Abstract
Background
Long term treatment with immunosuppressive agents results in nephrotoxicity in renal transplant recipients. We explored the effect of combination of Tacrolimus (TAC) and Sirolimus (SRL) on the immune system in renal transplant recipients.
Methods
10 stable renal transplant recipients were selected to participate in a pharmacokinetic study with a combination of TAC and SRL. Blood was drawn on day zero and 14 days post treatment. Lymphocyte proliferation was quantified by 3H-thymidine uptake assay (results expressed as counts per minute). The mRNA expression was studied by RT-PCR and serum levels of cytokines were quantified by ELISA and a cytokine bead array system.
Results
Lymphocyte proliferative response to PHA (p < 0.05), Con A (p < 0.006) and Anti-CD3 (p <0.005) were significantly decreased in patients who received both TAC and SRL compared to TAC alone. The mRNA expression of proinflammatory cytokines TNF-α (p < 0.05), cyclins G (p < 0.01) and E (p < 05) were decreased, and of TGF-β (p < 0.03) and p21 (p < 0.05) were increased in patients treated with this combination. Circulating levels of IFN-γ (p < 0.04), IL-4 (p < 0.02), and Il-2 (p < 0.03) were significantly inhibited and elevation of TGF-β (p < 0.04) was observed in patients treated with TAC and SRL combination.
Conclusion
These novel findings demonstrate that addition of SRL to TAC therapy enhances immuno modulation and causes increased immunosuppression providing a rationale for this concomitant therapy.
Background
Cyclosporine (CsA) and tacrolimus (TAC) are the primary immunosuppressive drugs used for the prevention of acute rejection after renal transplantation. The long-term treatment of patients with either CsA or TAC results in side effects such as nephrotoxicity, hypertension, and gingival hyperplasia [1,2]. Sirolimus (SRL) a new immunosuppressive agent is structurally similar to TAC binds to same binding protein FKBP, but its mechanism of action is different [3]. Both clinical and experimental studies demonstrated that SRL when used either with CsA or alone is associated with side effects such as hyperlipidemia, thrombocytopenia and nephrotoxicity [4-7]. Since SRL and TAC have affinity for the same binding site, it was thought to be ineffective when used together. However, experimental and clinical studies have refuted this assumption, and have successfully demonstrated the efficacy of this combination therapy [8-10].
Encouraged by our recent in vitro study [11] that demonstrated a significant inhibition of lymphocyte proliferation, IL-2 and induction of TGF-β expression by combination of sub-clinical concentration of SRL with either TAC or CsA, we conducted the current study on renal transplant recipients. A total of 10 stable renal transplant recipients were selected to participate in a pharmacokinetic (PK) and immune function study with combination of TAC and SRL therapy in human transplant. The current study describes the analysis of immune function in stable renal transplant recipients with SRL and TAC combination therapy.
Methods
Study subjects/Patients
Stable renal transplant recipients receiving TAC based immunosuppression were included in this study. Definition of "stable" transplant patients is based on post transplant care, renal function, acute rejection episodes. A total of 10 patients were enrolled after informed consent. Azathioprine/MMF (Mycophenolate Motefil) were discontinued prior to initiation of SRL and all patients were on corticosteroids. On day zero (24 h) after discontinuation of Aza/MMF), blood samples were collected at time zero (baseline) and 12 h after the administration of TAC for lymphocyte proliferation and cytokine expression at the gene and protein level. SRL was started on day 0 and patients were maintained on the same dose of TAC and SRL for 14 days. Blood samples were repeated on day 14 for immune functions, again at time zero (baseline and 12 h after). The graphic representation of the treatment protocol is shown in figure 1. The effect of this concomitant therapy on the immune function was studied by quantifying lymphocyte proliferation and the mRNA expression of TGF-β, TNF-α, IL-10, IL-6, IFN-γ by RT-PCR assay. Serum levels of cytokines (IFN-γ, TNF-α, IL-10, and IL-2) were quantified by a cytokine bead array system and TGF-β specific ELISA. Trough levels of TAC and SRL were not available as they were processed in central core laboratory as a part of multicenter study.
Figure 1.
Treatment protocol: A time line of treatment with TAC with MMF/AZA and steroids and TAC and SRL. Vertical arrows show times when blood samples were drawn.
