Abstract
Background
Cutaneous leishmaniasis (CL) is endemic in the Middle Eastern countries. New cases are emerging in areas previously free of the disease. In Jordan, the diagnosis of cases during the 1960s and 1970s was mainly reported in military hospitals in Amman. Endemicity of the disease was ascertained after reporting a total of 524 cases during 1973–1978.
Results
Leishmania major and Leishmania tropica were isolated from seventy-six autochthonous and imported cases of CL, during eight-year period. The highest infection rates recorded were in the central part of Jordan (60.5%), in males (72.4%) and in the age group 21–30 years (30.5%). Lesions were on the exposed sites of the body, mainly on the face (40%). Both Leishmania spp. were isolated from all parts of the country, although L. major was the predominant species (75% of cases) in all areas except in the north part of Jordan. Isoenzyme characterization of the isolates identified four previously undescribed zymodemes (Z). Four Leishmania major zymodemes were found, one of which was a new zymodeme (ZMON-103 variant in GLUD220); L. major ZMON-103 was the most common zymodeme. Four Leishmania tropica zymodemes were identified, of which three were previously unreported. Of these, ZMON-54 var PGD96–97 was isolated from autochthonous cases, whereas ZMON-59 var MDH100 and ZMON-75 var FH110 were obtained from both autochthonous and imported cases, or from an imported CL case, respectively.
Conclusion
The findings of this study indicate the emergence of the CL disease in new areas. New foci are reported, where the sporadic nature of the cases indicates recent spread of the disease to these areas and the urge for the implementation of control measures.
Background
Cutaneous leishmaniasis (CL) is still considered as an important health problem in many parts of the world especially the Mediterranean and Middle East countries [1-4]. Difficulty in identifying these parasites due to the similar morphology of amastigotes of different Leishmania species has been solved using isoenzyme characterization [5]. This is the most common method used to study the variability of Leishmania spp. in general. Several PCR assays using specific primers were reported to be extremely sensitive and promising in the detection and identification of the New World [6] or Old World [7,8] species of Leishmania directly in clinical samples. The first cases of CL in Jordan were reported by Adler and Theodor in 1929 [9]. No further reports appeared until 1968 when WHO reported 33 cases in East Jordan [10]. The endemicity of the region was recognized in the 1970s due to the increase in the number of cases diagnosed. A total of 524 cases were reported during 1973–1978, half of them occurred in the Jordan Valley [11]. Further information on human cases [12] sandfly vectors [13], and reservoir hosts [14] from several localities were reported on both the incidence and the spread of the disease to other geographical locations in Jordan. Cases of CL were reported from many locations in the country [2,11,12]; both L. major and L. tropica were reported. More cases of L. major were reported in the central part of the Jordan valley. Cases of L. tropica were more sporadic and reported mainly in the northern regions, species identification was based on the isoenzyme characterization [15].
While many facts of the epidemiology and clinical aspects of CL caused by L. major (dry type) and L. tropica (wet type) have been revealed, information on the parasites distribution and the epidemiologic factors governing their transmission are sparse. Zoonotic cutaneous leishmaniasis (ZCL) caused by L. major was reported for many years to be the only causative agent prevalent in Jordan mainly in rural areas [16]. In Jordan, the diagnosis of CL cases during the 1960s and 1970s was mainly reported in military hospitals in Amman [11]. Endemicity of the disease was ascertained after reporting a total of 524 cases during 1973–1978 [17]. Almost 50% of these cases came from the Jordan Valley, which is considered a hyper endemic region in Jordan. The Ministry of Health became increasingly aware of the seriousness of the leishmaniasis as a public health problem, procedures for the diagnosis and isolation of the parasite in cultures were added to their parasitologic procedures and isolates obtained were identified by isoenzyme characterization.
This study was initiated to investigate the emergence of the CL disease in new areas. It reports on the species identification and their zymodeme composition among Leishmania stocks isolated from human CL cases collected from different locations in the country during an eight year-period. Several new foci are reported for the first time indicating the spread of the disease. In addition, four previously undescribed Leishmania zymodemes are reported, of which three are endemic in Jordan and one isolated from an imported CL case.
Methods
Subjects
Patients clinically suspected of having CL were referred to the Ministry of Health, Central Laboratories in Amman, for parasitologic diagnosis during the period 1992–2000. These patients came from different governorates in Jordan mainly from cities and towns within 50 Km from Amman, the capital of Jordan.
For each case having cutaneous lesions (ulcers or scars), a questionnaire was completed to record the necessary information such as name, age, sex, sites of ulcer(s) on the body, address, date and place of acquiring the disease, previous travel history or location of work if other than the permanent address.
