Abstract
The ERG1 gene encodes a family of potassium channels. Mutations in human ERG1 lead to defects in cardiac repolarization, referred to as the long QT syndrome. Through homologous recombination in mouse embryonic stem cells the ERG1 B potassium channel transcript was eliminated while the ERG1 A transcript was maintained. Heterologous expression of ERG1 isoforms had previously indicated that the deactivation time course of ERG1 B is 10-fold more rapid than that of ERG1 A. In day-18 fetal +/+ myocytes, IKr exhibited two time constants of deactivation (3,933 ± 404 and 350 ± 19 ms at −50 mV), whereas in age-matched ERG1 B−/− mice the rapid component was absent. Biexponential deactivation rates (2,039 ± 268 and 163 ± 43 ms at −50 mV) were also observed in adult +/+ myocytes. In adult ERG1 B−/− myocytes no IKr was detected. Electrocardiogram intervals were similar in +/+ and −/− mice. However, adult −/− mice manifested abrupt spontaneous episodes of sinus bradycardia (>100 ms of slowing) in 6 out of 21 mice. This phenomenon was never observed in +/+ mice (0 out of 16). We conclude that ERG1 B is necessary for IKr expression in the surface membrane of adult myocytes. Knockout of ERG1 B predisposes mice to episodic sinus bradycardia.
Normal heart rhythm depends on the coordination of a wave of depolarization followed by a wave of repolarization with each cardiac cycle. Potassium channels play an important role in the repolarization phase. The mammalian heart contains a number of distinct potassium channel species, each with a unique role in repolarization (18, 24). The human ERG1 gene, through alternative mRNA processing, encodes a family of potassium channels that are important in the late stage (phase 3) of action potential repolarization. Mutations in this gene, which generally reduce sarcolemmal expression of ERG1, lead to the long QT2 syndrome in humans (5, 27, 46). The QT interval, derived from a surface electrocardiogram, is a measure of the time required to repolarize the heart. Patients with the long QT syndrome have a delay in the repolarization phase which predisposes them to arrhythmias that can be fatal (1, 38). Genetic defects account for a small portion of long QT patients, with most patients acquiring the disease secondarily to myocardial disease (17, 35) or treatment with a wide variety of pharmaceuticals that either intentionally or unintentionally block human ERG1 (37). Sudden cardiac death associated with the acquired form of the long QT syndrome is a common cause of premature mortality in affluent populations (19, 35).
Ion channels generally function by gating the flux of specific ions across membranes. The ERG1 potassium channel has two gates, referred to as the activation and inactivation gates, that regulate potassium ion flux according to membrane potential (28, 32). Upon depolarization the ERG1 activation gate opens, but this is followed by rapid closure mediated by the inactivation gate. As the myocyte repolarizes, the ERG1 potassium channel recovers from inactivation before the activation gate closes, resulting in a tail current. This current is not only important for completing repolarization but is also thought to prevent arrhythmias induced by early after-depolarizations (32).
Two alternatively processed forms of ERG1, differing at their NH2 termini, are found in both humans and mice (12, 15). The ERG1 A NH2 terminus is long, contains a Per-ARNT-Sim (PAS) domain, and confers slow deactivation, whereas the ERG1 B NH2 terminus is short and highly basic and confers rapid deactivation. As a result, the tail current of ERG1 A is larger than that of ERG1 B, and relative isoform expression may play an important role in heart rhythm. All published information relating to the effects of long QT2 syndrome mutations on ERG1 function has been applicable to the ERG1 A isoform alone. This ignores the fact that the majority of mutations are found in regions common to both isoforms. There are several reasons why the contribution of the individual isoforms has been largely ignored: (i) the ERG1 A transcript is relatively abundant in both human and mouse hearts, whereas the ERG1 B transcript is abundant in mouse hearts but scarce in human hearts (12, 13, 15); (ii) studies with antibodies to the common region or to the N terminus of ERG1 A have been interpreted to indicate that only the ERG1 A isoform is expressed at the protein level in adult mouse ventricle (25); and (iii) ERG1 A, when transfected into mammalian cells at 35°C, deactivates more rapidly than at 20°C in Xenopus oocytes and has kinetics similar to those seen for human cardiac myocytes at 36°C (42, 45). Alternatively, there is some evidence from human studies that ERG1 B could contribute to repolarizing currents. An exon 4 duplication that frameshifts ERG1 A at amino acid 200 (200fs) is equivalent to selectively knocking out the ERG1 A isoform in homozygous individuals (10). An individual homozygous for this truncation was rescued during the perinatal period and had long-term survival, while mice with a complete knockout of the ERG1 gene die during early development (14). Moreover, humans who can survive a complete knockout of ERG1 have never been identified. The study of Hoorntje et al. (10) lends credence to efforts aimed at identifying the biologic role of ERG1 B. In this regard, we present the ERG1 B−/− mouse.