Preparation of lymphocytes and Experimental Protocol
Peripheral blood mononuclear cells from the blood of renal transplants obtained at time zero (day # 0) and (day # 14) were separated by ficoll-hypaque as described by earlier [11]. Briefly, lymphocytes were washed twice in Phosphate Buffered Saline (PBS) and suspended in RPMI containing 5% FBS and Penicillin-Streptomycin. Lymphocytes were counted and adjusted to 1 × 106/ml and 200,000 cells were plated in a 96-well plate. Cells were cultured at 37 C in an ambient environment with 5% CO2 air with PHA (2 μg/ml), ConA (2 μg/ml) and anti-CD3 (100 ng/ml) for 60–70 h and last 16 hours with 3H-thymidine (1 μCi/well). The cells were then harvested on filter papers using a harvester, filter papers were dried and were then transferred to liquid scintillation vials and [3H] thymidine uptake were quantified as counts per minute (CPM) using a liquid scintillation counter.
Detection of mRNA by reverse transcription and polymerase chain reaction (RT-PCR)
Total RNA was isolated from lymphocytes isolated from blood obtained from patients using Trizol isolation System (Life Sciences, Invitrogen) and quality of RNA was verified by 260/280-nm ratio. 1 μg of RNA was reverse transcribed to cDNA using Superscript First Strand Synthesis system for RT-PCR (Life Technologies Rockville MD, USA). The amplification by polymerase chain reaction (PCR) was carried out using 1 μl of cDNA, and 2 μl each of 2.5 mM coding and non-coding oligonucleotide primers and Platinum PCR Supermix (Life Technologies Rockville MD, USA). The primer sequences of TGF-β, β-actin and p21 are described in our earlier studies [11,12]. The primer sequences for other genes are: TNF-α; coding 5'-AAGAATTCAAACTGGGGCCT-3' and noncoding: 5'-GGCTACATGG GAACAG CCTA-3' [13] Cyclin G: coding 5'-TGAAGCCTCAAAACATCACG-3' and noncoding 5'-CAGTGAT TGAAGCTGTG GGA-3' [14]; Cyclin E: coding 5'-TATACTTGCTGCTTC GGCCT-3' and non coding 5'-CTGCTCTGCTTC TTACCGCT-3' [15]; IL-6: coding 5' ATGAACTCCTTCTC CACAAGCGC-3' and non coding 5'-GAAGAGCCCTCA GGCTGGACTGC-3' [16]; IL-10: coding 5'-AAGCCTGACCACGCTTTCTA-3'; and non coding 5'-CTCAGCCT CCCAAGT AGCTG-3' [17]; IFN-γ: coding 5'-GCGAAAAAG GAGTCAGA TGC-3'; and non coding 5'-TGGGATCTTGCTTAGGTTGG-3' [18]; The PCR products were resolved in 1% agarose gel electrophoresis, ethidium bromide stained specific bands will be visualised under UV light and photographed. The densitometric analysis of the specific bands was made using Alpha-Imager (Alpha Innotech Corp. San Leandro CA USA) and data are represented as the ratio of the specific gene to β-actin.
Cytokine Bead Array
We studied the expression of circulating levels of cytokines IFN-γ, TNF-α, IL-10, IL-5, IL-4 and IL-2 in sera of patients using a new cytokine bead array system (BD Biosciences San Diego CA, USA). This method also named multiplexed bead assay is comprised of spectrally discrete particles, which can be used to quantitative soluble particles, in this case cytokines using a fluorescence based detection mechanism and flowcytometric analysis. The beads coated with IFN-γ, TNF-α, IL-10, IL-5, IL-4 and IL-2 react with test sera and standards, to which fluorescence dyes are then added. The flowcytometric analysis using special software allows the calculation of concentrations in the unknown sera.
Quantification TGF-β protein in sera
Circulating levels of TGF-β1 protein in patients' sera were quantified using the TGF-β specific ELISA (Promega, Madison, and USA), as described by us earlier [11].
Data Analysis
Statistical analyses were performed by using statistical program purchased from GraphPad Software, Inc.(San Diego, CA USA). The results are expressed as Mean ± SEM and a two-tailed p value of < 0.05 was considered significant.