Parasitologic procedures
Skin scrapings from the edge of the lesion were obtained from each patient. Part of it was immediately cultured in Evan's Modified Tobie's Medium with L-proline, foetal calf serum and antibiotics (gentamicin, nystatin or 5-Fluorocytosine as antifungal agent). Culture media were incubated at 22°C and examined at regular intervals to monitor the growth of the parasite or the presence of contaminants. The contaminated cultures were discarded. The remaining skin scraping portion was smeared on a slide for staining with Giemsa stain and examined microscopically for presence of amastigotes.
Isoenzyme characterization
Promastigote cultures isolated from specimens were air mailed to the Leishmania Reference Center of the Istituto Superiore di Sanità, Rome, Italy for cryopreservation and isoenzyme characterization. All Leishmania isolates were then cryopreserved, mass cultured in Brain Heart Infusion culture medium and characterized by starch-gel electrophoretic analysis of 13 isoenzymes (15 enzymic loci). The enzymes studied are: PGM, phosphogucomutase (E.C.2.7.5.1); GPI, glucose-phosphate isomerase (E.C.5.3.1.9); GOT, glutamate-oxaloacetate transmainase (E.C.2.6.1.1); ME, malic enzyme (E.C.1.1.1.40); 6PGD, glucose 6 phosphate dehydrogenase (E.C.1.1.1.49); G6PD, glucose 6 phosphate dehydrogenase (E.C.1.1.1.37); NP, nucleoside purine phosphorylase (E.C.3.2.2.1); MDH, malate dehydrogenase (E.C.1.1.1.37.), MPI, mannose phosphate isomerase (E.C.5.3.1.8); ICD, isocitrate dehydrogenase (E.C.1.1.1.42); DIA, diaphorase nicotinamide adenine dinucleotide (reduced form) (E.C.1.6.2.2); GLUD, glutamate dehydrogenase (E.C.1.4.1.3); FH, fumarate hydratase (E.C.4.2.1.2). The techniques employed and the zymodemes nomenclature adopted were those of Montpellier centre [18]. World Health Organization (WHO) reference strains of L. infantum zymodeme (Z) MON-1 (MHOM/TN/80/IPT1), L. donovani ZMON-2 (MHOM/IN/80/DD8), L. tropica ZMON-60 (MHOM/SU/74/K27) and L. major ZMON-4 (MHOM/SU/73/5-ASKH) were used as references. Furthermore, strains of L. tropica and L. major zymodemes previously isolated in Jordan and neighboring countries were also used as references. When a new zymodeme was identified, this was provisionally named as "variant" (var) from the most similar classified MON zymodeme, and the electrophoretic mobility of the variant enzyme(s) was reported along.
Phenetic analysis
For the taxonomic study 17 operational taxonomic units (OTUs) corresponding to the zymodemes, were used. Of these, 12 were included in the phylogenetic complexes reported by Rioux et al (1993)[19]; 6 in the L. tropica complex (ZMON-54, 59, 60, 75, 76 and 137), 5 in the L. major complex (ZMON-4, 26, 68, 103, 229) and 1 in the L. infantum complex (ZMON-1); 1 was a L. tropica zymodeme from the Arabic Peninsula (71-var PGD95, G6PD85, NP1450, NP2100, FH100); and finally, 4 were the new zymodemes detected during the present study. Apart from the three WHO reference zymodemes of L. infantum (MON-1), L. tropica (MON-60) and L. major (MON-4), all the other zymodemes were from the Middle East, ex Soviet Union, Afghanistan, Yemen and Arabic Peninsula.
The phenogram was built using the UPGMA method [20] based on the Jaccard similarity index values (Sj), as implemeted by the NTSYS-pc computer software.
Results
Epidemiologic data
Parasites were isolated in culture from skin ulcers with detailed sociodemographic data from 76 patients.
The age groups and sex distribution for both species are shown in Table 1. The highest infection rate of 72.4% was recorded in males compared to 27.6% in females. The age range of the patients was from 10 months to 64 years while that forL. tropica was from 7 months to 54 years. Infections with L. major were recorded in 48 males (84.2%) and in 9 females (15.8%). The opposite was recorded in L. tropica, where higher infection rates were recorded in females (63.2%) than in males (36.8%). The highest rates in both species were recorded in adults in the age group 21–30 years (30.5%) and in children less than five years (23.7%), followed by 10.5% in the age groups of 11–20, 31–40, and 41–50 years. The lowest rates (6.6% and 7.7%) were recorded in the age groups 5–10 years, and > 50 years respectively.
Table 1.