MATERIALS AND METHODS
Generation of ERG1 B-deficient mice.
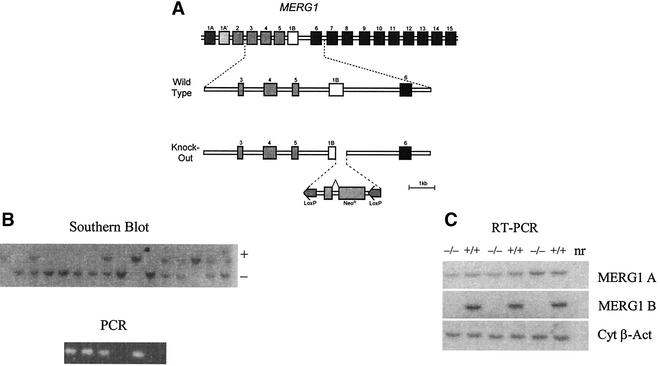
A genomic DNA clone was isolated from a mouse 129-SV/J genomic DNA library (16) by using a 540-bp probe from the 5′ cDNA sequence of mouse ERG1 B (12). A targeting construct containing a pol II-driven neomycin cassette flanked by loxP sites was cloned into lambda2TK (Fig. 1A) (7, 36). The resistance cassette replaced the coding sequence of exon 1 B and 420 bp of the downstream intron. Following construction the arms of the phage-targeting vector were removed via NotI digestion. The targeting vector was introduced into 5 × 106 to 7 × 106 R1 embryonic stem (ES) cells (23) by electroporation at 240 V and 500 μF (Bio-Rad Gene Pulser). Cells were plated in 100-mm-diameter dishes and were cultured for 48 h. Positive and negative selections were carried out by using 300 U of Geneticin (G418)/ml and 2 × 10−7 M 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil, respectively. Surviving clones were screened for morphology, and selected colonies were cloned in days 8, 9, and 10 of the selection. ES cell clones containing a modified ERG1 B allele were identified by Southern blot analysis with a 3′ probe external to the ERG1 B genomic DNA present in the vector. Potential targets were rescreened by Southern blot analysis. The karyotype of targeted ES cell clones was determined to verify their diploid status by using a previously described method (11). Three targeted ES cell clones demonstrating diploid karyotypes were injected into 3.5-day-postcoitum (dpc) C57BL/6 blastocysts, which were next transferred into 2.5-dpc CD1 foster mothers. One male chimera with 60% agouti pigmentation was bred to C57BL/6 females, and agouti pups were genotyped to confirm germ line transmission of the targeted ERG1 B allele. The genotypes from the F1 and F2 generation were determined by Southern blotting, while all subsequent matings were determined by PCR on DNA from tail biopsy specimens. For PCR analysis, oligonucleotides for neo cDNA (neo f, 5′-ATCTCCTGTCATCTCACCTTGC-3′; neo r, 5′-GCCAACGCTATGTCCTGATAGC-3′) and the ERG1 gene (erg1b f, 5′-ATGGCGATTCCAGCCGGGAA-3′; erg1b r 5′-TACCGAAGAACTCCCAACCT-3′) were used. PCR conditions were as follows: denaturation at 95°C for 1 min followed by 30 cycles of 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. ERG1 B heterozygotes were intercrossed to produce ERG1 B null mice. Analysis thus far has been carried out on the hybrid C57BL/6-129-SV/J background. All animals were housed in the animal care unit of the Department of Medicine, University of Calgary, Alberta, Canada, according to animal care guidelines.
FIG. 1.
Knockout strategy. (A) Homologous recombination in ES cells was used to replace the coding region of exon b and a portion of the downstream intron with a loxP-neo-loxP cassette. MERG1, mouse ERG1. (B) The results of Southern analysis on mouse tail DNA from the F2 generation probed with a 32P-labeled fragment of the mouse ERG1 gene DNA. The wild type gives a band of >9,000 bp, and the knockout gives a band of 5,000 bp. This Southern analysis identifies −/− mice and is confirmed by using PCR with oligonucleotide primers designed to hybridize to the deleted region of the ERG1 gene. (C) RNA analysis of ventricles obtained from wild-type (+/+) and ERG1 B knockout (−/−) mice. Oligonucleotides were designed to recognize the mouse ERG1 A or ERG1 B or cytoplasmic β-actin (Cyt β-Act) transcripts (12). Reverse transcription-PCR demonstrated that the ERG1 A mRNA transcript was intact, whereas the ERG1 B transcript was abolished. nr, no RNA.