Results
Clinical Parameters
The mean age of patients included in this study was 45 years (32–56 years). The study was conducted after a mean period of 49 months (16–70 months) post-transplantation. The mean plasma creatinine prior to SRL therapy was 1.3 mg/dl (1.0–1.7 mg/dl) and was 1.2 mg/dl (0.9–1.6 mg/dl) at the end of 14 days. The mean TAC dose and level prior to SRL therapy was 6.5 mg/day (4–10) and 8 ng (6–14 ng/ml). The TAC dose was not adjusted during the study period and mean TAC level at 14 days was 6 ng/ml (4–13 ng/ml). None of these recipients experienced acute rejection or any other side effects with this therapy. The exact SRL levels were not available as they were processed in central core laboratories, however were maintained at therapeutic level.
Effect of concomitant tacrolimus and rapamycin therapy on lymphocyte proliferation
The proliferation of lymphocytes from patients treated with TAC and SRL were compared with lymphocytes from patients treated with TAC and MMF/Aza. The results are shown in Figure 2 which, demonstrated a significant inhibition of proliferation of lymphocytes from patients with TAC and SRL compared to TAC plus MMF/Aza. (PHA: 17510 ± 4094 vs 10963 ± 3008 p < 0.05; ConA: 23995 ± 2449 vs 12124 ± 2946 p < 0.006; and anti-CD3: 27089 ± 1557 vs 16093 ± 3097 p < 0.005).
Figure 2.
Effect of different treatment protocols on lymphocyte proliferation: Peripheral blood mononuclear cells from the blood of renal transplants obtained on (day # 0) and on (day # 14) were separated by ficoll-hypaque. [3H]-thymidine uptake was quantified as counts per minute (CPM) using a liquid scintillation counter. The open bars represent the proliferation data (Mean ± SEM) of lymphocyte proliferation from patients treated with TAC/MMF/AZA and steroids and closed bars represent data after addition of SRL to TAC (day # 14) without MMF/AZA.
Effect of concomitant tacrolimus and rapamycin therapy on gene expression in lymphocytes
We compared the mRNA expression of cytokines; IFN-γ, TNF-α, Il-6, IL-10 and growth regulatory molecules; TGF-β, p21 and cyclins E and G in lymphocytes from patients treated with TAC + MMF/Aza and TAC + SRL.
i) TGF-β mRNA
As shown in figure 3, expression of TGF-β in lymphocytes from patients treated with both TAC and SRL expressed as ratio with β-actin (0.9 ± 0.12) significantly increased (p < 0.03), when compared to TAC and MMF/Aza (0.5 ± 0.12).
Figure 3.
TGF-β and p21 mRNA expression: TGF-β and p21 mRNA with housekeeping gene β-actin with TAC/MMF/Aza (day #0) and TAC and SRL (day# 14) are shown in the figure.
ii) p21 mRNA expression
The results are expressed as ratio of p21 with β-actin demonstrate a significant (p < 0.05) increase in patients treated with both TAC and SRL (1.02 ± 0.25), when compared with patients when they were treated with TAC and MMF/Aza (0.5 ± 0.24) {Figure 3}.
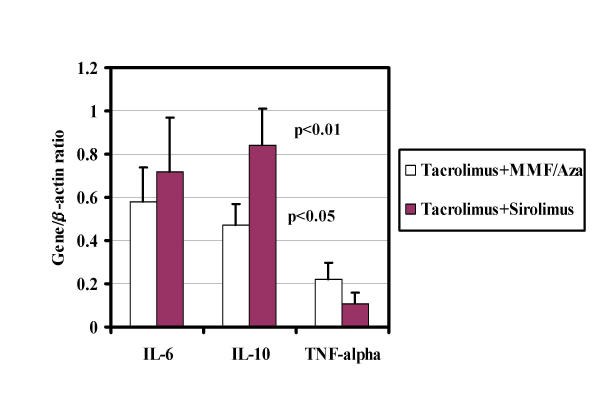
iii) IL-6 and IL-10 mRNA
Figure 4 illustrates comparison of the IL-6 and IL-10 mRNA expression in patients treated with TAC/MMF/Aza and TAC/SRL. The results are expressed as ratio with β-actin, a significant (p < 0.01) increase in IL-10 mRNA in patients treated with both TAC and SRL (0.84 ± 0.1), when compared with TAC and MMF/Aza (0.47 ± 0.1). Though an increase in IL-6 mRNA was observed in renal transplant recipients treated with TAC/SRL (0.72 ± 0.2) compared to patients treated with TAC/MMF/Aza (0.59 ± 0.16), the difference was not significant.