Distribution of 76 cutaneous leishmaniasis cases by age and sex
| Age group (years) | L. major | L. tropica | Total No. of cases (%) | ||
| M* | F** | M | F | ||
| < 5 | 12 | 2 | 2 | 2 | 18 (23.7) |
| 5–10 | 1 | 1 | 1 | 2 | 5 (6.6) |
| 11–20 | 3 | 2 | 1 | 2 | 8 (10.5) |
| 21–30 | 18 | 2 | 1 | 2 | 23 (30.5) |
| 31–40 | 5 | 1 | 1 | 1 | 8 (10.5) |
| 41–50 | 5 | 0 | 1 | 2 | 8 (10.5) |
| > 50 | 4 | 1 | 0 | 1 | 6 (7.7) |
| Total | 48 | 9 | 7 | 12 | 76 (100) |
* M: males ** F: females
Lesions were mainly located on the face and neck (47%) compared with (20%) on the hands and arms, and (17%) on the legs. Multiple lesions appeared on two or more sites of the body in 16% of the cases.
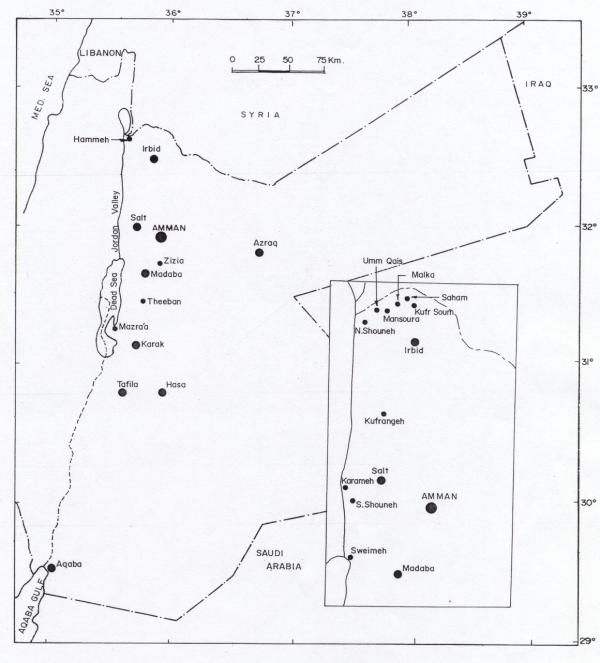
Patient's residence, working place, travel history and the time of appearance of the lesions were important data for determining the locations where the infections might have taken place. The numbers of positive cases in the north, central and south parts of Jordan are shown in Table 2. The highest infection rate (60.5%) was recorded in patients who are living or contracted the disease in the central part of Jordan (Figure 1) followed by the infection rate (14.5%) recorded for patients from the north. The lowest rate (13.2%) was recorded for patients living or contracted the disease in the south. The infection rate in patients who contracted the disease in the neighboring countries was 11.8 %. These patients worked and lived outside Jordan previous to the appearance of the lesions. The countries where they contacted the disease were Saudi Arabia (three in Taif and two in Riyadh), Iraq (two in Baghdad) and Syria (two in Aleppo); these countries are known to be endemic with CL.
Table 2.
Infection rates in patients from different regions of Jordan
| Region | No. of cases examined (%) | No. of positive (%) |
| North | 70 (21.9) | 11 (14.5) |
| Central | 180 (56.3) | 46 (60.5) |
| South | 44 (13.7) | 10 (13.2) |
| Imported | 26 (8.1) | 9 (11.8) |
Figure 1.
Distribution of cutaneous leishmaniasis cases in Jordan during 1992–2000.
Parasite species and zymodemes
L. major and L. tropica were isolated from patients coming from Jordan and neighbouring countries. The zymodemes of Leishmania spp. that were isolated varied from one region to another as reported in Table 3. A total of eight zymodemes were identified, of which four previously unreported. Zymodeme composition is reported in Tables 4 and 5. Three L. major zymodemes were identified as autochthonous in Jordan. In the North of the country ZMON-103 was isolated from Malka and ZMON-103 var GLUD220 (reference strain MHOM/JO/94/JCL50) was a newly described variant isolated from patients in Northern Jordan Valley. In the Central region three zymodemes were isolated: ZMON-103 (e.g. Amman, Salt, S. Shouneh), ZMON-103 var GLUD220 (S. Shouneh), and MON-229 (Karameh). Finally, L. major ZMON-103 was isolated from a patient from Karak in the South of the country.
Table 3.
