mmol/L) | 2.53 ± 0.74 | 2.69 ± 0.74 |
| HDL-C (mmol/L) | 1.13 ± 0.31 | 1.15 ± 0.28 |
| Triglycerides (mmol/L) | 1.75 ± 0.94 | 1.65 ± 0.83 |
| TC/HDL-C | 4.15 ± 1.08 | 4.16 ± 1.17 |
| Glucose (mmol/L) | 5.8 ± 1.4 | 5.8 ± 1.7 |
| Blood Pressure (mmHg) | 128/72 ± 21/11 | 127/72 ± 20/10 |
| Smokers (%) | 5 (3%) | 7 (5%) |
| Exercise Capacity (METs) | 9.8 ± 2.7 | 10.0 ± 2.5 |
| LTPA (kcal/week) | 3137 ± 2531 | 2963 ± 2183 |
| BMI (kg/m2) | 28.1 ± 4.2 | 27.0 ± 3.7* |
| Waist circumference (cm) | 95.5 ± 12.8 | 92.7 ± 11.0* |
| Diet (% daily kcal) | ||
| Protein | 19 ± 4 | 19 ± 4 |
| Carbohydrate | 56 ± 9 | 57 ± 8 |
| Fat | 22 ± 7 | 22 ± 7 |
| Quality of life | ||
| Perceived stress | 33 ± 7 | 32 ± 8 |
| Illness intrusive | 31 ± 14 | 31 ± 15 |
| Global Self-efficacy | 42 ± 4 | 42 ± 5 |
| Exercise self-efficacy | 66 ± 11 | 65 ± 13 |
* p < 0.05 compared to ELMI group (independent samples t-test).
Table 5.
Medication use at baseline and year one for the ELMI and UC groups expressed as percent values (with absolute numbers).
| Medication | ELMI (n = 151) | UC (n = 151) |
| Lipid lowering | 87% (131) | 80% (121) |
| β-Blocker | 70% (105) | 67% (101) |
| ACE Inhibitor | 43% (65) | 41% (63) |
| Calcium Channel Blocker | 26% (39) | 22% (33) |
| Diuretic | 19% (28) | 15% (23) |
| ASA | 89% (135) | 84% (127) |
| Hypoglycemic Agents | 13% (19) | 17% (25) |
| Angiotensin Receptor Blocker | 5% (7) | 3% (5) |
| Anti-anginal | 19% (29) | 13% (20) |
| Hormone Replacement Therapy (% women) | 19% (5) | 26% (7) |
Discussion
The ELMI trial is designed to test a new method of health services delivery that can be readily used in and adapted to clinical settings throughout North America. To accomplish this, the ELMI incorporates a number of techniques proven successful in previous studies (case management, face-to-face visits, telephone follow-ups and incorporation of proven behavioural strategies)[3,6], in addition to the Lifestyle and Risk Factor Report, the Treatment Algorithms and close communications with the ELMI participants' family physicians.
Most of the contact between the case manager and the ELMI participants will be via telephone. This will allow for a greater frequency of contacts than would be reasonable for face-to-face visits, thereby permitting more patients to be managed by one case manager at any given time. We estimate that each ELMI participant will receive a minimum of 15 hours and a maximum of 23 hours of direct intervention contact over four years.