Figure 4.
IL-6, IL-10 and TNF-α mRNA expression: The ratios of IL-6, IL-10 and TNF-α with housekeeping gene β-actin with TAC/MMF/Aza (day #0) and TAC and SRL (day # 14) are shown in the figure.
iv) TNF-α mRNA
We also studied the effect of SRL on pro-inflammatory cytokine TNF-α in lymphocytes from these patients (Figure 4). The results expressed as ratio with β-actin, demonstrate a marked decrease (p < 0.05) in TNF-α levels in lymphocytes from patients treated with TAC and SRL (0.22 ± 0.08) compared to patients treated with TAC/MMF/Aza (0.11 ± 0.05).
v) Cyclin mRNA expression
The results of cyclins G and E mRNA expressed as ratio with β-actin shown in Figure 5. A significant decrease (p < 0.01) in Cyclin G mRNA in lymphocytes from patients treated with TAC and SRL (0.23 ± 0.05) compared to patients treated with TAC/MMF/Aza (0.08 ± 0.01). Similarly, a significant decrease (p < 0.01) in Cyclin E mRNA was noted with TAC and SRL (0.2 ± 0.04) compared to TAC/MMF/Aza (0.1 ± 0.01). Combination of decreased expression of cyclin and increased p21 mRNA expression correlates with the decreased lymphocyte proliferation.
Figure 5.
Cyclins G and E mRNA expression: The ratios of Cyclins G and E with housekeeping gene β-actin with TAC/MMF/Aza (day #0) and TAC and SRL (day #14) are shown in the figure.
Circulating levels of cytokines
Combination of TAC and SRL resulted in a significant inhibition of cytokines in sera of patients compared to patients treated with TAC/MMF/Aza. The results shown in Figure 6 are expressed as pg/ml; IFN-γ(9 ± 2 vs 6 ± 1.6, p < 0.04), IL-4 (7 ± 0.35 vs 5.8 ± 0.36, p < 0.02) and IL-2 (7 ± 0.36 vs 6 ± 0.26 p < 0.03) without any change in the levels of TNF-α, IL-5 and IL-10.
Figure 6.
Circulating levels of proinflammatory cytokines: Significantly decreased levels of IFN-γ (p < 0.04), IL-4 (p < 0.02) and IL-2 (p < 0.03) in patients treated with TAC and SRL compared to TAC/MMF/Aza are shown.
TGF-β protein expression in sera of patients
Since we observed a significant increase in TGF-β mRNA expression in lymphocytes from patients treated with combination of TAC and SRL compared to TAC/MMF/Aza, we also studied circulating levels of total TGF-β protein in acid activated sera from these patients. The results (figure 7) demonstrate statistically increased levels (p < 0.04) of TGF-β in renal transplant recipients after addition of SRL to TAC (107 ± 17.7 ng/ml vs 135 ± 18 ng/ml). These results demonstrate that decreased lymphocyte proliferation in part may be due to increased levels of TGF-β in these patients.
Figure 7.
Circulating levels of TGF-β protein: A significant (p < 0.04) increase in circulating levels of TGF-β in patients treated with SRL and TAC are shown compared to TAC/MMF/Aza.