The isolated Leishmania spp, their zymodemes identification and infection location
| Infection location | L. major zymodemes | L. tropica zymodemes |
| North | MON-103, MON-103 var GLUD220* | MON-54 var PGD96–97 |
| Central | MON-103, MON-103 var GLUD220*, MON-229 | MON-137, MON-54 var PGD96-97 |
| South | MON-103 | MON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110* |
| Other countries | ||
| Aleppo, Syria | MON-75 FH110 * | |
| Riyadh, Saudi Arabia | MON-26 | MON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110* |
| Taif, Saudi Arabia | MON-59 var MDH100, PGD95, G6PD85, GLUD80, FH110* |
* Newly described enzymatic variant
Table 4.
Enzyme profiles of the Leishmania tropica zymodemes identified from autochthonous and imported CLcases in Jordan
| Zymodeme MON | ENZYMES | ||||||||||||||
| MDH | ME | IDH | PGD | G6PD | GLUD | DIA | NP1 | NP2 | GOT1 | GOT2 | PGM | FH | MPI | PGI | |
| 137 | 100 | 110 | 100 | 98 | 85 | 80 | 100 | 450 | 110 | 140 | 85 | 88 | 100 | 110 | 76 |
| 54 var* | 112 | 95 | 100 | 96–97 | 82 | 80 | 120 | 450 | 90 | 135 | 90 | 100 | 110 | 100 | 76 |
| 59 var** | 100 | 100 | 100 | 95 | 85 | 80 | 100 | 450 | 100 | 135 | 90 | 100 | 100 | 110 | 76 |
| 75 var*** | 112 | 95 | 100 | 95 | 82 | 95 | 100 | 450 | 100 | 135 | 90 | 100 | 110 | 101 | 76 |
* ZMON-54 PGD96–97 var; ** ZMON59 MDH100, PGD95, G6PD85, GLUD80, MPI110 var; *** ZMON-75 FH110 var
Table 5.
Enzymes profiles of the Leishmania major zymodemes identified from autochthonous and imported CL cases in Jordan
| Zymodemes MON | ENZYMES | ||||||||||||||
| MDH | ME | IDH | PGD | G6PD | GLUD | DIA | NP1 | NP2 | GOT1 | GOT2 | PGM | FH | MPI | PGI | |
| 26 | 160 | 88 | 100 | 122 | 94 | 200 | 100 | 400 | 90 | 110 | 110 | 118 | 79 | 150 | 77 |
| 103 | 160 | 88 | 100 | 122 | 94 | 200 | 100 | 400 | 100 | 110 | 110 | 118 | 79 | 150 | 77 |
| 103 var* | 160 | 88 | 100 | 122 | 94 | 220 | 100 | 400 | 100 | 110 | 110 | 118 | 79 | 150 | 77 |
| 229 | 160 | 90 | 100 | 122 | 94 | 200 | 100 | 400 | 90 | 110 | 110 | 118 | 79 | 150 | 77 |
*: ZMON-103 var GLUD220
Three L. tropica zymodemes were identified from autochthonous CL cases in Jordan. L. tropica ZMON-54 var PGD96–97 (reference strain MHOM/JO/94/JCL34) was isolated from the North (e.g. Um Qais, Hammeh,) of the country. In the Central region (e.g. S. Shouneh) ZMON-54 var PGD96–97 and ZMON-137 were isolated. A newly described variant, ZMON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110 (reference strain MHOM/JO/92/JCL27) was isolated from the South (Tafila).
Both Leishmania species were also isolated from patients who contracted the disease during their work in the neighboring countries. L. major ZMON-26 was isolated from two patients living in Riyadh, Saudi Arabia. L. tropica ZMON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110 was isolated from two patients who contracted the disease in Taif, Saudi Arabia. L. tropica ZMON-75 var FH110 (reference strain MHOM/SY/93/JCL29), which is a newly described zymodeme, AND was isolated from a patient who contracted the disease in Aleppo, Syria.
L. major ZMON-103 was also isolated from four jirds that were caught in the central rural area.
Phenetic analysis
The phenogram in Figure 2 shows 2 principal branches clearly individualized, with a third branch supported by L. infantum (A). The first branch (B) is highly polymorphic with a first branch at Sj value as low as 0.27, and includes all the zymodemes of L. tropica. The second one (C) is more monomorphic (Sj = 0.70) and includes the group of L. major zymodemes. L. infantum links to L. tropica delimiting two principal clusters of this species: a) the first one is made of 7 zymodemes that show links between the WHO reference MON-60 (from ex Soviet Union) and MON-76 (from Syria); between MON75 (from ex Soviet Union) and the new zymodeme MON-75 var FH110 (Sj = 0.76); and between the close pair MON-54 (from Israel) and the new zymodeme MON-54 var PGD96–97 (Sj = 0.87); MON-59 (from Afghanistan) is grouped as a single entity; b) the second cluster is made of 3 zymodemes, of which MON-137 (from Israel) links to a group of very similar zymodemes MON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110 and MON-71 var PGD95, G6PD85, NP1450, NP2100, FH100 (from Arabic Peninsula) (Sj = 0.76). As regards L. major, two principal clusters are identified: a) the first closely links zymodemes MON-4 (WHO reference from ex Soviet Union) and MON 26 (from Yemen) (Sj = 0.87) to ZMON-229 from Jordan; b) the second identifies the paired MON-103 from Jordan and the new zymodeme MON-103 var GLUD220 (Sj = 0.87). As external group MON-68 from Israel links L. major to L. infantum. (Sj = 0.17), confirming the high distance between the two species [21]
Figure 2.