The incorporation of the Lifestyle and Risk Factor Report will: 1) educate and empower the patient and 2) communicate patients' progress to their primary care physicians. Previous literature has revealed that educating and empowering patients can lead to increased self-efficacy, which in turn can lead to successful behaviour change.[32,33] Delivering this report to the participant's primary care physician will not only facilitate communication (sometimes overlooked between specialist programs and primary care) but has been demonstrated to assist in the treatment of patients.[34]
A unique aspect of the ELMI trial involves the design and incorporation of the Treatment Algorithms. These will provide a consistent management guide across the ELMI, while recognizing that each individual has a unique attributable risk, thereby allowing appropriate allocation of resources. Another important aspect of the study relates to the use of current published clinical treatment guidelines (the same guidelines that are used in usual care) and the 'hands-off' approach to medication changes. These represent departures from previous multi-factorial IHD interventions, in that those studies used treatment targets that were more aggressive than usual care at the time and prescribed medications directly.[3,35] This method maintains optimal patient care by providing the primary care physician with the support and experience of the cardiologist, thus helping to reduce the treatment gap.[20,36]
Given that both of the CRPs from which participants were recruited draw their populations locally, it is possible that some family physicians may have patients in the ELMI group as well as in the UC group at any given time. While these family physicians will receive comprehensive updates on their patients in the ELMI group in addition to possible recommendations from the study cardiologist, we do not foresee contamination to those patients of the same physician in the UC group to be a major concern. The Treatment Algorithms distributed to the ELMI group participants' family physicians are meant to inform physicians about the nature of the study. Those algorithms that may require physician intervention are based on current guidelines that each physician has likely received on previous occasions from various other sources (British Columbia Medical Association, pharmaceutical marketing and medical journals). Recommendations from the program cardiologist are sent to the physicians regarding action for their patients in the ELMI group. These will be specific to each patient's current condition and medications and will not be applicable to other patients. To assess the possibility of treatment group cross contamination within a physician's practice post-study subgroup analysis will investigate whether the outcomes of those participants in the UC group whose family physicians also have patients in the ELMI group differ from the remainder of the UC group participants.
The study inclusion criteria were designed to recruit a cohort representative of current CRP populations. The main inclusion criterion for this study was documented diagnosis of IHD. Based on the high percentage of participants recruited (29% of the entire population and 48% of the eligible population), this cohort is representative of a large portion of the CRP population. Compared to other cohorts, our population has similar characteristics to that reported in a survey of CRP participants with respect to age, proportion of previous MI, CABG, and diabetes.[37] Women were not over sampled in this study and therefore represent only 17.5% of the randomized study population. It has been well documented that women are under-represented in CRP [38]; even when they do attend CRPs, they are more likely to drop out than men.[39] This may account for the low representation of women in the study. This also places limits on performing subgroup analysis based on gender.
Since the design of this study, two reports investigating interventions following CRP have been published .[40,41] In the first study, 40 patients were randomized to a one-year intervention consisting of either monthly counselling sessions by a nurse or usual care. At the end of the study, participants in the intervention group had significantly lower TC and LDL-C values compared to the usual care group.[40] This coincided with a significantly greater proportion of intervention participants using lipid-lowering medications (84% vs. 50%, p < 0.05). The authors concluded that the intervention resulted in greater medication compliance and lower lipid values.
The second study investigated 31 patients exiting a CRP who were randomly assigned to either a home-based maintenance program or to usual care. Patients were followed for nine months.[41] The home-based maintenance program consisted of an initial visit to the participant's home, followed by telephone calls from an exercise physiologist. The calls were conducted every two weeks during the course of the study. At the end of the study, there were no significant differences observed between the two groups with respect to weight, serum lipids or exercise capacity. The authors speculated that the lack of deterioration of risk factors in the usual care group may have accounted for the lack of significant results. Despite the recent report of these two studies, their inconsistency of results, small sample sizes and limited application to the multi-factorial nature of IHD make the implementation of the ELMI trial that much more timely.
Since this study begins after completion of a CRP, the study cohort presents with IHD risk factors and lifestyle behaviours at, or very close to, the recommended targets. Given that the study intervention is not as concentrated as the initial CRP, it is possible that this could affect the ability to detect a difference between the two groups. Current literature, however, indicates that risk factors and lifestyle behaviours decline following completion of a CRP.[7-9,42] Thus, the intervention is designed to maintain the gains from the initial CRP, anticipating that participants in the UC group will become non-adherent to diet, exercise, smoking cessation and medications, resulting in a deterioration of their risk factor profile.
