Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and other aryl hydrocarbon receptor (AhR) ligands suppress 17β-estradiol (E)-induced responses in the rodent uterus and mammary tumors and in human breast cancer cells. Treatment of ZR-75, T47D, and MCF-7 human breast cancer cells with TCDD induces proteasome-dependent degradation of endogenous estrogen receptor α (ERα). The proteasome inhibitors MG132, PSI, and PSII inhibit the proteasome-dependent effects induced by TCDD, whereas the protease inhibitors EST, calpain inhibitor II, and chloroquine do not affect this response. ERα levels in the mouse uterus and breast cancer cells were significantly lower after cotreatment with E plus TCDD than after treatment with E or TCDD alone, and our results indicate that AhR-mediated inhibition of E-induced transactivation is mainly due to limiting levels of ERα in cells cotreated with E plus TCDD. TCDD alone or in combination with E increases formation of ubiquitinated forms of ERα, and both coimmunoprecipitation and mammalian two-hybrid assays demonstrate that TCDD induces interaction of the AhR with ERα in the presence or absence of E. In contrast, E does not induce AhR-ERα interactions. Thus, inhibitory AhR-ERα cross talk is linked to a novel pathway for degradation of ERα in which TCDD initially induces formation of a nuclear AhR complex which coordinately recruits ERα and the proteasome complex, resulting in degradation of both receptors.
Estrogenic hormones induce their tissue-specific responses through binding the estrogen receptor (ER), which is a ligand-activated transcription factor and a member of the nuclear receptor (NR) superfamily (3, 15). The two ER subtypes (ERα and ERβ) and other NRs exhibit modular structures containing N-terminal activation function 1 (AF1) and C-terminal AF2, which also contains the ligand-binding domain, a DNA-binding domain (DBD), and an adjacent hinge region. Most early-stage mammary tumors are ER positive and are responsive to endocrine therapies which target ERα and/or E biosynthesis (5, 13, 47). Selective ER modulators, such as tamoxifen, are extensively used for treating early-stage breast cancer, and the primary modes of action of selective ER modulators involve competitive binding to the ER and subsequent inhibition of one or more steps in ER-mediated transactivation. Several studies show that there are important mechanistic differences among antiestrogens, and this is consistent with their tissue-specific ER antagonist-agonist activities (20, 52, 53). For example, the “pure” antiestrogens ICI 164,384 and/or ICI 182,780 not only bind ERα with high affinity but induce a rapid proteasome-dependent degradation of the receptor, and this is observed in breast cancer cells and human tumors (41, 52). Rapid degradation of ERα protein in cells or tumors treated with ICI 164,384 or 182,780 may play an important role in the antiestrogenic activity of these compounds.
Studies in this laboratory have investigated inhibition of ER signaling through cross talk with the ligand-activated aryl hydrocarbon receptor (AhR) (reviewed in references 36 and 44), and selective AhR modulators are highly effective inhibitors of mammary tumor growth in rodent models (32, 44). The environmental toxicant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a potent AhR agonist, and treatment of hepatoma cells with TCDD induces rapid proteasome-dependent degradation of the AhR (7, 30, 35, 40). Similar results have been observed in T47D and MCF-7 human breast cancer cells treated with TCDD or selective AhR modulators, and this is also accompanied by proteasome-dependent degradation of ERα (32, 54). This study further investigates the mechanisms of AhR-mediated degradation of ERα protein in breast cancer cells and the contributions of this pathway to inhibitory AhR-ERα cross talk. In breast cancer cells, TCDD alone and in combination with E induces rapid degradation of ERα, and AhR interaction with ERα in a mammalian two-hybrid assay is dependent on TCDD but not E. Interactions of ligand-bound AhR with ERα are accompanied by enhanced formation of ubiquitinated forms of ERα, proteasome-dependent degradation of ERα, and decreased E-induced transactivation. Thus, ligand activation of the nuclear AhR coordinately recruits both ERα and proteasomes which degrade both receptors and thereby enhance inhibitory AhR-ERα cross talk in breast cancer cells.
MATERIALS AND METHODS
Chemicals, constructs, cells, and animals.
TCDD was prepared in this laboratory and shown to be >99% pure by gas chromatographic analysis. 17β-Estradiol (E), chloroquine, cycloheximide, cell culture chemicals, and media were purchased from Sigma. Calpain inhibitor II (Cal II), EST, MG132, PSI, PSII, bis-indolylmaleimide I, manumycin A, U0126, PP2, wortmannin, and KT5720 were purchased from Calbiochem. Primary antibodies for human ERα, AhR, Sp1, CYP1A1, c-Fos, and ubiquitin proteins were purchased from Santa Cruz. Alexa Fluor 594 goat anti-rabbit was purchased from Molecular Probes. Fluorescein isothiocyanate-conjugated goat anti-rabbit was purchased from Zymed Laboratories. The rat c-Fos pSP65 expression vector was kindly provided by Tom Curran (Roche Research Center), and the human phuAhR expression vector was a kind gift from Christopher Bradfield (University of Wisconsin School of Medicine). The pERE3 reporter construct containing three tandem consensus ERE sites linked to a luciferase gene was created by cloning an oligonucleotide containing this sequence into the BamHI-HindIII-cut pXP-2 plasmid (American Type Culture Collection, Manassas, Va.) 30 bp upstream from a TATA box. The sequence of the ERE was GGTCACAGTGACC. The ERα-GAL4-AD and AhR-GAL4-DBD fusion proteins were constructed using the mammalian Matchmaker two-hybrid kit (Clontech) pVP16 and pM fusion vectors. The expression plasmids for ERα with deletions of amino acids 1 to 178 (ERαΔAF1), 185 to 252 (ERαΔDBD), and 282 to 595 (ERαΔAF2) were kindly provided by Pierre Chambon (Institute de Genetique et Biologie Moleculaire et Cellulaire). T47D, MCF-7, and ZR-75 human breast cancer cells were obtained from the American Type Culture Collection and cultured as previously described (54). B6C3F1 mice were obtained from Charles River Laboratories. The mice were kept in a temperature-controlled conditioned room with a 14-h light and 10-h dark photocycle. Rodent chow and water was supplied ad libitum.
Protein isolation and Western analysis.
Cells were seeded into 35-mm six-well tissue culture plates in phenol red-free medium (Dulbecco's modified Eagle's medium [DMEM] Ham F-12) containing 2.5% charcoal-stripped fetal bovine serum (FBS). After 24 h, cells were treated and harvested at designated time points and lysed in ice-cold lysis buffer (50 mM HEPES [pH 7.5], 500 mM NaCl, 10% [vol/vol] glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA) supplemented with protease inhibitor cocktail (Sigma). Equal amounts of protein from each treatment group were boiled in 1× Laemmli buffer (50 mM Tris-HCl, 2% sodium dodecyl sulfate [SDS], 0.1% bromphenol blue, 175 mM β-mercaptoethenol), separated by SDS-10% polyacrylamide gel electrophoresis (SDS-PAGE), and electrophoresed to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked in Blotto (5% milk, Tris-buffered saline [10 mM Tris-HCl, pH 8.0, 150 mM NaCl], and 0.05% Tween 20) and probed with primary antibodies ERα G-20 (1:1,000), AhR N-19 (1:200), Sp1 PEP2 (1:5,000), CYP1A1 G-18 (1:1,000), and c-Fos H-125 (1:1,000). Following incubation with peroxidase-conjugated secondary antibody, immunoglobulins were visualized using the ECL detection system (NEN). Quantitation was performed using a Sharp JX-330 scanner and Zero-D Scanalytics software (Scanalytics Corp.). The human ERα, human phuAhR, and rat c-Fos pSP65 expression vectors were used to in vitro translate standards for Western blotting using the TNT T7 quick-coupled transcription/translation system (Promega). Human recombinant Sp1 protein (Promega) was added to the in vitro-translated mixtures as the Sp1 standard.
Transfection assays.
ZR-75 cells were seeded onto 12-well plates at a concentration of 2.75 × 105 cells per well in phenol red-free DMEM Ham F-12 supplemented with 2.5% charcoal-stripped FBS. After 18 h, cells were transfected by the calcium phosphate method with 500 ng of pERE3 luciferase reporter plasmid and 250 ng of pCDNA3.1-β-gal (Invitrogen) as the control vector. Cells were treated for 36 h and assayed for luciferase (Promega) and β-galactosidase (Tropix) activity in a Packard luminometer according to the manufacturer's instructions. Protein-protein interactions between ERα and AhR were examined in ZR-75 cells by using the ERα-GAL4-AD (vpER) and AhR-GAL4-DBD (pmAhR) fusion constructs. ZR-75 cells were transfected by the calcium phosphate method with 500 ng of 5XGAL-luciferase, 250 ng of pmAhR, 100 ng of vpER, 250 ng of pCDNA3.1-β-gal as the control vector, or empty pM and pVP vectors as DNA mass balance controls. Cells were treated for 36 h, harvested, and assayed as described above. The effects of TCDD on proteasome-dependent degradation of ERα mutants were determined in T47D cells. Cells were seeded onto 35-mm six-well culture plates at a concentration of 6 × 105 cells per well in DMEM Ham F-12 without phenol red, supplemented with 2.5% charcoal-stripped FBS. After 18 h, cells were transfected by the calcium phosphate method with 500 ng of ERαΔAF1, ERαΔDBD, or ERαΔAF2 expression plasmids. After a 12-h recovery period, cells were treated with dimethyl sulfoxide (DMSO; control) or 10 nM TCDD for 6 h. Cells were collected for transfected and endogenous ERα protein analysis using Western blotting buffers as described in “Protein isolation and Western analysis.” Fifty-microgram protein aliquots were electrophoresed for each treatment and transfection group.