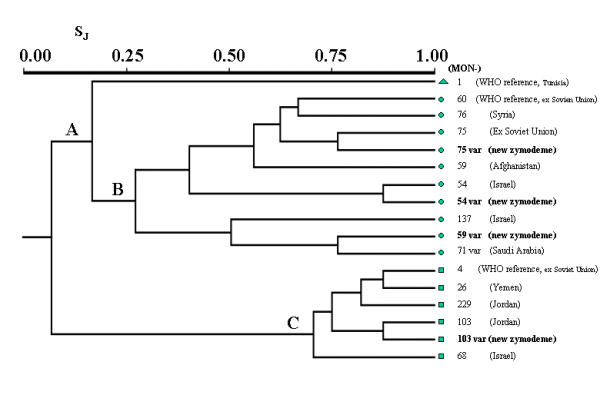
Phenogram built on 15 enzymatic loci from 3 WHO Leishmania reference strains and 14 Leishmania zymodemes from Middle East and Central Asia (similarity Jaccard index (Sj); UPGMA method). Three groups are identified: Leishmania infantum (A, triangle), L. tropica (B, circles) and L. major (C, squares).
Discussion
This study reports on cases of human CL caused by L. major and L. tropica from different regions in Jordan. Figure 1 shows the locations where cases are reported in this study. Cases are reported for the first time from cities in the north (e.g., Kufrsoum, Kufrangeh, and Saham), the central region (e.g. Azraq, Falha) and the south (e.g., Aqaba, Al Hasa, Karak, and Tafila). The sporadic nature of the cases in these areas indicates the recent spread of the disease to these areas. Clustering of cases was recorded in endemic areas along the Jordan Valley; highest number of cases was reported mostly in the central part. Several studies reported on the endemicity of CL on both sides of the Jordan Valley [11,13,22]. The disease is endemic for many years in Jericho (Jericho boil), the endemicity of the disease has been maintained due to the presence of the vector Phlebotomus papatasi, and the reservoir host Psammomys obesus in that area [22].
The highest number of cases reported in our study was mainly in the central part of Jordan (Karameh, S. Shouneh and Sweimeh). These results are in agreement with previous studies, which also reported higher infection rates in Sweimeh in the Jordan Valley [17]. The number of cases diagnosed in one military hospital in Amman during 1979 through 1981 was 207 cases, where 71% of these came from the same area, Sweimeh in the Jordan Valley. A report of an outbreak of zoonotic CL in 80 nonimmuned soldiers in Southern Jordan confirmed the endemicity of the disease in the Jordan Valley [23].
The infection rates reported in the north particularly along the Jordan Valley came second. Isolation of the parasite from new areas indicates that the geographical distribution of CL in Jordan is far wider than previously thought.
The higher infection rates (72.4%) recorded in males compared to females (27.6%) in all age groups were also reported by others [12]. This difference might be explained by more exposure of males to the sandflies bites during their travel and work activities in the endemic areas. The high rates in children less than five years old (23.7%) might be explained by non immunity and frequent exposure to bites of the sandflies inside their homes since most of the cases in this age group were from the central endemic areas (e.g., S. Shouneh and Sweimeh) and other cities in the south (e.g., Aqaba and Al Hasa). The low rates recorded in the age group > 50 years (7.7%) might be explained by development of immunity over time as a result of previous exposure, which is well known in CL. The low rate in the age group 5–10 years (6.6%) could not be explained. It might be due to the exclusion of some of the cases in this age group among other cases due to incomplete information about the patients.