Discussion
A number of studies have demonstrated that long treatment of both CsA and TAC is associated many side effects such as nephrotoxicity, hypertension, and gingival hyperplasia [1,2]. Rapamycin clinically termed, as Sirolimus (SRL) is a new immunosuppressive agent, which is structurally similar to TAC, binds to same binding protein FKBP, but has different mechanism of action [3]. Both clinical and experimental studies demonstrated that SRL when used either with CsA or alone is associated with side effects like hyperlipidemia, thrombocytopenia and nephrotoxicity [4-7]. SRL and TAC compete for the same binding site and hence it was thought they could not be used concomitantly. However, recent experimental and clinical studies have refuted this assumption, and have successfully used this combination therapy [8-10]. Therefore there is need of an alternate strategy to use these drugs to treat renal transplant recipients. Recently [11], we have demonstrated the efficacy of combination of low doses of SRL with either CsA or TAC to inhibit lymphocyte proliferation. The results from this study also demonstrate the increased immuno modulation in renal transplant recipients by this therapy. The synergistic effect of SRL and CsA has been demonstrated in animal models and clinical transplantation [19,20]. This prompted us to explore the mechanistic action of SRL and TAC when used in combination. Patients when received a concomitant therapy of TAC and SRL exhibited a significant inhibition of their lymphocytes when cultured with mitogens; PHA, ConA and anti-CD3, compared to the proliferation of lymphocytes obtained from when they were treated with TAC and MMF/Aza. Such a sustained effect is expected to prevent graft rejection, demonstrating the efficacy of this combination in renal transplant recipients.
To analyse the mechanism of the effect of concomitant therapy of TAC and SRL on lymphocyte proliferation, we studied the mRNA expression of TGF-β, p21, IFN-γ, TNF-α, Il-6, IL-10, cyclins G and E. The results demonstrated a significant increase in TGF-β mRNA expression (p < 0.03) with TAC and SRL. The expression of p21 mRNA was also increased in renal transplant recipients with this therapy. These results are consistent with our previous studies [21,22], that TGF-β mediates in part the immunosuppressive effects of CsA and TAC. Current study supports our hypothesis that the immunosuppression results in an increased expression of cyclin inhibitor p21, which by inhibiting the expression of cyclins controls the allo-immune activation. Since p21 is one of the most potent cyclin inhibitor, these results may explain the significant inhibition of proliferation of lymphocytes may in part be due to the increased p21 expression. The expression of cyclin E and G, which controls the cell cycle progression and G0/S and G1 phase of cell cycle, was decreased with TAC and SRL therapy. The expression of cyclins also corresponds to the decreased lymphocyte proliferation with SRL and TAC therapy.
We noted an increased expression of IL-6 and IL-10 mRNA in lymphocytes from patients with concomitant therapy with TAC and SRL. A number of experimental and clinical studies [23,24] have demonstrated IL-10 expression to be beneficial and correlates with increased graft survival. The results from this study demonstrate down regulation of cyclins, and up-regulation of cyclin inhibitor p21 and IL-10 mRNA expression.
IL-6, like IL-10 has also been considered to possess both pro- and anti-inflammatory properties [25,26]. In this study, we observed an increase in mRNA expression of IL-6. Acute rejection has been associated with increased intragraft IL-6 protein and mRNA production [27]. However, other studies have failed to demonstrate any correlation between IL-6 production and rejection [28]. The current study demonstrate that a concomitant therapy of TAC and SRL increases expression of IL-6 mRNA in lymphocytes and its significant remains to be evaluated.
We also quantified circulating levels of TGF-β, IL-2, IL-4, TNF-α, and IFN-γ. The results from this study demonstrate that addition of SRL to TAC therapy results in a significant (p < 0.04) increase in circulating levels of TGF-β. We have earlier demonstrated that TGF-β in part mediates the immunosuppressive effects of these drugs [11,12,21,22] and therefore the increased levels of TGF-β may partially explain the increased inhibition of mitogen induced lymphocyte proliferation. Though the role of TGF-β has been correlated with drug induced nephrotoxicity [29] it is early to speculate if this combination will also result in an increased nephrotoxic effects. We are unable to demonstrate the nephrotoxicity with this combination due to short course of therapy.
The levels of IFN-γ, IL-4 and IL-2 were decreased substantially with SRL and TAC therapy {IFN-γ (p < 0.04), IL-4 (p < 0.02) and IL-2 (p < 0.03)}. This indicates that the inhibition of lymphocyte proliferation with SRL also resulted in decreased inflammatory response.