Nuclear extract preparation and EMSA.
MCF-7 and ZR-75 cells were seeded at a density of 5 × 106 cells/plate in 60-mm tissue culture plates using DMEM Ham F-12 without phenol red, supplemented with 2.2 g of sodium bicarbonate/liter and antibiotic-antimycotic solution, and 2.5% charcoal-stripped FBS, pH 7.4. After 24 h, cells were treated for 3 h with 10 nM E or 10 nM E plus 10 nM TCDD following a 30-min pretreatment with DMSO, 10 μM MG132, or 10 μM Cal II. Nuclear extracts were obtained using the NE-PER nuclear and cytoplasmic extraction kit (Pierce) according to the manufacturer's instructions. Three micrograms of nuclear protein from each treatment group was incubated for 10 min at 25°C with 500 ng of poly(dI-dC) in 30 μl of HEGDK+ (25 mM HEPES, 1.5 mM EDTA, 10% glycerol, 1 mM DTT, 100 mM KCl) and 32P-end-labeled ERE probe (5′-GTC CAA AGT CAG GTC ACA GTG ACC TGA AAG TT-3′). ERα D-12 antibody and normal mouse immunoglobulin G (IgG) were used for supershift controls. Samples were electrophoresed on a 5% polyacrylamide gel at 120 V in 90 mM Tris, 90 mM borate, 2 mM EDTA (pH 8.0), dried, and visualized by autoradiography.
Northern blot analysis.
T47D cells were seeded into 100-mm tissue culture plates and, after 24 h, cells were treated for 6 h with DMSO or 10 nM TCDD following a 30-min pretreatment with DMSO, 10 μM MG132, or 10 μM PSI. Thirty micrograms of total RNA from each treatment was loaded onto a 1.2% agarose, 10% formaldehyde gel and electrophoresed in 1× morpholinepropanesulfonic acid (MOPS) buffer. Separated RNA was transferred to Zeta-Probe GT (Bio-Rad) membrane by capillary action in 1× MOPS for 48 h, UV cross-linked for 15 min, dried at 80°C for 1 h, and probed at 42 to 65°C according to the Zeta-Probe GT protocol. Membranes were scanned on a STORM 860 PhosphorImager (Molecular Dynamics). Band intensities of AhR, Arnt, and ERα were normalized to values obtained for the β-tubulin loading control. Expression vectors used for generating RNA probes for Northern blot analysis were generously supplied by the following: AhR (Kristy Dolwick, Northwestern University Medical School), Arnt (Rosally Agbunag, University of California), hERα (Ming-Jer Tsai, Baylor College of Medicine), and β-tubulin (Masahito Negishi, National Institute of Environmental Health Sciences, National Institutes of Health).
Kinase inhibitors and cycloheximide studies.
ZR-75 cells were seeded as described in the Western immunoblotting protocol above and treated for 3 h with DMSO, E, TCDD, or ET following a 30-min pretreatment with DMSO, 10 μM PP2, 1 μM bis-indolylmaleimide I, 1 μM KT5720, 2 μM manumycin A, 5 μM U0126, 400 nM wortmannin, methanol (MeOH), or 25 μM cycloheximide. Cells were harvested and immunoblotted as described above in the “Protein isolation and Western analysis” protocol.
Coimmunoprecipitation.
MCF-7 and ZR-75 cells were seeded into 150-mm tissue culture plates in maintenance medium and allowed to grow to approximately 90% confluence. Cells were treated with DMSO or 10 nM TCDD for 30 min, and nuclear extracts for each treatment group were obtained using the NE-PER nuclear and cytoplasmic extraction kit with the addition of protease inhibitor cocktail. Duplicate aliquots of 550 μg (MCF-7) and 525 μg (ZR-75) of equal volume were used for the experiments. The nuclear protein was diluted fivefold in ice-cold phosphate-buffered saline (PBS) containing protease inhibitor cocktail to a final volume of 1 ml, followed by the addition of 30 μl of protein A/G PLUS-agarose beads (Santa Cruz). The reactions were placed on a rocker at 4°C for 3 h, followed by centrifugation at 600 × g at 4°C for 5 min. A 900-μl aliquot of supernatant was removed from each sample and placed into a new Eppitube on ice. Mouse monoclonal anti-ERα D-12 (1 μg) or normal mouse IgG (1 μg) was added to either replicate treatment set, followed by the addition of 30 μl of protein A/G PLUS-agarose beads. The samples were then placed on a rocker at 4°C for 12 h, followed by centrifugation at 600 × g at 4°C for 5 min. The supernatant was removed by aspiration, and the pellets were washed once with 1 ml of ice-cold PBS followed by centrifugation at 600 × g at 4°C for 5 min. The agarose pellet was then resuspended in 50 μl of 1× Laemmli buffer, boiled, and centrifuged. The supernatant was separated by SDS-10% PAGE, electrophoresed to PVDF membrane, and visualized by ECL as described above.
Ubiquitinated ERα immunoprecipitation.
ZR-75 cells were seeded as described in the coimmunoprecipitation protocol above and treated with DMSO or 10 nM E, 10 nM TCDD or ET, or 10 nM ICI 182,780 for 6 h. Duplicate aliquots of 300 μg were immunoprecipitated with anti-Sp1 PEP2 (1 μg) or anti-ERα D-12 (1 μg) as described above. Immunoprecipitates were washed with two cycles of 1 ml of ice-cold radioimmunoprecipitation assay buffer followed by 1 ml of ice-cold PBS using centrifugation at 600 × g at 4°C for 5 min. The agarose pellet was resuspended in 50 μl of 1× Laemmli buffer, boiled, and centrifuged, The supernatant was separated by SDS-10% PAGE, electrophoresed to PVDF membrane, blotted with anti-ubiquitin P4D1 (Sigma), and visualized by ECL as described above. The membrane was then stripped using stripping buffer (62.5 mM Tris-HCl, 112 mM 2-mercaptoethanol, 20% SDS [wt/vol]; pH 6.8) at 60°C for 1 h and reprobed with anti-ER α D-12 and anti-Sp1 PEP2 consecutively.
Effects of siRNA for the AhR.
ZR-75 cells were cultured in DMEM Ham F-12 containing 5% FBS in six-well plates until 50 to 60% confluent. Based on results of ongoing studies, a maximal decrease in the AhR protein was observed using 7 μl of a 20 μM solution of the small inhibitory RNA (siRNA), and this amount was transfected into ZR-75 cells using oligofectamine reagent (Invitrogen, Carlsbad, Calif.). The final concentration of siRNAs in each well was 140 nM. Thirty-six hours after transfection, cells were treated with DMSO, 10 nM E2, or 10 nM TCDD for 5 h, and nuclear extracts were obtained and analyzed by Western blot analysis for AhR, ERα, and Sp1 proteins essentially as described elsewhere (1). Replicate (three) experiments were carried out to quantitate the effects of siRNA for the AhR on TCDD-induced downregulation of ERα. The siRNA oligonucleotides for the AhR and scrambled siRNA were as follows: scramble siRNA, 5′-GCG CGC UUU GUA GGA UUC G TT and TT CGC GCG AAA CAU CCU AAG C-5′; siRNA for AhR, 5′-UAC UUC CAC CUC AGU UGG C TT and TT AUG AAG GUG GAG UCA ACC G-5′; siRNA for lamin A/C, 5′-CUG GAC UUC CAG AAG AAC A TT and TT GAC CUG AAG GUC UUC UUG U-5′.
Immunofluorescence.
For uterine immunohistochemistry, 25-day-old mice were injected intraperitoneally with 200 ng of E in 100 μl of corn oil, 1 μg of TCDD in 100 μl of corn oil, ET, or corn oil alone. Twelve hours after treatment, mice were euthanized by CO2 asphyxiation. Uteri were removed, fixed in 4% paraformaldehyde overnight, washed with 70% ethanol, paraffin embedded, and sectioned at a 5-μm thickness onto positively charged slides and, after subsequent processing, slides were immunostained with ERα H-184 antibodies and analyzed by immunofluorescence as indicated below.
For immunocytochemistry, ZR-75 cells were seeded onto four-well glass chamber slides at a density of 75,000 cells per well in RPMI maintenance medium. After 24 h, cells were treated with DMSO, 10 nM E, 10 nM TCDD, or ET for 24 h. Slides were then fixed for 10 min in −20°C MeOH, air dried, and washed for 5 min in PBS-0.3% Tween. Slides were blocked for 1 h with 5% goat serum in antibody dilution buffer (1% bovine serum albumin-PBS-0.3%Tween-31% glycerol [vol/vol] [pH to 8.0] with 0.5 M Na2CO3 [pH 9.5]). A 1:100 dilution of anti-ERα H-184-5% goat serum-antibody dilution buffer, or 5% goat serum-antibody dilution buffer alone (control) was added to the samples and placed in a humidified chamber overnight at 4°C. Slides were then washed three times for 30 min in PBS-Tween and blocked again for 1 h with 5% goat serum-antibody dilution buffer. Alexa Fluor 594 goat anti-rabbit secondary antibody was added at a 1:1,000 dilution in 5% goat serum-antibody dilution buffer to all samples for 1 h at room temperature. Slides were washed three times for 30 min in PBS-Tween and once for 15 min in deionized water and mounted as above. Immunofluorescence preparations were evaluated with a Zeiss Axioplan2 microscope (Carl Zeiss) fitted with a Hamamatsu-C5810 chilled 3CCD color camera (Hamamatsu Corporation). Images of at least three different fields from three different sections per treatment group containing uterine luminal epithelium and stromal cells were captured using identical settings. Fluorescence intensity measurements of ER in both epithelial and stromal cells were obtained following subtraction of background staining determined from the control prepared without primary antibody. Values of mean fluorescence intensity ± the standard error (SE) were analyzed statistically.