In a study of the distribution of phlebotomine sandfly species in Jordan [16], Phlebotomus papatasi, the vector of L. major was collected from both domestic and rural areas in many localities in Jordan. It was reported as the predominant or the abundant species in localities where cases were reported in this study such as the Jordan Valley, suburbs of Amman and in the newly reported localities such as Azraq, and Aqaba. Evidence on the role of P. papatasi as a vector of L. major was provided after the isolation of the parasite from P. papatasi females collected from the Southern Jordan Valley [13]. L. major was also isolated from four Psammomys obesus, the principle reservoir host caught in endemic foci in Jordan [14]. CL is also known to be endemic in the neighboring countries where nine patients contracted the disease [24-26]. L. major was the predominant species (75%) that was isolated from all localities in this study and in previous studies [13,14]. CL caused by L. tropica is less common in Jordan as well as in the other endemic neighboring countries [27,28]. L. tropica was mainly isolated from the north, few cases from the central region, and one case in the south. It was also isolated from cases imported from Saudi Arabia and Syria where it is endemic [24,29].
The isoenzyme characterization of the isolates identified four L. major zymodemes MON-26, MON-103 (the predominant zymodeme), and MON-103 var GLUD220 (newly described enzymatic variant), MON-229 and MON-26, from an imported CL case from Saudi Arabia. As already reported for this species, a general enzymatic homogeneity among the strains was observed, variations being limited to 1–2 enzymes only (Tab. 5, Fig. 2). L. major zymodeme MON-26 is known as the agent of ZCL in the sub-Saharan Sahel to the Near and Middle East [30].
Four L. tropica zymodemes were identified, MON-137, MON-54 var PGD96–97, MON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110, and MON-75 var FH110, the last three being newly described zymodemes. Zymodeme MON-59 var MDH100, PGD95, G6PD85, GLUD80, MPI110 was identified in both autochthonous (Tafila) and in imported cases from Saudi Arabia, while zymodeme MON-75 var FH110 was only identified from imported cases from Aleppo, Syria. L. tropica zymodemes differed in one to five enzymes, confirming the high degree of intraspecific polymorphism reported for this species (Tab. 4, Fig. 2). Diversification into "small variants" is apparently the result of recent mutations, possibly associated with genetic exchange [31]. Unlike L. major, which is known to have a zoonotic transmission cycle, the transmission cycle of L. tropica is mainly anthroponotic and associated to urban environment. If the transmission cycle in the areas where it was isolated is man-fly-man, then the geographical distribution of intraspecific variants will be greatly influenced by the movement of the infected individuals. Thus a zymodeme may be spread from city to city, becoming established if the species of the sandfly vector is present. This could explain the wide distribution of zymodeme MON-54 var PGD96–97 identified in cases from the north and the central regions. An explanation for the greater variation noticed in L. tropica than L. major may be that L. tropica simply has greater genetic diversity than L. major.
The importance of finding an isoenzymatic variant could be applied to identify a geographical entity, with the possibility of identifying the origin of the infection especially in highly polymorphic Leishmania species such as L. tropica, when an appropriate panel of isoenzymes is analyzed. The phenetic analysis confirms this hypothesis linking zymodemes for similarity distance according their geographical origin. Then most zymodemes from Middle East are progressively linked (Figure 2). Although in some Leishmania species the enzymatic variation of an isolate could also be used as a clinical marker (for example NP1140 in L. infantum could be used as a marker for cutaneous, rather than visceral leishmaniasis ; however, it seems that no "clinical" association is evident for different L. tropica zymodemes.
To our knowledge, this is the first study that reports on zymodemes of both L. major and L. tropica isolated from different parts of the country. L. tropica with isoenzyme profiles characteristics resembling MON-54 that was reported for eight isolates from towns in the north [15] is confirmed by isolating ZMON-54 var PGD96–97 from the same towns in this study. The isolation of L. major ZMON-103 from Salt in this study and L. tropica ZMON-137 from Salt district in another study [32], indicates the presence of both spp. in the same area and calls for further investigation on the status of the disease in that area.
Conclusions
Based on the findings of this study, the new localities where cases were reported should be added to the list of CL foci. The data clearly demonstrate the clustering of cases in endemic areas and the spread of the disease to new areas that were not previously reported indicated by the sporadic nature of the disease in these areas. Further studies on the vector and reservoir host are needed to investigate the transmission cycle in these areas.
Authors' contribution
Nimri L. prepared the questionnaire, carried out culture of Leishmania, microscopic identification, data analysis, and drafted the manuscript. Soubani R. carried out the specimens collection, data recording, and culture of Leishmania. Gramiccia M. carried out the isoenzyme characterization and the phenetic analysis. All authors read and approved the final manuscript.
Competing interests
None declared
Acknowledgments
Acknowledgements
The authors are grateful to engineer Fawwaz Nimri, consultant of the Authority of Natural Resources for his help in drawing Jordan Map. This study received financial support from the Deanship of Research at Jordan University of Science and Technology, Grant # 19/94 and 172/20.
Contributor Information
Laila Nimri, Email: nimri@just.edu.jo.
Radwan Soubani, Email: soubani@nets.com.jo.