Conclusions
In conclusion, these results provide evidence to potential efficacy of TAC and SRL combination therapy in renal transplant recipients. The addition of SRL to the immunosuppressive regimen of Tac resulted in decreased lymphocyte proliferation exhibiting decreased allo-immune response. Also, mRNA expression of proinflammatory cytokines TNF-α, Interferon-γ and cyclins G1, and E were significantly decreased and the expression of TGF-β and p21 were increased. The addition of SRL significantly decreased the circulating levels of pro-inflammatory cytokines and conversely increased the circulating levels of TGF-β, partially explaining the increased immunosuppression in these patients. However, due to short span of this treatment, the nephrotoxic effects remains unclear. These novel findings provide a mechanism and rationale for a concomitant therapy with TAC and SRL in renal transplant patients.
Author 1 initials AK planned and carried out all the studies with the help of Author 2 initials MP, Author 3 initials CB assisted in collecting blood samples. Author 4 initials JW carried out Cytokine Bead Array analysis for serum cytokine levels and Author 5 initials S H recruited patients for this study and clinically managed these patients.
Acknowledgments
Acknowledgements
This work was supported in part by research grant from the National Institutes of Health RO1AI41703.
Contributor Information
Ashwani Khanna, Email: akkhanna@mcw.edu.
Matthew Plummer, Email: mplummer@mcw.edu.
Katherine Bromberek, Email: cbromber@mcw.edu.
Jeffrey Woodliff, Email: jwoodlif@mcw.edu.
Sundaram Hariharan, Email: hari@mcw.edu.
References
- Kahan BD. Cyclosporine. N Engl J Med. 1989;321:1725–1728. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Plosker GL, Foster RH. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59:323–389. doi: 10.1159/000012190. [DOI] [PubMed] [Google Scholar]
- Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/S0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- Trotter JF, Wachs ME, Trouillot TE, Bak T, Kugelmas M, Everson G, Kam I. Dyslipidemia during sirolimus therapy in liver transplant recipients occurs with concomitant cyclosporine but not tacrolimus. Liver Transpl. 2001;7:401–408. doi: 10.1053/jlts.2001.23916. [DOI] [PubMed] [Google Scholar]
- Dominguez J, Mahalati K, Kiberd B, McAlister VC, MacDonald AS. Conversion to rapamycin immunosuppression in renal transplant recipients: report of an initial experience. Transplantation. 2000;70:1244–1247. doi: 10.1097/00007890-200010270-00021. [DOI] [PubMed] [Google Scholar]
- Meier-Kriesche HU, Kaplan B. Toxicity and efficacy of sirolimus: relationship to whole-blood concentrations. Clin Ther. 2000;22:B93–100. doi: 10.1016/S0149-2918(00)89026-8. [DOI] [PubMed] [Google Scholar]
- Andoh TF, Burdmann EA, Fransechini N, Houghton DC, Bennett WM. Comparison of acute rapamycin nephrotoxicity with cyclosporine and FK506. Kidney Int. 1996;50:1110–1117. doi: 10.1038/ki.1996.417. [DOI] [PubMed] [Google Scholar]
- Qi S, Xu D, Peng J, Vu MD, Wu J, Bekersky I, Fitzsimmons WE, Peets J, Sehgal S, Daloze P, Chen H. Effect of tacrolimus (FK506) and sirolimus (rapamycin) mono- and combination therapy in prolongation of renal allograft survival in the monkey. Transplantation. 2000;69:1275–1283. doi: 10.1097/00007890-200004150-00012. [DOI] [PubMed] [Google Scholar]
- Vu MD, Qi S, Xu D, Wu J, Fitzsimmons WE, Sehgal SN, Dumont L, Busque S, Daloze P, Chen H. Tacrolimus (FK506) and sirolimus (rapamycin) in combination are not antagonistic but produce extended graft survival in cardiac transplantation in the rat. Transplantation. 1997;64:1853–1856. doi: 10.1097/00007890-199712270-00039. [DOI] [PubMed] [Google Scholar]
- McAlister VC, Gao Z, Peltekian K, Domingues J, Mahalati K, MacDonald AS. Sirolimus-tacrolimus combination immunosuppression. Lancet. 2000;355:376–377. doi: 10.1016/S0140-6736(99)03882-9. [DOI] [PubMed] [Google Scholar]