Statistics.
All quantitative data were analyzed by an analysis of variance followed by Fisher's protected least significant difference test for significance (P < 0.05). Data are expressed as means ± SE (n ≥ 3).
RESULTS
TCDD induces proteasome-dependent degradation of ERα in breast cancer cell lines.
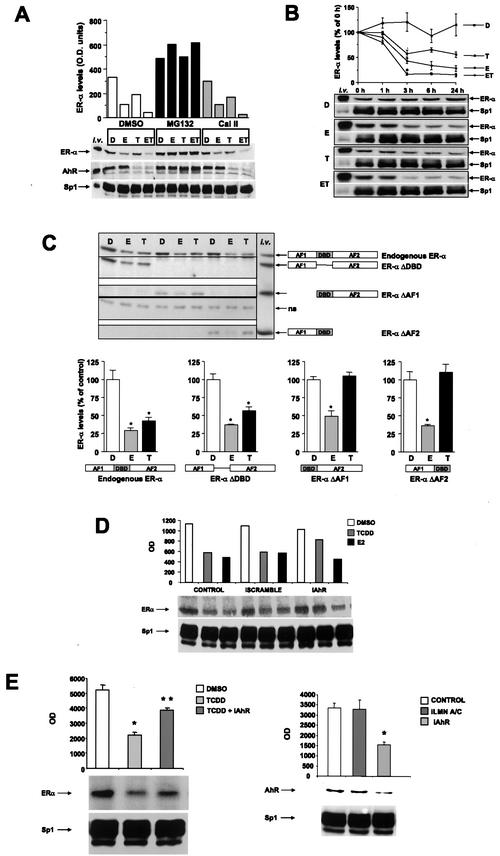
ZR-75 human breast cancer cells express ERα and the AhR, and results in Fig. 1A summarize the effects of treatment with DMSO (D), 10 nM TCDD (T), and 10 nM E alone and in combination (ET) on levels of ERα, AhR, and Sp1 proteins in the presence of solvent (DMSO), proteasome (MG132), or protease (Cal II) inhibitors. E induced degradation of ERα (but not AhR or Sp1), and this response was inhibited by proteasome but not protease inhibitors. In cells treated with T or ET, there was coordinate degradation of both ERα and the AhR (but not Sp1) proteins which was blocked by proteasome but not protease inhibitors. Most significantly, there was a decrease in ERα levels in all three cell lines cotreated with ET compared to that of treatment with E alone. The effects of the D, E, T, and ET treatments are also illustrated in a time course study on ERα degradation in ZR-75 cells (Fig. 1B). There was a rapid and sustained degradation of ERα by TCDD and significantly (>50%) lower ERα levels in cells treated with ET compared to cells treated with E alone, and comparable results were obtained in MCF-7 and T47D breast cancer cells. These data complement results of previous studies in MCF-7 and T47D cell lines, which also express AhR and ERα proteins (54). Thus, in three ER-positive breast cancer cell lines, TCDD activation of AhR results in proteasome-dependent degradation of both AhR and ERα proteins, whereas E induces degradation of ERα but not the AhR; cotreatment with ET enhanced ERα degradation, whereas Sp1 levels were unchanged in all treatment groups. The effects of TCDD and E on degradation of ERα mutants with deletions of the DBD (ERαΔDBD), AF1 (ERαΔAF1), and AF2 (ERαΔAF2) were investigated by transient transfection in T47D cells (Fig. 1C). TCDD- and E2-induced degradation of endogenous wild-type ERα served as a positive control. The results showed that in cells treated with TCDD, deletion of the DBD did not affect degradation, whereas deletion of AF1 or AF2 eliminated this response. In contrast, E2 induced degradation of wild-type ERα, ERαΔDBD, ERαΔAF1, and ERαΔAF2, showing that in T47D cells, multiple domains of ERα are targeted for degradation by proteasomes. In contrast, degradation of ERα induced by TCDD was not observed for mutants with deletions of AF1 or AF2, suggesting that TCDD and E2 induce distinct patterns of ERα degradation. A previous study showed that E2-induced degradation of ERα mutants by proteasomes in HeLa cells was DBD dependent (52), indicating that cell context is also important for ERα degradation by proteasomes. siRNAs can be used for gene silencing in mammalian cells (10, 16, 17), and studies in this laboratory have applied this technique for study of ERα/Sp1 and AhR action in breast cancer cells (1; M. Abdelrahim, unpublished results). Transfection of ZR-75 cells with siRNA for the AhR inhibited TCDD-induced, but not E2-induced, degradation of ERα protein compared to control (solvent-treated) cells or cells transfected with scrambled siRNA (Fig. 1D and E). Sp1 was used as a control, and levels of this protein were unaffected by any of the treatments. Results illustrated in Fig. 1E show that siRNA for the AhR induced degradation of the AhR but not Sp1 protein or lamin A/C (data not shown), whereas siRNA for lamin A/C did not affect AhR protein but decreased lamin A/C protein (data not shown). Similar results were obtained in MCF-7 cells (data not shown). Thus, AhR expression in ZR-75 cells is required for TCDD-induced degradation of ERα, and this has also been previously observed in AhR-deficient MCF-7 cells (54).
FIG.1.
Activated AhR causes degradation of ERα in multiple breast cancer cell lines through the proteasome pathway. (A) ZR-75 cells were treated for 3 h with DMSO (D), 10 nM E, 10 nM TCDD (T), or their combination (ET) following a 30-min pretreatment with vehicle control (EtOH or DMSO), proteasome (MG132), or protease (Cal II) inhibitors at 10 μM concentrations for each. Whole-cell extracts from the different treatment groups were analyzed by Western blot analysis for ERα (G-20), AhR (N-19), and Sp1 (PEP2) proteins as described in Materials and Methods. Relative ERα protein levels for each cell line are illustrated in bar graphs. (B) Time course degradation of ERα in ZR-75 cells. Cells were treated with DMSO, 10 nM E, 10 nM TCDD, or 10 nM ET for the designated times, and whole-cell extracts from the different treatment groups (D, T, E, and ET) were analyzed for ERα and Sp1 protein by Western blot analysis as described in Materials and Methods. In vitro-translated ERα, AhR, and pure Sp1 protein (i.v.) were used as controls. (C) Degradation of wild-type and variant ERα in T47D cells. Cells were transfected with ERα variants treated with 10 nM E or 10 nM T, and levels of endogenous ERα and transfected ERα variants were determined by Western blot analysis as described in Materials and Methods. Results are expressed as means ± SE for three replicate determinations, and levels of wild-type or variant ERα significantly (P < 0.05) lower than the controls (DMSO) are indicated with an asterisk. Western blot analysis of these proteins is indicated (upper left). (D) Effects of siRNA for the AhR on degradation of ERα. siRNA or scrambled siRNA was transfected in ZR-75 cells and the effects of 10 nM TCDD or E2 on ERα protein were determined by Western blot analysis of whole-cell extracts as described in Materials and Methods. TCDD and E2 induced degradation of ERα in control cells and cells transfected with scrambled siRNA, whereas siRNA for AhR blocked the effects of TCDD but not E2. These data were observed in duplicate experiments. (E) Degradation of ERα and AhR. Cells were treated with DMSO, E, or T and siRNAs for the AhR or lamin A/C, and levels of ERα/Sp1 (right) or AhR/Sp1 (left) protein were determined in replicate (three) experiments. TCDD alone significantly (P < 0.05) decreased levels of ERα, and TCDD plus siRNA for AhR (iAhR) significantly (P < 0.05) reversed the effects of TCDD. siRNAs for the AhR (but not lamin A/C) significantly (P < 0.05) decreased AhR levels. Results are expressed as means ± SE relative to control (DMSO) protein levels.