Whole-cell voltage clamp electrophysiology.
Single ventricular myocytes from day-18 fetal mice and 6-week-old adult mice were enzymatically isolated by using methods described previously (39, 41). K+ currents were recorded with conventional whole-cell voltage clamp techniques by using an Axopatch 200B amplifier and pCLAMP 8 software (Axon Instruments). The bath solution contained 140 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES, 5.5 mM glucose, and 0.2 μM nisodipine (pH 7.4 with NaOH). The pipette solution contained 110 mM K-aspartate, 10 mM KCl, 5 mM MgCl2, 5 mM ATP-Na2, 10 mM EGTA, 10 mM HEPES, and 1 mM CaCl2 (pH 7.2 with KOH). A −10-mV liquid junction potential was corrected. The holding potential was −50 mV. The delayed rectifying K+ current (IK) was activated by depolarization from a holding potential of −50 mV to potentials from −30 to +50 mV in 20-mV increments. The membrane potential was then repolarized to −50 mV to record a slowly decaying outward tail current. All data were recorded at 32°C.
Recording ECGs.
To record electrocardiograms (ECGs), conscious, unsedated adult mice were placed in a custom-designed restraining tube. Multiple perforations were cut in the tube so that the forelimbs and hind limbs would protrude through the perforations. The limbs were coated with electrocardiographic gel, and electrodes were placed on the surface of the skin. To minimize the stress associated with being restrained, mice were allowed to accustom themselves to the tube for 5 min; ECG measurements were then obtained for 1 min. All ECGs were calibrated with a 1-mV test pulse. ECGs were sampled at 10,000 Hz with an anti-aliasing filter (5,000 Hz), and data was recorded from direct current to 5,000 Hz in unsedated conscious mice (41). Mouse ECGs were analyzed by using custom software (Bio-Di-Electrics Inc). A second-order continuous wavelet transformation was used to locate the R wave. R waves were aligned. To avoid contamination of the signal average process by rare premature beats, beats with RR intervals that would impinge on the QT interval were excluded from the signal averaging. The QT interval was measured as the time when repolarization returns to the isoelectric point. The isoelectric point was defined as the position of the PR segment (41). Similarly, Mitchell et al. (20) assigned the end of the T wave as the isoelectric point, which follows a terminal negative T-wave vector in the surface ECGs.
To delineate the bradycardic episodes within the 1-min RR interval sequence, we examined the RR interval histograms and found all distributions to be bimodal. The start of each bradycardic episode occurred when the RR interval sequence exceeded the high mode of the distribution; this bradycardic episode was considered terminated when the RR interval sequence dropped below the low mode.
RESULTS
Knockout.
Engineering of the mouse ERG1 B-specific knockout is illustrated in Fig. 1A. Three appropriately recombined cell lines were identified (Fig. 1B) from which one male chimeric mouse was obtained. It was bred with C57BL/6 mice to yield the ERG1 B−/− mice used in this study. The ERG1 B+/+ mice were bred from the same heterozygotes. The ERG1 B−/− mice were viable and fertile and did not show any gross physical or behavioral abnormalities. Reverse transcription-PCR (Fig. 1C) demonstrated that the ERG1 A mRNA transcript was intact, despite the presence of a NEO cassette in place of the coding region of exon 1B. ERG1 B mRNA expression was abolished.
Effect of the ERG1 B knockout on IK in fetal ventricular myocytes.
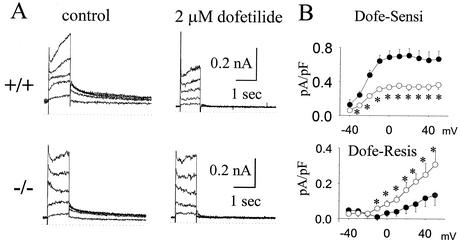
IK was recorded for day-18 fetal mouse ventricular myocytes (Fig. 2A). Most of the tail current was blocked by 2 μM dofetilide, suggesting that IKr dominated this current, which is the ERG1-encoded current. Figure 2B shows the current-voltage relationship of dofetilide-sensitive and dofetilide-resistant tail currents. The dofetilide-sensitive tail current was activated at voltages positive to −30 mV and reached a plateau at around 0 to 10 mV (n = 9; Fig. 2B), in keeping with the properties of IKr and the ERG1-related currents (39). In ERG1 B−/− mice the peak dofetilide-sensitive tail current (Fig. 2B) was significantly smaller than that in wild-type mice at all relevant potentials (n = 8; Fig. 2A, bottom). In contrast, the dofetilide-resistant current of ERG1 B−/− mice was significantly increased compared to that of wild-type mice (Fig. 2B, bottom). The dofetilide-resistant current is outwardly rectified, in keeping with the features of IKs (39).