Marina Gramiccia, Email: gramiccia@iss.it.
References
- Al-Zahrani MA, Peters W, Evans DA, Smith V, Ching Chin I. Leishmania infecting man and wild animals in Saudi Arabia. 6. Cutaneous leishmaniasis of man in the south west. Trans Roy Soc Trop Med Hyg. 1989;83:621–628. doi: 10.1016/0035-9203(89)90376-3. [DOI] [PubMed] [Google Scholar]
- Khoury S, Saliba EK, Oumeish OY, Tawfig MR. Epidemiology of cutaneous leishmaniasis in Jordan 1983–1992. Inter J Dermatol. 1996;35:566–559. doi: 10.1111/j.1365-4362.1996.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Schlein Y, Warburg A, Schnur LF, Le Blancq SM, Gunders AE. Leishmaniasis in Israel: reservoir hosts, sandfly vectors and leishmanial strains in the Negev, Central Arava and along the Dead Sea. Trans Roy Soc Trop Med Hyg. 1984;78:480–484. doi: 10.1016/0035-9203(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Tayeh A, Jalouk L, Cairncross S. Twenty years of cutaneous leishmaniasis in Aleppo, Syria. Trans Roy Soc Trop Med Hyg. 1997;91:657–659. doi: 10.1016/s0035-9203(97)90509-5. [DOI] [PubMed] [Google Scholar]
- Chance ML, Walton BC. Biochemical characterization of Leishmania . Geneva: UNDP/World Bank/WHO. 1982. pp. 275–280.
- Ashford DA, Bozza M, Freire M, Miranda JC, Sherlock I, Eulalio C, Lopes U, Fernandes O, Degrave W, Barker RH, Jr, Badaro R, David JR. Comparison of the polymerase chain reaction and serology for the detection of canine visceral leishmaniasis. Am J Trop Med Hyg. 1995;53:251–255. doi: 10.4269/ajtmh.1995.53.251. [DOI] [PubMed] [Google Scholar]
- Eisenberger CL, Jaffe CL. Leishmania: identification of old world species using permissively primed intergenic polymorphic-polymerase chain reaction. Exp Parasitol. 1999;91:70–77. doi: 10.1006/expr.1999.4355. [DOI] [PubMed] [Google Scholar]
- Belli A., Rodrequez B, Aviles H, Harris E. Simplified polymerase chain reaction detection of new world Leishmania in clinical specimens in cutaneous leishmaniasis. Am J Trop Med Hyg. 1998;58:102–9. doi: 10.4269/ajtmh.1998.58.102. [DOI] [PubMed] [Google Scholar]
- Adler S, Theodor D. The distribution of sandflies and leishmaniasis in Palestine, Syria and Mesopotamia. Ann Trop Med Parasitol. 1929;23:269–306. [Google Scholar]
- World Health Organization WHO regional committee for the Eastern Mediterranean 3rd session, 1950 on leishmaniasis, 1939–1948, Mimeograph RC 3/EM/14. WHO Statistical Report 21. Geneva. 1968.
- Oumeish O, Saliba EK, Allawi TF. Cutaneous leishmaniasis: an endemic disease in Jordan. Jordan Med J. 1982;16:55–61. [Google Scholar]
- Saliba EK, Oumeish O, Haddadin J, Ashford RW. Cutaneous leishmaniasis in Mowaqqar area, Amman governorates, Jordan. Ann Trop Med Parasitol. 1985;79:139–146. doi: 10.1080/00034983.1985.11811900. [DOI] [PubMed] [Google Scholar]
- Janini R, Saliba E, Khoury S, Oumeish O, Adwan S, Kamhawi S. Incrimination of Phlebotomus papatasi as a vector of Leishmania major in the southern Jordan Valley. Med Vet Entomol. 1995;9:420–422. doi: 10.1111/j.1365-2915.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Saliba EK, Disi AM, Ayed RE, Saleh N, Al-Younes H, Oumeish O, Al-Ouran R. Rodents as reservoir hosts of cutaneous leishmaniasis in Jordan. Ann Trop Med Parasitol. 1994;88:617–622. doi: 10.1080/00034983.1994.11812912. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Abdel-Hafez SK, Arabgi A. A new focus of cutaneous leishmaniasis caused by Leishmania tropica in northern Jordan. Tran Roy Soc Trop Med Hyg. 1995;89:255–257. doi: 10.1016/0035-9203(95)90526-x. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Abdel-Hafez SK, Molyneux DH. A comprehensive account of species composition, distribution and ecology of Phlebotomine sandflies in Jordan. Parasitol. 1995;2:163–172. [Google Scholar]