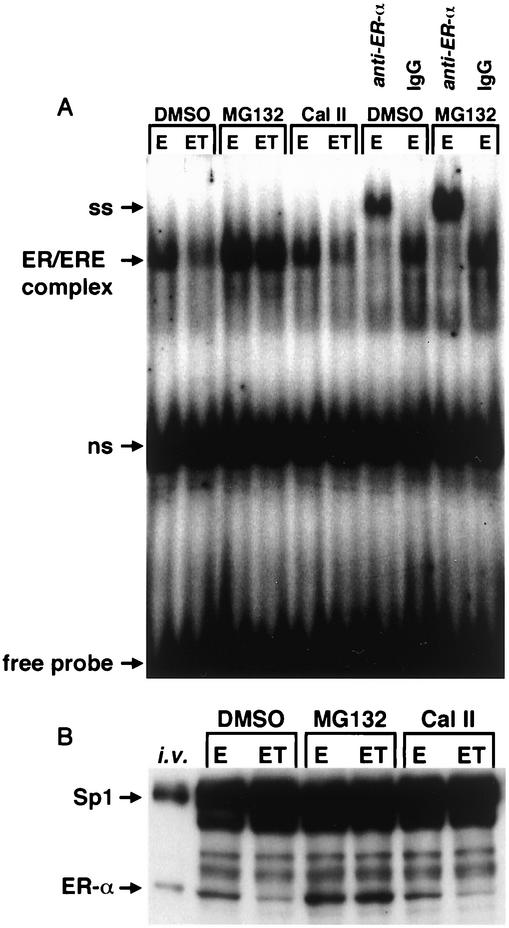
The effects of E and ET treatment on binding of nuclear extracts from MCF-7 cells to [32P]ERE were investigated in gel mobility shift assays (Fig. 2A). Nuclear extracts from cells treated with ET in solvent alone or in combination with Cal II gave a retarded band with decreased intensity compared to the band obtained using extracts from cells treated with E alone, and these data corresponded to results of immunoblot analysis of ERα protein in the same extracts (Fig. 2B). In contrast, the proteasome inhibitor MG132 blocked ERα protein degradation in cells treated with E or ET, and nuclear extracts exhibited increased retarded band intensities (compared to DMSO) (Fig. 2A) and increased immunoreactive ERα protein (Fig. 2B). Antibody supershift experiments with ERα antibody or nonspecific IgG confirmed that the retarded band complex contained ERα protein (Fig. 2A). The effects of the various treatments on Sp1 protein were also determined as a control experiment, since levels of Sp1 are relatively constant in low-passage breast cancer cells (54). The results (Fig. 2) show that E, ET, and the proteasome and protease inhibitors did not affect Sp1 protein expression, indicating specificity for the AhR-ERα interactions.
FIG. 2.
Decreased ERα protein-DNA binding parallels diminished ERα protein levels. (A) Gel mobility shift assay. MCF-7 cells were treated for 3 h with 10 nM E or 10 nM E plus 10 nM T (ET) following a 30-min pretreatment with DMSO, 10 μM MG132, or 10 μM Cal II, and binding of extracts to [32P]ERE was analyzed in a gel mobility shift assay as described in Materials and Methods. Antibody supershift (SS) experiments used ERα antibody (D-12) or nonspecific mouse IgG. ns, nonspecific binding. (B) Immunoreactive ERα or Sp1 proteins in nuclear extracts. The same nuclear extracts from the treated cells from panel A were also analyzed by Western blot analysis as described in Materials and Methods. i.v., in vitro-translated ERα and pure Sp1 protein control lane.
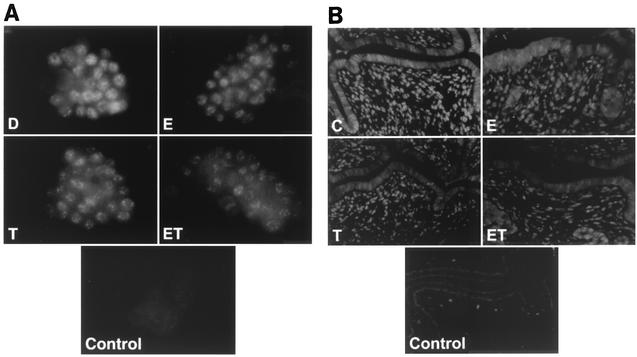
The effects of treatments on cellular ERα levels were investigated by immunostaining ZR-75 cells with ERα antibodies (Fig. 3A). In cells treated with DMSO, there was widespread staining of ERα, which was located in the nuclei. Treatment of the cells with 10 nM E, 10 nM T, or their combination for 24 h caused a decrease in immunostaining of ERα and, in the cotreatment group, only low levels of nuclear ERα were detected. These results confirm that treatment of MCF-7 cells with ET not only decreased immunoreactive ERα protein but also decreased binding of nuclear extracts to [32P]ERE, suggesting that limiting levels of nuclear ERα in cells treated with ET may contribute to inhibition of E-induced gene and reporter gene expression by TCDD (reviewed in reference 44). We also investigated the effects of E and ET in the immature female mouse uterus, which is also responsive to inhibitory AhR-ERα cross talk (51). There was extensive swelling of uterine epithelial and stromal cells after treatment with E for 12 h (Fig. 3B); however, in animals cotreated with ET, the swelling response was decreased and expression of ERα protein was lower in both stromal and epithelial cells. This response in vivo paralleled the effects observed in ZR-75 cells. Quantification of ERα fluorescence intensity revealed that treatment with E, T, or cotreatment with ET results in levels of ERα which are significantly lower than those observed after treatment with corn oil alone (P < 0.001). The mean ERα fluorescence intensity values obtained from uterine sections were 56.41 ± 5.12, 30.26 ± 5.42, 27.88 ± 5.06, and 13.46 ± 3.48 for corn oil control, E, T, and ET, respectively.
FIG. 3.
AhR-mediated ERα degradation occurs in situ. (A) ZR-75 cells were treated with DMSO (D), 10 nM E, 10 nM TCDD (T), or ET for 24 h, and ERα was immunostained as described in Materials and Methods. Field width is 150 μm. (B) Immature (25-day-old) B6C3F1 mice were injected intraperitoneally with corn oil (C), 200 ng of E, or 1 μg of TCDD (T) or ET for 12 h. Uteri were fixed and immunostained as described in Materials and Methods. Field width is 240 μm. Control fields represent background without ERα H184 antibody.
Inhibitory AhR-ERα cross talk: role of proteasome-dependent degradation of ERα.
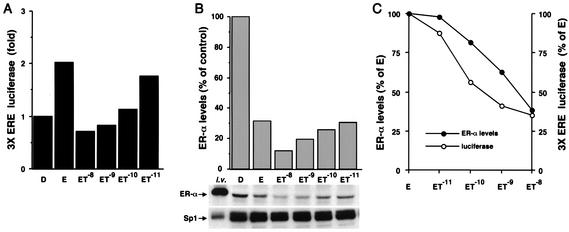
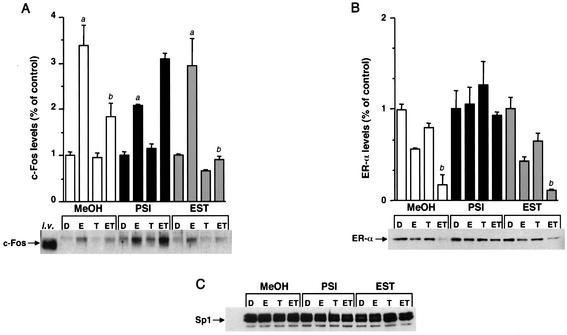
TCDD inhibits transactivation of several E-induced genes in breast cancer cells (9, 14, 18, 21, 25, 38), and different mechanisms of inhibitory AhR-ER cross talk have been proposed (36, 44). The results summarized in Fig. 4A show that E induces luciferase activity in ZR-75 cells transfected with pERE3 alone and, in cells cotreated with E and different concentrations of TCDD, there was a concentration-dependent decrease in hormone-induced activity. In a parallel experiment, treatment of ZR-75 cells with 10 nM E alone decreased ERα levels to 30% of those observed in control (DMSO) cells (Fig. 4B), and this was associated with maximal transactivation (Fig. 4A). These results are consistent with previous reports and reflect the notion of spare ERα levels coupled with proteasome-dependent activation of ERα (2, 11, 29, 49). However, in ZR-75 cells treated with E plus different concentrations of TCDD, there was a further decrease in ERα protein below levels observed in cells treated with E alone (Fig. 3B). Figure 4C illustrates the concentration-dependent effects of TCDD on levels of ERα protein as a percentage of ERα levels in cells treated with 10 nM E alone and on luciferase activity as a percentage of the E-induced response. These parallel curves suggest that in the cells cotreated with ET, inhibition of E-induced transactivation by TCDD was related to the enhanced AhR-mediated degradation of ERα. These data suggest that cotreatment with ET results in limiting levels of ERα, as observed in cells treated with the antiestrogen ICI 182,780, which also induces proteasome-dependent degradation of ERα (41, 52). Previous studies have demonstrated that TCDD inhibits induction of c-fos gene expression by E and induction of E-responsive constructs containing c-fos gene promoter inserts, as shown in Fig. 4A for pERE3 (9). The results in Fig. 5A demonstrate that TCDD also inhibits induction of c-Fos protein by E, and this was observed in the presence of a solvent control (MeOH) or after addition of the protease inhibitor EST. TCDD alone did not affect c-Fos protein levels. The pattern of ERα protein levels in these same treatment groups (Fig. 5B) also showed that in cells treated with ET, both c-Fos and ERα protein levels were maximally decreased. The importance of ERα protein levels in regulation of c-Fos was confirmed in cells treated with the proteasome inhibitor PSI, where inhibition of E-induced c-Fos protein by TCDD was not observed (Fig. 5A), and this correlated with high ERα levels which were maintained in the presence of PSI (Fig. 5B). In contrast, levels of Sp1 protein were unaffected by the different treatments (Fig. 5C). The results in Fig. 5A indicate that the proteasome inhibitor PSI increases Fos protein levels in the various treatment groups compared to levels observed in solvent (MeOH) or protease-treated cells. These data suggest that c-Fos protein is regulated by proteasomes in MCF-7 cells, and this is consistent with the reported regulation of c-Fos protein in other cells lines by the ubiquitin-proteasome pathway (48, 50). Thus, inhibition of E-induced c-fos gene and reporter gene expression by TCDD (9) is also accompanied by decreased c-Fos protein levels, and these responses at the protein (and gene) level correlated with the limiting ERα levels observed in breast cancer cells after cotreatment with ET. Therefore, inhibitory AhR-ERα cross talk in breast cancer cells is dependent on ET-induced degradation of ERα protein by activation of proteasomes.