FIG. 2.
Impact of ERG1 B knockout on IK recorded for fetal mice. (A) Representative families of current traces of IK from fetal-day-18 ventricular myocytes from +/+ and ERG1 B−/− mice recorded before (left) and after (right) administration of 2 μM dofetilide. The Ic was elicited by depolarization to −30 to +50 mV in steps of 20 mV from a holding potential of −50 mV, and then their tail currents were recorded after returning to a potential of −50 mV. (B) The mean current-voltage relationships of dofetilide-sensitive (top) and -resistant (bottom) currents recorded from +/+ (black circle) and −/− (white circle) mice. Asterisks designate P values of <0.05.
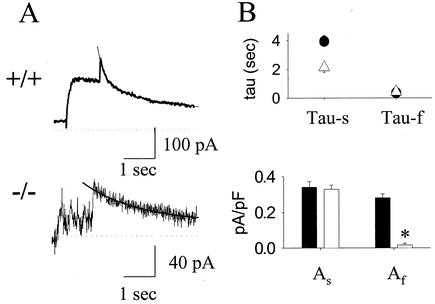
Deactivation time constants (tau) of the dofetilide-sensitive tail currents were further analyzed. For wild-type mice the decay of the tail current could only be fit to a biexponential function with two time constants of 3,933 ± 404 and 350 ± 19 ms (Fig. 3A and B, top, n = 24). In contrast, in ERG1 B−/− mice deactivation could be fit to a biexponential function in only 2 out of 11 mice (tau = 2,142 ± 334 ms and 427 ± 206 ms; Fig. 3A and B, top), and 9 out of 11 mice did not manifest the fast component. The rare observation of a rapid component in the −/− mice might be explained by the expression of mouse ERG1 A′ (15). Figure 3B (bottom) shows that the current densities (current [picoamps] normalized to capacitance [picofarads]) of the slow component were similar between +/+ and −/− mice (0.34 ± 0.03 versus 0.32 ± 0.02 pA/pF), whereas the mean density of the fast component in −/− mice was reduced by >90% (0.28 ± 0.02 pA/pF in +/+ mice versus 0.02 ± 0.01 pA/pF in −/− mice; P < 0.001).
FIG. 3.
Effect of ERG1 B knockout on deactivation kinetics of dofetilide-sensitive currents in day-18 fetal +/+ and −/− mice. (A) Representative dofetilide-sensitive tail current in +/+ (top) and −/− (bottom) mice. Solid smooth lines show the curves fitted to the biexponential (top) or monoexponential (bottom) function in +/+ and −/− mice, respectively. (B) The mean time constants (tau-f and tau-s, top panel) and magnitudes (As and Af, bottom panel) of both fast (f) and slow (s) components of the dofetilide-sensitive current. The wild type is shown in black and the −/− mutant is shown in white. Asterisks designate P values of <0.001.
Impact of the ERG1 B knockout on IK in adult ventricular myocytes.
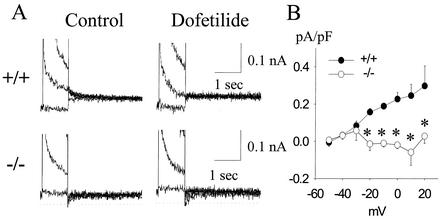
In adult +/+ mice, small but reproducible dofetilide-sensitive IK tail currents were recorded (Fig. 4A, top); however, this current was absent in −/− mice (Fig. 4A, bottom). In +/+ mice, the mean deactivation tau was well fit to a biexponential equation with mean taus of 2,039 ± 268 and 163 ± 43 ms at −50 mV (n = 7). No dofetilide-resistant tail currents were observed in either +/+ or −/− mice. Figure 4B shows the mean current-voltage relationship of the dofetilide-sensitive tail current in +/+ (n = 12) and −/− mice (n = 13). The data indicate that dofetilide-sensitive current (IKr) is virtually abolished in −/− mice.
FIG. 4.
Effect of ERG1 B knockout on IK recorded from adult cardiac myocytes. Representative IK current traces and current-voltage relationships in cardiac myocytes of adult mice. (A) Representative family of traces for +/+ (top panels) and −/− (bottom panels) mice before (left) and after (right) 2 mM dofetilide. (B) Mean current-voltage relationships of dofetilide-sensitive currents in +/+ (black circle) and −/− (white circle) mice. Asterisks designate P values of <0.05.