A possible limitation of this study may be the use of global risk scores as the primary outcome. It is currently accepted that global risk is superior to the measure and interpretation of any single risk factor.[15] Using a global risk score will allow for ease of interpretation of effect, as it is difficult to interpret contradictory changes of individual risk factors (for example, BP decreases while LDL-C increases). However, no global risk indicator exists that is applicable to secondary prevention populations or that represents an outcome of change in global risk. For this reason, we have chosen to use two independent global risk scores to reflect changes in global risk. Although these risk scores were originally designed to predict future risk of events, this should not pose a problem, as we will assess the change in these risk scores rather than their predictive value.
It is generally accepted that a reduction in either of these global risk scores is favourable, regardless of the patient population. Even though other IHD risk factors that are targeted by the ELMI (obesity, sedentary behaviour and diet) are not included in the FRA and Procam risk scores, modification of these factors will exert an indirect affect on the risk score. In addition, all risk score components and other IHD risk factors will be analyzed as secondary outcomes. The collection of cardiovascular events will allow for possible validation of these two global risk scores in a secondary population.
Conclusions
The ELMI trial will investigate an individualized multi-factorial intervention aimed at preventing the deterioration in lifestyle behaviours and risk factors that occurs following a CRP. Recruitment and randomization have resulted in a cohort of men and women who are representative of those patients completing a typical CRP. With a cohort of over 300 men and women and a duration of four years, completion of the ELMI trial will make it one of the largest randomized, multi-factorial lifestyle and risk factor intervention studies conducted thus far. To date, no other study has tested an intervention aimed at preventing the lifestyle adherence and risk factor deterioration that follow cardiac rehabilitation. If the null hypothesis of the ELMI trial is disproved, this will have far-reaching implications for current CRP practices. Conversely, if the null hypothesis is accepted, this will provide valuable information with respect to the long-term durability of CRP.
Competing interests
None declared
Author contributions
Author SAL contributed to the study's design and is responsible for the implementation and analysis of the study.
Author AI contributed to the design of the medical management protocols and provides input into the medical management of the participants.
Author WL contributed to the design of the behavioural aspects of the intervention.
Author AB aided in recruitment of participants and implementation of the exercise management of the intervention.
Author MK supervised and interpreted the exercise stress tests (blinded to group assignment).
Author JJS contributed to the statistical analyses of the study and sample size determination.
Author PHP contributed to the design of the study.
Author JJF supervised the study's design and provides input into the medical management of the participants.
All authors read and approved the manuscript.
Acknowledgments
Acknowledgements
This work was supported by the British Columbia Health Research Foundation. Dr. Lear's salary support was provided by the Medical Research Council of Canada and the Heart and Stroke Foundation of Canada.
Contributor Information
Scott A Lear, Email: slear@providencehealth.bc.ca.
Andrew Ignaszewski, Email: aignaszewski@providencehealth.bc.ca.
Wolfgang Linden, Email: wlinden@vanhosp.bc.ca.
Anka Brozic, Email: abrozic@vanhosp.bc.ca.
Marla Kiess, Email: mkiess@rovidencehealth.bc.ca.
John J Spinelli, Email: jspinelli@bccancer.bc.ca.
P Haydn Pritchard, Email: haydn@unixg.ubc.ca.
Jiri J Frohlich, Email: jifr@unixg.ubc.ca.