FIG. 4.
ERα levels determine the magnitude of hormone-induced transactivation. (A) ZR-75 cells were transfected with pERE3 treated with DMSO (D), 10 nM E, or E plus decreasing concentrations of TCDD (ET) for 36 h, and luciferase activity was determined as described in Materials and Methods. Results are expressed as means for duplicate determinations for each treatment group. (B) In parallel duplicate experiments, whole-cell extracts were collected, and ERα protein levels in the various treatment groups described in the legend for panel A were analyzed by Western blot analysis as described in Materials and Methods. i.v., in vitro-translated ERα and pure Sp1 control lane. Results are expressed as means for duplicate determinations for each treatment group. (C) TCDD cotreatment with E mediates a parallel decrease in ERα protein and transactivation. The data from panels A and B were used to plot the effects of increasing concentrations of TCDD on the percent decrease of ERα protein (•) and transactivation (○) below that observed in cells treated with E alone (set at 100%).
FIG. 5.
ERα levels determine regulation of c-Fos protein expression. MCF-7 cells were pretreated for 30 min with methanol vehicle (MeOH), 10 μM PSI, or 10 μM EST. Cells were pretreated with T for 3 h prior to addition of 10 nM E (total DMSO did not exceed 0.03%), and c-Fos, ERα, and Sp1 proteins were detected by Western blot analysis from the same PVDF membrane, as described in Materials and Methods. i.v., in vitro-translated rat c-Fos control. Results are expressed as means ± SE for three determinations for each treatment group. Significant induction of E-induced c-Fos protein is indicated by the letter a above the bar. Significant inhibition of E-induced c-Fos protein and decreased ERα levels by ET (compared to E) are indicated by the letter b above the bar (P < 0.05).
TCDD-induced ubiquitination of ERα.
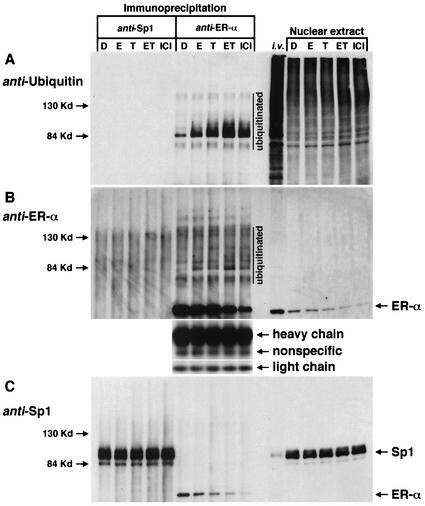
Proteasome-dependent degradation of proteins including ERα is a multistep process involving the 26S proteasome complex, in which proteins targeted for degradation are first conjugated with ubiquitin. Results illustrated in Fig. 6 summarize studies that investigated ubiquitination of ERα in ZR-75 cells by using immunoprecipitation and Western blot analysis of whole-cell lysates or nuclear extracts from cells treated with solvent (DMSO), 10 nM E, 10 nM TCDD or ET, or 10 nM ICI 182,780. Results of previous studies show that cells treated with ICI 182,780 undergo a high level of ubiquitination and protein degradation (52), and this treatment group served as a positive control, whereas Sp1 protein levels are relatively constant and served as an additional negative control protein for this study. Duplicate aliquots of nuclear extracts from different treatment groups were immunoprecipitated with ERα or Sp1 antibodies and then analyzed for ubiquitinated proteins by Western blot analysis using ubiquitin antibodies (Fig. 6A). In vitro-translated ERα and human recombinant Sp1 protein and 15-μg aliquots of nuclear extract served as controls for this experiment. Minimal to nondetectable levels of ubiquitinated proteins were detected in the lanes immunoprecipitated with Sp1 antibodies. However, a series of ubiquitinated bands (>84 kDa) were detected in the lanes immunoprecipitated with ERα antibodies, and increased staining of ubiquitinated bands was observed in cells treated with E, TCDD, ET, and ICI 182,780 compared to DMSO (control). The in vitro and nuclear extract control lanes show multiple ubiquitinated bands as expected, given the propensity of this regulatory protein. The same PVDF membrane was then stripped and reprobed with ERα antibodies (Fig. 6B). The results confirm that ERα was immunoprecipitated, and the pattern of ERα protein intensities was comparable to those observed in the nuclear extracts. Higher-molecular-weight bands are also visible in this panel, and some of these may correspond to the ubiquitinated bands observed in Fig. 6A. This same membrane was then reprobed with Sp1 antibodies (Fig. 6C), and Sp1 protein levels were similar in all treatment groups; this was also observed in the nuclear extract control. These data confirm that proteasome-dependent degradation of ERα induced by the ligand (TCDD)-activated AhR gave enhanced ubiquitination of ERα; moreover, the higher-molecular-weight ubiquitinated ERα bands are also observed in cells after treatment with E or ICI 182,780.
FIG. 6.
AhR-mediated ubiquitination of ERα. ZR-75 cells were treated with DMSO (D), 10 nM E, 10 nM TCDD (T) or ET, or 10 nM ICI 182,780 for 6 h, and nuclear extracts (300-μg aliquots) were immunoprecipitated with Sp1 (PEP2) or ERα (D-12) antibodies. In vitro-translated ERα and pure Sp1 protein (i.v.) and 15 μg of nuclear extract from each treatment group were loaded as controls. Western blot analysis was performed as described in Materials and Methods. (A) The PVDF membrane probed with ubiquitin antibody (P4D1). (B) The same membrane, stripped and reprobed with ERα antibody (D-12). (C) The same membrane reprobed with Sp1 antibody (PEP2). Loading controls using heavy chain, nonspecific, and light chain IgG are also shown.
AhR-mediated degradation of ERα: modulation of gene expression, effects of protein synthesis, and kinase inhibitors.
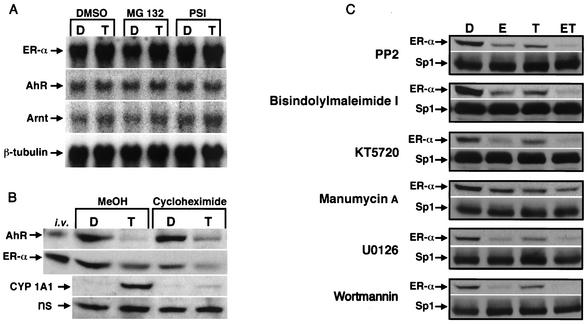
Since TCDD activates proteasome-dependent degradation of both AhR and ERα protein, we also investigated the effects of 10 nM TCDD alone (DMSO) and in combination with proteasome inhibitors (MG132 and PSI) on ERα, AhR, Arnt, and β-tubulin mRNA levels (Fig. 7A). The results show that ERα mRNA levels were similar in solvent control (D) or TCDD (T)-treated in cells in the presence or absence of proteasome inhibitors, and this was confirmed in four separate experiments. Moreover, AhR, Arnt, and β-tubulin mRNA levels were also comparable in the different treatment groups, confirming that TCDD does not downregulate ERα (or the AhR) protein by decreasing mRNA levels. The role of protein synthesis in activation of proteasomes by TCDD was investigated in ZR-75 cells treated with solvent (D) or 10 nM TCDD (T) alone (MeOH) or in the presence of 25 μM cycloheximide (Fig. 7B). The results show that TCDD induces degradation of AhR and ERα in the presence or absence of cycloheximide. In contrast, cycloheximide inhibits induction of CYP1A1 protein by TCDD, and this is consistent with previous studies on this Ah-responsive gene in which TCDD induces both mRNA and protein levels. These results suggest that activation of proteasome-dependent degradation of AhR and ERα by TCDD does not involve new protein synthesis.
FIG. 7.
Proteasome-dependent degradation of ERα; role of mRNA levels, protein synthesis, and kinase inhibitors. (A) mRNA levels. T47D cells were treated for 6 h with DMSO (D) or TCDD (T) following a 30-min pretreatment with DMSO, 10 μM MG132, or 10 μM PSI, and ERα, AhR, Arnt, and β-tubulin mRNA levels were determined as described in Materials and Methods. (B) Effects of cycloheximide. ZR-75 cells were treated for 3 h with DMSO (D) or 10 nM TCDD (T) following a 30-min pretreatment with MeOH or 25 μM cycloheximide, and whole-cell lysates were analyzed for AhR, ERα, and CYP1A1 proteins by Western blot analysis as described in Materials and Methods. ns, nonspecific protein. (C) Effects of kinase inhibitors. ZR-75 cells were treated with DMSO (D), 10 nM E, 10 nM TCDD (T), and ET for 3 h following a 30-min pretreatment with 10 μM PP2, 1 μM bis-indolylmaleimide 1, 1 μM KT5720, 2 μM manumycin A, 5 μM U0126, or 400 nM wortmannin. ERα and Sp1 proteins were determined by Western blot analysis as described in Materials and Methods.