- Khanna AK. Mechanism of the combination immunosuppressive effects of rapamycin with either cyclosporine or tacrolimus. Transplantation. 2000;70:690–694. doi: 10.1097/00007890-200008270-00027. [DOI] [PubMed] [Google Scholar]
- Khanna A, Hosenpud J. Cyclosporine induces the expression of the cyclin inhibitor p21. Transplantation. 1999;67:1262–1268. doi: 10.1097/00007890-199905150-00011. [DOI] [PubMed] [Google Scholar]
- Haegeman G, Content J, Volckaert G, Derynck R, Tavernier J, Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986;159:625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, deVries JE, Roncarolo MG, Mosmann TR, Moore KW. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci USA. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PW, Leung DW, Pennica D, Yelverton E, Najarian R, Simonsen CC, Derynck R, Sherwood PJ, Wallace DM, Berger SL, Levinson AD, Goeddel DV. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982;295:503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts JM. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Horne MC, Goolsby GL, Donaldson KL, Tran D, Neubauer M, Wahl AF. Cyclin G1 and cyclin G2 comprise a new family of cyclins with contrasting tissue-specific and cell cycle-regulated expression. J Biol Chem. 1996;271:6050–6061. doi: 10.1074/jbc.271.11.6050. [DOI] [PubMed] [Google Scholar]
- Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, Kohr WJ, Aggarwal BB, Goeddel DV. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312:724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Qi S, Xu D, Peng J, Liu D, Chen H. Synergistic effect of rapamycin and cyclosporine in prevention of acute kidney allograft rejection in the mouse. Microsurgery. 1999;19:344–347. doi: 10.1002/(SICI)1098-2752(1999)19:7<344::AID-MICR11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kahan BD, Podbielski J, Napoli KL, Katz SM, Meier-Kriesche HU, Van Buren CT. Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantation. Transplantation. 1998;66:1040–1046. doi: 10.1097/00007890-199810270-00013. [DOI] [PubMed] [Google Scholar]
- Khanna A, Cairns V, Becker CG, Hosenpud JD. Transforming Growth Factor-beta (TGF-β) mimics and anti-TGF-β antibody abrogates the in-vivo effects of Cyclosporine: demonstration of a direct role of TGF-β in immunosuppression and nephrotoxicity of CsA. Transplantation. 1999;67:882–888. doi: 10.1097/00007890-199903270-00016. [DOI] [PubMed] [Google Scholar]
- Khanna A, Cairns V, Hosenpud JD. Tacrolimus induces increased expression of transforming growth factor-beta in lymphoid and non-lymphoid cells. Transplantation. 1999;67:614–619. doi: 10.1097/00007890-199902270-00021. [DOI] [PubMed] [Google Scholar]
- Itano H, Mora BN, Zhang W, Ritter JH, McCarthy TJ, Yew NS, Mohanakumar T, Patterson GA. Lipid-mediated ex vivo gene transfer of viral interleukin 10 in rat lung allotransplantation. J Thorac Cardiovasc Surg. 2001;122:29–38. doi: 10.1067/mtc.2001.114636. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Wang C, Carpenter D, Okada Y, Nicolaidou E, Toyoda M, Trento A, Jordan SC. Prolongation of allograft survival with viral IL-10 transfection in a highly histoincompatible model of rat heart allograft rejection. Transplantation. 2001;71:686–691. doi: 10.1097/00007890-200103150-00020. [DOI] [PubMed] [Google Scholar]
- Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Flamme AC, Pearce EJ. The absence of IL-6 does not affect Th2 cell development in vivo, but does lead to impaired proliferation, IL-2 receptor expression, and B cell responses. J Immunol. 1999;162:5829–5837. [PubMed] [Google Scholar]
- Newstead CG, Lamb WR, Brenchley PE, Short CD. Serum and urine IL-6 and TNF-alpha in renal transplant recipients with graft dysfunction. Transplantation. 1993;56:831–835. doi: 10.1097/00007890-199310000-00010. [DOI] [PubMed] [Google Scholar]
- Jain S, Furness PN, Nicholson ML. The role of transforming growth factor beta in chronic renal allograft nephropathy. Transplantation. 2000;69:1759–1766. doi: 10.1097/00007890-200005150-00001. [DOI] [PubMed] [Google Scholar]