References
- Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:945–950. doi: 10.1001/jama.260.7.945. [DOI] [PubMed] [Google Scholar]
- O'Connor GT, Buring JE, Yusuf S, Goldhaber SZ, Olmstead EM, Paffenbarger RS, Hennekens CH. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. doi: 10.1161/01.cir.80.2.234. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, Williams PT, Johnstone IM, Champagne MA, Krauss RM, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation. 1994;89:975–990. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- Ades PA, Pashkow FJ, Nestor JR. Cost-effectiveness of cardiac rehabilitation after myocardial infarction. J Cardiopulm Rehabil. 1997;17:222–231. doi: 10.1097/00008483-199707000-00002. [DOI] [PubMed] [Google Scholar]
- DeBusk RF, Miller NH, Superko HR, Dennis CA, Thomas RJ, Lew HT, Berger WE, Heller RS, Rompf J, Gee D, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120:721–729. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]
- Moore SM, Ruland CM, Pashkow FJ, Blackburn GG. Women's patterns of exercise following cardiac rehabilitation. Nurs Res. 1998;47:318–324. doi: 10.1097/00006199-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Willich SN, Muller-Nordhorn J, Kulig M, Binting S, Gohlke H, Hahmann H, Bestehorn K, Krobot K, Voller H. Cardiac risk factors, medication, and recurrent clinical events after acute coronary disease; a prospective cohort study. Eur Heart J. 2001;22:307–313. doi: 10.1053/euhj.2000.2294. [DOI] [PubMed] [Google Scholar]
- Brubaker PH, Warner JG, Rejeski WJ, Edwards DG, Matrazzo BA, Ribisl PM, Miller HS, Herrington DM. Comparison of standard- and extended-length participation in cardiac rehabilitation on body composition, functional capacity, and blood lipids. Am J Cardiol. 1996;78:769–773. doi: 10.1016/S0002-9149(96)00418-3. [DOI] [PubMed] [Google Scholar]
- Lear SA, Ignaszewski A, Laquer E, Pritchard PH, Frohlich JJ. Extensive Lifestyle Management Intervention Following Cardiac Rehabilitation: Pilot Study. Rehabil Nursing. 2001;26:227–232. doi: 10.1002/j.2048-7940.2001.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Assman G. Munich: MMV Medizin Verlag. Second 1993. Lipid Metabolism Disorders and Coronary Heart Disease, [Google Scholar]
- Prochaska JO, DiClemente CC. Transtheoretical therapy: Toward a more integrative model of change. Psychotherapy Theory Res Pract. 1982;19:276–288. [Google Scholar]
- Bandura A. Self-efficacy. Englewood Cliffs, NJ: Prentice-Hall. 1986.
- Anonymous National Cholesterol Education Program. Second Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II). Circulation. 1994;89:1333–1445. doi: 10.1161/01.cir.89.3.1333. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Fodor G, McPherson R, Genest J, Langner N. Rationale for and outline of the recommendations of the Working Group on Hypercholesterolemia and Other Dyslipidemias: interim report. Dyslipidemia Working Group of Health Canada. Can J Cardiol. 1998;14:17A–21A. [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. New Eng J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- Dawson KG. The clinical management of non-insulin dependent diabetes mellitus. British Columbia Medical Journal. 1997;39:80–83. [Google Scholar]
- Smith SC, Jr, Blair SN, Criqui MH, Fletcher GF, Fuster V, Gersh BJ, Gotto AM, Gould KL, Greenland P, Grundy SM, et al. Preventing heart attack and death in patients with coronary disease. Circulation. 1995;92:2–4. [PubMed] [Google Scholar]
- Wolff M, Bower DJ, Marbella AM, Casanova JE. US family physicians' experiences with practice guidelines. Fam Med. 1998;30:117–121. [PubMed] [Google Scholar]
- Fodor JG, Frohlich JJ, Genest JJ, Jr, McPherson PR. Recommendations for the management and treatment of dyslipidemia. Report of the Working Group on Hypercholesterolemia and Other Dyslipidemias. CMAJ. 2000;162:1441–1447. [PMC free article] [PubMed] [Google Scholar]
- Anonymous Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Richardson MT, Leon AS, Jacobs DR, Jr, Ainsworth BE, Serfass R. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47:271–81. doi: 10.1016/0895-4356(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Stuff JE, Garza C, Smith EO, Nichols BL, Montandon CM. A comparison of dietary methods in nutritional studies. Am J Clin Nutr. 1983;37:300–306. doi: 10.1093/ajcn/37.2.300. [DOI] [PubMed] [Google Scholar]