Activation of kinase pathways is required for proteasome-dependent degradation of some proteins, and the results in Fig. 7C summarize effects of several kinase inhibitors on ERα and Sp1 protein levels. Cells were treated with DMSO (D), 10 nM E, 10 nM TCDD or ET, and several inhibitors (kinases), including 10 μM PP2 (src), 1 μM bis-indolylmaleimide 1 (protein kinase C), 1 μM KT 5720 (protein kinase A), 2 μM manumycin A (ras), 5 μM U0126 (mitogen-activated protein kinase kinase [MAPKK]), or 400 nM wortmannin (phosphatidylinositol 3-kinase), for 3 h and whole-cell extracts were analyzed for ERα and Sp1 by Western blot analysis. The pattern of downregulation of ERα by TCDD was unaffected by the kinase inhibitors, and similar effects were observed in cells treated with E alone and ET, although the magnitude of the response was decreased in cells cotreated with the ras inhibitor manumycin A. The MAPKK inhibitor PD98059 blocked activation of proteasomes by TCDD (data not shown); however, this compound and related 3′-methoxy-substituted flavonoids inhibit nuclear translocation and activation of the AhR by TCDD (39), suggesting that this response is also required for AhR-mediated activation of proteasomes.
Ligand activation of the AhR is required for AhR-ERα interactions.
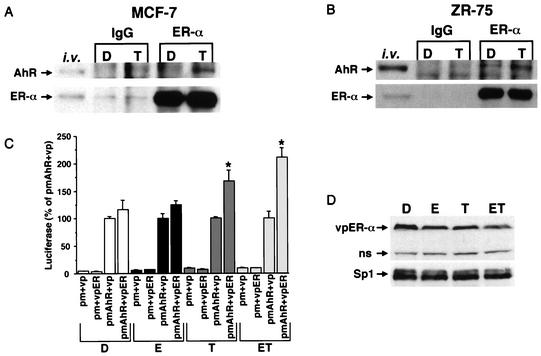
Klinge and coworkers (21) first showed that a glutathione S-transferase (GST)-ERα chimeric protein interacted with the AhR (but not Arnt) in a pull-down assay, and this interaction was AhR ligand (β-naphthoflavone) dependent. The results in Fig. 8 show that ERα and AhR antibodies (but not IgG) immunoprecipitated both the AhR and ERα protein in nuclear extracts from MCF-7 (Fig. 8A) or ZR-75 (Fig. 8B) cells treated with 10 nM TCDD. There was also evidence for some interactions of ERα and the AhR in solvent (DMSO)-treated ZR-75 cells; however, a more intense band was observed in the extract from cells treated with TCDD. Interaction of these proteins was further investigated in a mammalian two-hybrid assay using an expression plasmid containing the DBD of the yeast GAL4 protein (amino acids 1 to 147) fused to full-length AhR (pm-AhR), and a plasmid expressing the VP16 (amino acids 411 to 455) activation domain fused to ERα; empty vectors (pm and vp) served as controls. The GAL4-responsive construct (pGAL4) contains five tandem yeast GAL4 response elements linked to a luciferase reporter gene. In cells treated with DMSO (solvent control) or E and cotransfected with pmAhR plus vp (empty vector) or vpER, comparable activities were observed, indicating that the high activity was primarily due to unliganded pmAhR, and this was not enhanced by vpER in the absence or presence of E. The high basal activity of pmAhR in ZR-75 cells is in contrast to the lower response observed for pmAhR (mouse) in COS cells, which was similar to the activity of pm alone (19). This may be due, in part, to breast cancer cell expression of cofactors such as p300, steroid receptor coactivators, and TATA binding protein, which interact with the AhR (26, 33, 43). The activity of pm-AhR plus vp was not increased in cells treated with TCDD alone or ET; however, a significant increase (over pmAhR plus vp) was observed when cells were cotransfected with pmAhR plus vpER. Thus, ER-AhR interactions in the mammalian two-hybrid assay were dependent on TCDD, and these results were consistent with interactions of these proteins in the pull-down assay (21). The pattern of vpER degradation in transfected cells (Fig. 8D) was similar to that observed for wild-type ERα (Fig. 1). Previous studies showed that E induced degradation of transfected vpER and ERα in HeLa cells, and this study confirms that TCDD alone or in combination with E also induces degradation of both proteins in breast cancer cells. Thus, TCDD-induced degradation of ERα (and vpER) is linked to physical interactions of AhR and ERα.
FIG. 8.
Activated AhR interacts with ERα. MCF-7 (A) and ZR-75 (B) cells were treated with DMSO (D) or TCDD (T) for 30 min. Nuclear extracts were isolated and immunoprecipitated with nonspecific mouse or goat IgG, anti-ERα (D12), or the AhR. AhR and ERα proteins were analyzed by Western blot analysis as described in Materials and Methods. In vitro-translated AhR and ERα were also used as markers. (C) Mammalian two-hybrid interactions of pm-AhR and vp-ER in ZR-75 breast cancer cells. ZR-75 cells were transfected with pGAL4, pm plus vp (empty vectors), pm plus vpER, pmAhR plus vp, or pmAhR plus vpER, treated with DMSO (D), 10 nM E (E), 10 nM (TCDD), or E plus TCDD (ET), and luciferase activity was determined as described in Materials and Methods. Results are expressed as means ± SE for three separate determinations for each treatment group. Luciferase activity significantly (P < 0.05) higher than that observed in cells transfected with pmAhR plus vp is indicated with an asterisk. (D) Levels of vpER were also determined by Western blot analysis in cells transfected with pmAhR and vpER. ns, nonspecific.
DISCUSSION
The ubiquitin-proteasome pathway targets proteins for degradation and plays a critical role in multiple biochemical processes, including differentiation, cell cycle regulation, transport, embryogenesis, immune responses, apoptosis, and control of signal transduction (8, 12, 24, 34). Proteins targeted for degradation by proteasomes are first ubiquitinated by enzyme-catalyzed formation of polyubiquitin-protein conjugates, which are substrates for ATP-dependent proteolysis by the 26S proteasome complex. Proteasomes play an important role in regulating cellular expression of ERα and other NR proteins; however, there is considerable variability in proteasome activation and modulation of these nuclear transcription factors (2, 4, 6, 11, 23, 27-29, 45, 46, 49, 55). For example, both the androgen and vitamin D receptors are degraded by proteasomes to maintain basal levels of these proteins, and receptor agonists or proteasome inhibitors increase receptor levels through inhibition of proteasome-dependent degradation in several cell lines (28, 45, 46). In contrast, synthetic or endogenous ligands for the progesterone receptor, thyroid hormone receptor, retinoic acid receptor (RAR), and ERα activate proteasome-dependent receptor degradation (4, 6, 23, 27, 28, 45, 46, 55).
Ligands for the AhR also activate proteasome-dependent degradation of ERα protein, and this process is not observed in benzo(a)pyrene-resistant MCF-7 cells that do not express AhR (54); AhR antagonists that inhibit formation of the nuclear AhR complex also block AhR-mediated degradation of ERα (data not shown). siRNA oligonucleotides can be used for gene silencing in mammalian cells (10, 16, 17), and this technique has been used for investigating ERα/Sp1-mediated transactivation and inhibitory AhR-ERα cross talk in breast cancer cells (1; M. Abdelrahim, unpublished results). Transfection of siRNA for the AhR induces a 50 to 70% decrease in AhR levels in MCF-7 cells and also inhibits degradation of ERα by TCDD but not E2 (Fig. 1D and E) These data complement results of previous studies in AhR-deficient MCF-7 cells, where TCDD did not induce degradation of ERα (54). Results shown in Fig. 1 to 3 confirm that the AhR agonist TCDD alone or in combination with E induces rapid degradation of endogenous ERα in at least three ERα-positive breast cancer cell lines (T47D, ZR-75, and MCF-7), and these responses are blocked by proteasome inhibitors but not protease inhibitors. This represents a novel interaction between ERα and the basic helix-loop-helix AhR protein, in which ligands for the AhR activate proteasome-dependent degradation of both AhR and ERα, whereas ERα agonists induce proteolysis of ERα but not the AhR. Interestingly, we also observed a punctate pattern of nuclear ERα staining in cells treated with E, TCDD, and ET (Fig. 3A), and this has only been observed in previous studies using ERα-specific ligands (49). The effects of ET in the mouse uterus (Fig. 3B) showed that there was a significant decrease in E-induced swelling of epithelial and stromal cells, and ET also decreased ERα staining in epithelial and stromal cells. These responses in vivo paralleled results in cell culture (Fig. 3A) and are consistent with inhibitory AhR-ERα cross talk previously observed in the rodent uterus (42, 51).
This study investigates the mechanism and role of AhR-dependent degradation of endogenous ERα protein in mediating inhibition of E-induced gene and reporter gene and protein expression in breast cancer cells. E activates degradation of ERα, and this is accompanied by high transactivation (Fig. 4) (29). Since both E and TCDD induce proteasome-dependent degradation of ERα, cells cotreated with ET express <20% of ERα protein levels observed in untreated cells and lower levels than in cells treated with E alone. Both ET and the direct-acting pure antiestrogen ICI 182,780 induce substantial degradation and ubiquitination of ERα (Fig. 6), suggesting that cellular levels of ERα are limiting and are insufficient for hormone-induced transactivation (Fig. 4 and 5). Thus, inhibitory AhR-ERα cross talk in these cells is linked to activation of proteasome-dependent degradation of ERα by ET.