- The Hashemite Kingdom of Jordan, Department of Statistics Morbidity Statistics of Hospitals, 1973-1978
- Rioux JA, Lanotte G, Serres E, Pratlong F, Bastien P, Perières J. Taxonomy of Leishmania: Use of isoenzymes. Sugestions for a new classification. Ann Hum Parasitol. 1990;65:111–125. doi: 10.1051/parasite/1990653111. [DOI] [PubMed] [Google Scholar]
- Rioux JA, Lanotte G. Apport de la cladistique à l'analyse du genre Leishmania Ross, 1903 (Kinetoplastida, Trypanosomatidae). Corollaires écoépidémiologiques. Biosystema. 1993;8:79–90. [Google Scholar]
- Sneath PHA, Sokal RR. The principle and practice of numerical classification. In: Kennedy D, Park RB, editor. Numerical Taxonomy. San Francisco: Freeman; 1973. p. 537. [Google Scholar]
- Soccol VT, Lanotte G, Rioux JA, Pratlong F, Martini-Dumas A, Serres E. Monophyletic origin of the genus Leishmania Ross, 1903. Ann Parasitol Hum Comp. 1993;68:107–108. [PubMed] [Google Scholar]
- Schlein Y, Warburg A, Schnur LF, Gunders AE. Leishmaniasis in the Jordan Valley II. Sandflies and transmission in the central endemic area. Trans Roy Soc Trop Med Hyg. 1982;76:582–586. doi: 10.1016/0035-9203(82)90215-2. [DOI] [PubMed] [Google Scholar]
- Jumaian N, Kamhawi SA, Halalsheh M, Abdel-Hafez SK. Short report: outbreak of cutaneous leishmaniasis in a nonimmune population of soldiers in Wadie Araba, Jordan. Am J Trop Med Hyg. 1998;58:160–162. doi: 10.4269/ajtmh.1998.58.160. [DOI] [PubMed] [Google Scholar]
- Ashford RW, Rioux JA, Jalouk L, Khiami A, Dye C. Evidence for long term increase in the incidence of Leishmania tropica in Aleppo, Syria. Trans Roy Soc Trop Med Hyg. 1993;87:247–249. doi: 10.1016/0035-9203(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Douba M, Mowakeh A, Wali A. Current status of cutaneous leishmaniasis in Aleppo, Syria Arab Republic. Bull WHO. 1997;75:253–259. [PMC free article] [PubMed] [Google Scholar]
- Peters W, Al-Zahrani MA. The leishmaniasis – a public health problem in Saudi Arabia. Saudi Med J. 1987;8:333–343. [Google Scholar]
- Klaus S, Axelrod O, Jonas F, Frankenburg S. Changing patterns of cutaneous leishmaniasis in Israel and neighboring territories. Trans Roy Soc Trop Med Hyg. 1987;88:649–650. doi: 10.1016/0035-9203(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Rioux JA, Ashford RW, Khiami A. Ecoepidemiology of leishmaniasis in Syria 3. Leishmania major infection in Psammomys obesus provides clues to life history of the rodent and possible control measures. Ann Hum Parasitol. 1992;67:163–165. doi: 10.1051/parasite/1992676163. [DOI] [PubMed] [Google Scholar]
- Kreutzer RD, Grogl M, Neva FA, Fryauff DJ, Magill AJ, Aleman-Munoz M. Identification and genetic comparison of leishmanial parasites causing viscerotropic and cutaneous disease in soldiers returning from operation Desert Storm. Am J Trop Med Hyg. 1993;49:357–363. doi: 10.4269/ajtmh.1993.49.357. [DOI] [PubMed] [Google Scholar]
- Khiami A, Dereure J, Pratlong F, Martini A, Rioux J'A. La leishmaniose cutane humaine a Leishmania major MON-26 au environs de Damas (Syrie). Bull Soc Exp Pathol. 1991;84:340–344. [PubMed] [Google Scholar]
- Pratlong F, Rioux JA, Dereure J, Mahjour J, Gallego M, Guilvard E, Lanotte G, Perieres J, Martini A, Saddiki A. Leishmania tropica in Morocco. IV. Intrafocal enzyme diversity. Ann Parasitol Hum Comp. 1991;66:100–104. doi: 10.1051/parasite/1991663100. [DOI] [PubMed] [Google Scholar]
- Saliba EK, Saleh N, Oumeish O, Khoury S, Bisharat Z, Al-Ouran R. The endemicity of Leishmania tropica (zymodeme MON-137) in the Eira-Yaraqa area of Salt District, Jordan. Ann Trop Med Parasitol. 1997;91:453–459. doi: 10.1080/00034989760815. [DOI] [PubMed] [Google Scholar]