- Linn MW. Modifiers and Perceived Stress Scale. J Consult Clin Psychol. 1986;54:507–513. doi: 10.1037//0022-006X.54.4.507. [DOI] [PubMed] [Google Scholar]
- Devins GM. Illness intrusiveness and the psychosocial impact of lifestyle disruptions in chronic life-threatening disease. Adv Ren Replace Ther. 1994;1:251–263. doi: 10.1016/s1073-4449(12)80007-0. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Chumlea WC, Bouchard C, Himes JH, Lohman TG, Martin AD, Mitchell CD, Mueller WH, Roche AF, Seefeldt VD. Circumferences. In: TH Loham, editor. Anthropometric Standardization Reference Manual. Champaign: Human Kinetics; 1988. pp. 39–54. [Google Scholar]
- McGuinness C, Seccombe DW, Frohlich JJ, Ehnholm C, Sundvall J, G Steiner. Laboratory standardization of a large international clinical trial: the DAIS experience. DAIS Project Group. Diabetes Atherosclerosis Intervention Study. Clin Biochem. 2000;33:15–24. doi: 10.1016/S0009-9120(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- Campeau L. Letter: Grading of angina pectoris. Circulation. 1976;54:522–523. [PubMed] [Google Scholar]
- Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kalberer B, Weiss C, von Hodenberg E, Schlierf G, Schuler G, et al. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96:2534–2541. doi: 10.1161/01.cir.96.8.2534. [DOI] [PubMed] [Google Scholar]
- Meland E, Maeland JG, Laerum E. The importance of self-efficacy in cardiovascular risk factor change. Scand J Public Health. 1999;27:11–17. doi: 10.1080/14034949950153841. [DOI] [PubMed] [Google Scholar]
- Pellino T, Tluczek A, Collins M, Trimborn S, Norwick H, Engelke ZK, Broad J. Increasing self-efficacy through empowerment: preoperative education for orthopaedic patients. Orthop Nurs. 1998;17:48–51. [PubMed] [Google Scholar]
- Lowensteyn I, Joseph L, Levinton C, Abrahamowicz M, Steinert Y, Grover S. Can computerized risk profiles help patients improve their coronary risk? The results of the Coronary Health Assessment Study (CHAS). Prev Med. 1998;27:730–737. doi: 10.1006/pmed.1998.0351. [DOI] [PubMed] [Google Scholar]
- De Busk RF. Why is cardiac rehabilitation not widely used? West J Med. 1992;156:206–208. [PMC free article] [PubMed] [Google Scholar]
- Feely J. The therapeutic gap – compliance with medication and guidelines. Atherosclerosis. 1999;147:S31–S37. doi: 10.1016/S0021-9150(99)00253-1. [DOI] [PubMed] [Google Scholar]
- Thomas RJ, Miller NH, Lamendola C, Berra K, Hedback B, Durstine JL, Haskell W. National Survey on Gender Differences in Cardiac Rehabilitation Programs. Patient characteristics and enrollment patterns. J Cardiopulm Rehabil. 1996;16:402–412. doi: 10.1097/00008483-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Blackburn GG, Foody JM, Sprecher DL, Park E, Apperson-Hansen C, Pashkow FJ. Cardiac rehabilitation participation patterns in a large, tertiary care center: evidence for selection bias. J Cardiopulm Rehabil. 2000;20:189–195. doi: 10.1097/00008483-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Halm M, Penque S, Doll N, Beahrs M. Women and cardiac rehabilitation: referral and compliance patterns. J Cardiovasc Nurs. 1999;13:83–92. doi: 10.1097/00005082-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Labrador MP, Merz CNB, Pass R. A randomized trial of risk factor case management in coronary artery disease patients following cardiac rehabilitation (Abstr). Circulation. 1998;98:I811. [Google Scholar]
- Brubaker PH, Rejeski WJ, Smith MJ, Sevensky KH, Lamb KA, Sotile WM, Miller HS. A home-based maintenance exercise program after center-based cardiac rehabilitation: effects on blood lipids, body composition, and functional capacity. J Cardiopulm Rehabil. 2000;20:50–56. doi: 10.1097/00008483-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Suter PM, Suter WN, Perkins MK, Bona SL, Kendrick PA. Cardiac rehabilitation survey: maintenance of lifestyle changes and perception of program value. Rehabil Nurs. 1996;21:192–195. doi: 10.1002/j.2048-7940.1996.tb01704.x. [DOI] [PubMed] [Google Scholar]