Preliminary studies in this laboratory did not observe E- or TCDD-induced degradation of transfected wild-type or mutant ERα in HeLa cells (data not shown). Moreover, our data showed that E increased transfected ERα levels, indicating that transfected ERα may behave differently from endogenous ERα. Therefore, this study used E-responsive breast cancer cell lines which express functional AhR and ERα as models for studying the mechanisms of AhR-dependent degradation of ERα through activation of proteasomes. Proteasome-dependent degradation of ERα is dependent on multiple factors, including ligand structure, cell context, and the form of ERα expressed (i.e., wild type, chimeric, or mutant) (2, 11, 29, 52, 54). For example, one study reported that ligand-mediated downregulation of ERα is linked to coactivator recruitment and coordinate degradation of both receptor and coactivator proteins in HeLa cells transfected with ERα or mutant ERα expression plasmids (29). In contrast, another study indicated that mutations which block AF2-interacting coactivator recruitment did not block proteasome-dependent degradation of mutant ERα or mutant chimeric vp-ERα protein in HeLa cells (52). Moreover, it was also reported that E2 did not induce proteasome-dependent degradation of mutant ERα containing a DBD deletion in HeLa cells (52), whereas our results show that TCDD- and E2-induced degradation of ERα is DBD independent and that the response for TCDD requires both AF1 and AF2 of ERα (Fig. 1C). These results clearly distinguish between activation of proteasomes by TCDD or E2 through their respective receptors, since different domains of ERα are required. These results also show that the effects of E2 on degradation of ERα are dependent on cell context (53). Current studies are investigating specific regions within AF1 and AF2 of ERα which are required for AhR-dependent degradation of ERα by proteasomes.
TCDD induces kinase activities in some tissues and cells (31); however, results of ongoing studies indicate that TCDD does not activate the src-MAPKK or -phosphatidylinositol 3-kinase pathways in ER-positive breast cancer cells (data not shown). Therefore, the results in Fig. 7 showing that a series of kinase inhibitors did not affect AhR-mediated degradation of ERα suggest that activation of kinase-dependent phosphorylation of ERα by TCDD is not required for degradation of ERα by proteasomes. In contrast, MAPK-dependent phosphorylation of S294 of the progesterone receptor is required for proteasome degradation of this receptor in T47D cells (27), whereas RARα1 degradation by proteasomes is inhibited by phosphorylation (23).
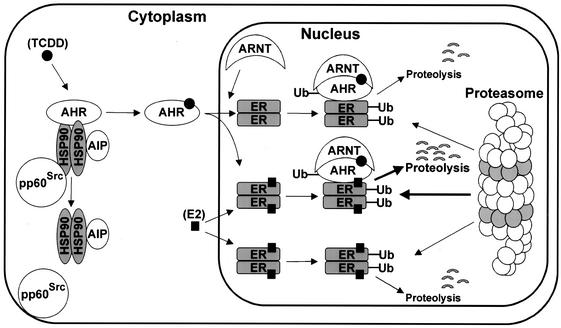
The AhR and ERα can be coimmunoprecipitated in both MCF-7 and ZR-75 cells (Fig. 8), and this interaction was enhanced by TCDD, suggesting a possible mechanism of AhR-induced degradation of ERα that involves initial ligand (TCDD)-dependent interaction of AhR with ERα. These interactions were further investigated in a mammalian two-hybrid assay using pmAhR [GAL4(DBD)-AhR fusion protein] and vpERα (VP16-ERα fusion protein) and treated with solvent (D), E, or TCDD alone or in combination. Interactions of AhR and ERα were dependent on TCDD but not E. This was similar to results of previous studies using GST-AhR and ERα in pull-down assays (21) and suggests an explanation for the unidirectional ligand-dependent activation of proteasomes in AhR-ERα cross talk. E does not induce AhR-ERα interactions in the mammalian two-hybrid assay (Fig. 8) and, therefore, activates proteasome-dependent degradation of ERα but not the AhR (Fig. 1). Moreover, since the AhR and ERα are cytosolic and nuclear proteins, respectively, addition of E cannot induce colocalization of both proteins in the nucleus. In contrast, TCDD induces nuclear localization of the AhR, AhR-ERα interactions, and proteasome-dependent degradation of the receptors. These results suggest a mechanism (Fig. 9) in which ligand binding to AhR induces formation of the nuclear AhR, which recruits both ERα and proteasomes, resulting in ubiquitination of AhR and ERα and degradation of both proteins. This process is highly specific, since Sp1 protein is not affected by TCDD, even though Sp1 binds to both AhR and ERα (22, 37). Currently, we are using this model to further investigate AhR-mediated degradation of specific domains of ERα and to determine the role of protein-protein interactions in activating the proteasome pathway for degradation of one or both interacting proteins.
FIG. 9.
Proposed model for AhR-mediated degradation of ERα through activation of the proteasome pathway. Cytosolic AhR is activated by TCDD ligand, which induces disassociation of the heat shock protein 90 (hsp 90), AhR-interacting protein (AIP), and pp60Src kinase complex. Activated AhR translocates to the nucleus, which enables direct interaction with nuclear ERα, subsequent ubiquitination, and proteolysis by the 26S proteasome complex. Both ERα ubiquitination and proteolysis are increased in cells cotreated with ET. E alone induces degradation of nuclear ERα but not the cytosolic AhR, and this may be due to lack of receptor colocalization.
Acknowledgments
The financial assistance of the National Institutes of Health (ES04176 and ES09106), the Texas Agricultural Experiment Station, and the Sid Kyle Endowment are gratefully acknowledged.
REFERENCES
- 1.Abdelrahim, M., I. Samudio, R. Smith, R. Burghardt, and S. Safe. 2002. Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J. Biol. Chem. 277:28815-28822. [DOI] [PubMed]
- 2.Alarid, E. T., N. Bakopoulos, and N. Solodin. 1999. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol. Endocrinol. 13:1522-1534. [DOI] [PubMed] [Google Scholar]
- 3.Beato, M., P. Herrlich, and G. Schutz. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851-857. [DOI] [PubMed] [Google Scholar]
- 4.Boudjelal, M., Z. Wang, J. J. Voorhees, and G. J. Fisher. 2000. Ubiquitin/proteasome pathway regulates levels of retinoic acid receptor γ and retinoid X receptor α in human keratinocytes. Cancer Res. 60:2247-2252. [PubMed] [Google Scholar]
- 5.Brueggemeier, R. W. 1994. Aromatase inhibitors—mechanisms of steroidal inhibitors. Breast Cancer Res. Treat. 30:31-42. [DOI] [PubMed] [Google Scholar]
- 6.Dace, A., L. Zhao, K. S. Park, T. Furuno, N. Takamura, M. Nakanishi, B. L. West, J. A. Hanover, and S. Cheng. 2000. Hormone binding induces rapid proteasome-mediated degradation of thyroid hormone receptors. Proc. Natl. Acad. Sci. USA 97:8985-8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davarinos, N. A., and R. S. Pollenz. 1999. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J. Biol. Chem. 274:28708-28715. [DOI] [PubMed] [Google Scholar]
- 8.DeMartino, G. N., and C. A. Slaughter. 1999. The proteasome, a novel protease regulated by multiple mechanisms. J. Biol. Chem. 274:22123-22126. [DOI] [PubMed] [Google Scholar]
- 9.Duan, R., W. Porter, I. Samudio, C. Vyhlidal, M. Kladde, and S. Safe. 1999. Transcriptional activation of c-fos protooncogene by 17β-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol. Endocrinol. 13:1511-1521. [DOI] [PubMed] [Google Scholar]
- 10.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 11.El Khissiin, A., and G. Leclercq. 1999. Implication of proteasome in estrogen receptor degradation. FEBS Lett. 448:160-166. [DOI] [PubMed] [Google Scholar]
- 12.Freemont, P. S. 2000. Ubiquitination: RING for destruction? Curr. Biol. 10:R84-R87. [DOI] [PubMed] [Google Scholar]
- 13.Fuqua, S. A., J. Russo, S. E. Shackney, and M. E. Stearns. 2000. Estrogen, estrogen receptors and selective estrogen receptor modulators in human breast cancer. J. Women's Cancer 2:21-32. [Google Scholar]
- 14.Gillesby, B., M. Santostefano, W. Porter, Z. F. Wu, S. Safe, and T. Zacharewski. 1997. Identification of a motif within the 5′-regulatory region on pS2 which is responsible for Ap1 binding and TCDD-mediated suppression. Biochemistry 36:6080-6089. [DOI] [PubMed] [Google Scholar]
- 15.Hall, J. M., J. F. Couse, and K. S. Korach. 2001. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 276:36869-36872. [DOI] [PubMed] [Google Scholar]
- 16.Hannon, G. J. 2002. RNA interference. Nature 418:244-251. [DOI] [PubMed] [Google Scholar]
- 17.Harborth, J., S. M. Elbashir, K. Bechert, T. Tuschl, and K. Weber. 2001. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J. Cell Sci. 114:4557-4565. [DOI] [PubMed] [Google Scholar]
- 18.Harper, N., X. Wang, H. Liu, and S. Safe. 1994. Inhibition of estrogen-induced progesterone receptor in MCF-7 human breast cancer cells by aryl hydrocarbon (Ah) receptor agonists. Mol. Cell. Endocrinol. 104:47-55. [DOI] [PubMed] [Google Scholar]
- 19.Jain, S., K. M. Dolwick, J. V. Schmidt, and C. A. Bradfield. 1994. Potent transactivation domains of the Ah receptor and the Ah receptor nuclear translocator map to their carboxyl termini. J. Biol. Chem. 269:31518-31524. [PubMed] [Google Scholar]
- 20.Katzenellenbogen, J. A., B. W. O'Malley, and B. S. Katzenellenbogen. 1996. Tripartite steroid hormone receptor pharmacology—interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol. Endocrinol. 10:119-131. [DOI] [PubMed] [Google Scholar]
- 21.Klinge, C. M., K. Kaur, and H. I. Swanson. 2000. The aryl hydrocarbon receptor interacts with estrogen receptor α and orphan receptors COUP-TFI and ERRα1. Arch. Biochem. Biophys. 373:163-174. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, A., K. Sogawa, and Y. Fujii-Kuriyama. 1996. Cooperative interaction between AhR·Arnt and Sp1 for the drug-inducible expression of CYP1A1 gene. J. Biol. Chem. 271:12310-12316. [DOI] [PubMed] [Google Scholar]
- 23.Kopf, E., J.-L. Plassat, V. Vivat, H. de Thé, P. Chambon, and C. Rochette-Egly. 2000. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors α and γ through the ubiquitin-proteasome pathway. J. Biol. Chem. 275:33280-33288. [DOI] [PubMed] [Google Scholar]
- 24.Kornitzer, D., and A. Ciechanover. 2000. Modes of regulation of ubiquitin-mediated protein degradation. J. Cell Physiol. 182:1-11. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan, V., W. Porter, M. Santostefano, X. Wang, and S. Safe. 1995. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol. Cell. Biol. 15:6710-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar, M. B., R. W. Tarpey, and G. H. Perdew. 1999. Differential recruitment of coactivator RIP140 by Ah and estrogen receptors: absence of a role for LXXLL motifs. J. Biol. Chem. 274:22155-22164. [DOI] [PubMed] [Google Scholar]
- 27.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, X. Y., M. Boudjelal, J. H. Xiao, Z. H. Peng, A. Asuru, S. Kang, G. J. Fisher, and J. J. Voorhees. 1999. 1,25-Dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol. Endocrinol. 13:1686-1694. [DOI] [PubMed] [Google Scholar]
- 29.Lonard, D. M., Z. Nawaz, C. L. Smith, and B. W. O'Malley. 2000. The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol. Cell 5:939-948. [DOI] [PubMed] [Google Scholar]
- 30.Ma, Q., and K. T. Baldwin. 2000. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activation and DNA binding of AhR. J. Biol. Chem. 275:8432-8438. [DOI] [PubMed] [Google Scholar]
- 31.Ma, X., and J. G. Babish. 1993. Acute 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure results in enhanced tyrosylphosphorylation and expression of murine hepatic cyclin dependent kinases. Biochem. Biophys. Res. Commun. 197:1070-1077. [DOI] [PubMed] [Google Scholar]
- 32.McDougal, A., M. Wormke, J. Calvin, and S. Safe. 2001. Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective Ah receptor modulator. Cancer Res. 61:3901-3907. [PubMed] [Google Scholar]
- 33.Nguyen, T. A., D. Hoivik, J. E. Lee, and S. Safe. 1999. Interactions of nuclear receptor coactivator/corepressor proteins with the aryl hydrocarbon receptor complex. Arch. Biochem. Biophys. 367:250-257. [DOI] [PubMed] [Google Scholar]
- 34.Pickart, C. M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 35.Pollenz, R. S., and E. R. Barbour. 2000. Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor-mediated gene regulation. Mol. Cell. Biol. 20:6095-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter, W., and S. Safe. 1998. Estrogenic and antiestrogenic compounds, p. 267-283. In A. Puga and K. B. Wallace (ed.), Molecular biology approaches to toxicology. Techbooks, Fairfax, Va.
- 37.Porter, W., B. Saville, D. Hoivik, and S. Safe. 1997. Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol. Endocrinol. 11:1569-1580. [DOI] [PubMed] [Google Scholar]
- 38.Porter, W., F. Wang, R. Duan, C. Qin, E. Castro-Rivera, and S. Safe. 2001. Transcriptional activation of heat shock protein 27 gene expression by 17β-estradiol and modulation by antiestrogens and aryl hydrocarbon receptor agonists: estrogenic activity of ICI 164,384. J. Mol. Endocrinol. 26:31-42. [DOI] [PubMed] [Google Scholar]
- 39.Reiners, J. J. J., J. Y. Lee, R. E. Clift, D. T. Dudley, and S. P. Myrand. 1998. PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol. Pharmacol. 53:438-445. [DOI] [PubMed] [Google Scholar]
- 40.Roberts, B. J., and M. L. Whitelaw. 1999. Degradation of the basic helix-loop-helix/Per-ARNT-Sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. J. Biol. Chem. 274:36351-36356. [DOI] [PubMed] [Google Scholar]
- 41.Robertson, J. F., R. I. Nicholson, N. J. Bundred, E. Anderson, Z. Rayter, M. Dowsett, J. N. Fox, J. M. Gee, A. Webster, A. E. Wakeling, C. Morris, and M. Dixon. 2001. Comparison of the short-term biological effects of 7α-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)-nonyl]estra-1,3,5,(10)-triene-3,17β-diol (Faslodex) versus tamoxifen in postmenopausal women with primary breast cancer. Cancer Res. 61:6739-6746. [PubMed] [Google Scholar]
- 42.Romkes, M., and S. Safe. 1988. Comparative activities of 2,3,7,8-tetrachlorodibenzo-p-dioxin and progesterone on antiestrogens in the female rat uterus. Toxicol. Appl. Pharmacol. 92:368-380. [DOI] [PubMed] [Google Scholar]
- 43.Rowlands, J. C., I. J. McEwan, and J. A. Gustafsson. 1996. Trans-activation by the human aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator proteins: direct interactions with basal transcription factors. Mol. Pharmacol. 50:538-548. [PubMed] [Google Scholar]
- 44.Safe, S., A. McDougal, M. S. Gupta, and K. Ramamoorthy. 2001. Selective Ah receptor modulators (SAhRMs): progress towards development of a new class of inhibitors of breast cancer growth. J. Women's Cancer 3:37-45. [Google Scholar]
- 45.Santiso-Mere, D., T. Sone, G. M. Hilliard, J. W. Pike, and D. P. McDonnell. 1993. Positive regulation of the vitamin D receptor by its cognate ligand in heterologous expression systems. Mol. Endocrinol. 7:833-839. [DOI] [PubMed] [Google Scholar]
- 46.Sheflin, L., B. Keegan, W. Zhang, and S. W. Spaulding. 2000. Inhibiting proteasomes in human HepG2 and LNCaP cells increases endogenous androgen receptor levels. Biochem. Biophys. Res. Commun. 275:144-150. [DOI] [PubMed] [Google Scholar]
- 47.Smith, C. L., and B. W. O'Malley. 1999. Evolving concepts of selective estrogen receptor action: from basic science to clinical applications. Trends Endocrinol. Metab. 10:299-300. [DOI] [PubMed] [Google Scholar]
- 48.Stancovski, I., H. Gonen, A. Orian, A. L. Schwartz, and A. Ciechanover. 1995. Degradation of the proto-oncogene product c-Fos by the ubiquitin proteolytic system in vivo and in vitro: identification and characterization of the conjugating enzymes. Mol. Cell. Biol. 15:7106-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenoien, D. L., K. Patel, M. G. Mancini, M. Dutertre, C. L. Smith, B. W. O'Malley, and M. A. Mancini. 2001. FRAP reveals that mobility of oestrogen receptor-α is ligand- and proteasome-dependent. Nat. Cell Biol. 3:15-23. [DOI] [PubMed] [Google Scholar]
- 50.Tsurumi, C., N. Ishida, T. Tamura, A. Kakizuka, E. Nishida, E. Okumura, T. Kishimoto, M. Inagaki, K. Okazaki, and N. Sagata. 1995. Degradation of c-Fos by the 26S proteasome is accelerated by c-Jun and multiple protein kinases. Mol. Cell. Biol. 15:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umbreit, T. H., and M. A. Gallo. 1988. Physiological implications of estrogen receptor modulation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Lett. 42:5-14. [DOI] [PubMed] [Google Scholar]
- 52.Wijayaratne, A. L., and D. P. McDonnell. 2001. The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J. Biol. Chem. 276:35684-35692. [DOI] [PubMed] [Google Scholar]
- 53.Wijayaratne, A. L., S. C. Nagel, L. A. Paige, D. J. Christensen, J. D. Norris, D. M. Fowlkes, and D. P. McDonnell. 1999. Comparative analyses of mechanistic differences among antiestrogens. Endocrinology 140:5828-5840. [DOI] [PubMed] [Google Scholar]
- 54.Wormke, M., M. Stoner, B. Saville, and S. Safe. 2000. Crosstalk between estrogen receptor α and the aryl hydrocarbon receptor in breast cancer cells involves unidirectional activation of proteosomes. FEBS Lett. 478:109-112. [DOI] [PubMed] [Google Scholar]
- 55.Zhu, J., M. Gianni, E. Kopf, N. Honore, M. Chelbi-Alix, M. Koken, F. Quignon, C. Rochette-Egly, and H. de The. 1999. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor α (RARα) and oncogenic RARα fusion proteins. Proc. Natl. Acad. Sci. USA 96:14807-14812. [DOI] [PMC free article] [PubMed] [Google Scholar]