Effect of the ERG1 B knockout on in vivo electrocardiography.
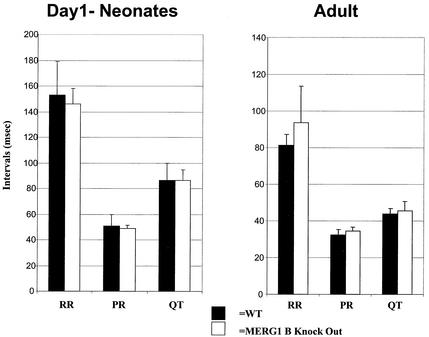
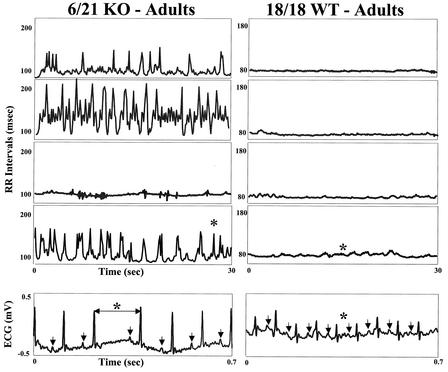
Figure 5 shows mean ECG interval data comparing +/+ and −/− mice. The mean sinus cycle length, PR, and QT intervals are similar for +/+ and −/− neonatal or adult mice. However, spontaneous and abrupt bradycardias were observed in 6 of 21 −/− mice (Fig. 6). The asterisks in Fig. 6 show an example of the ECG sequence manifesting an abrupt change in sinus cycle length. The arrows show the P waves (the surface manifestation of atrial depolarization) for each beat. Note that there is no change in PR interval and that there are no episodes of atrioventricular block (that is, each P wave is followed by a QRS [the surface manifestation of ventricular activation]). In the animals that showed episodic bradycardias, the median number of episodes/minute was 28. These bradycardias were never observed in +/+ littermates, and ECGs were sampled for exactly the same intervals and at the same time of day, making a sampling artifact unlikely.
FIG. 5.
Effect of the ERG1 B knockout on in vivo electrocardiography. The mean electrocardiographic intervals in +/+ and −/− mice are shown for day 1 neonates and adults. WT, wild type.
FIG. 6.
Spontaneous and abrupt bradycardias. Shown are observations for −/− mice on the left and for +/+ littermates on the right from representative individual animals. The −/− mice occasionally exhibit abrupt bradycardias (6 out of 21), while the wild-type (WT) mice do not. Sinus RR intervals are on the vertical axis, and time is on the horizontal axis. The asterisks show a sample bradycardia which is expanded to show the ECGs associated with that event. An abrupt change in sinus cycle length with little or no change in PR interval is shown. KO, knockout.
DISCUSSION
Models of human long QT2 syndrome in mice.
The long QT2 syndrome results from mutations in the human ERG1 potassium channels that manifest as prolongation of ventricular repolarization and predispose to torsade de pointes ventricular tachycardia and sudden cardiac death (1, 38). Even within families there is substantial variability in the QT phenotype of individuals that carry the mutation (26, 29). While mutations in human ERG1 can present as sudden infant deaths, most deaths occur in young adults, indicating that the relative physiological importance of the ERG1 potassium channel is modulated by the redundancy of other potassium channels and other genetic or environmental factors (29). The role of human ERG1 is not limited to the ventricle. Episodic sinus bradycardia is part of the long QT2 syndrome phenotype in some families with ERG1 mutations, and abrupt bradycardias can trigger arrhythmic events (30, 44). In review, there are two determinants for the development of arrhythmias associated with long QT2 syndrome: prolongation of repolarization and the bradycardias which can trigger torsade de pointes.
In mice, the ERG1-encoded IKr current is the dominant repolarizing current at fetal day 18. Treatment with dofetilide, a specific IKr blocker, results in early after-depolarizations and a failure to repolarize (41). Moreover, in vivo treatment of fetal mice with IKr blockers produced concentration-dependent embryonic bradycardia and arrhythmia resulting in circulatory failure, which explains the associated embryonic death of mice (6, 31). In addition, simultaneous knockout of all of the isoforms of ERG1 results in intrauterine deaths and failure of the heart or the brain to develop normally (14). Thus, ERG1 appears to play an important role in normal growth and development of heart and brain.
In the present study we have found that deletion of ERG1 B in mice does not lengthen the QT interval in either neonatal or adult mice and therefore does not provide a good model for this aspect of the human long QT2 syndrome. Since deleting ERG1 B removes the fast component of IKr, one might have expected a lengthened QT interval. However, in fetal ERG1 B−/− murine ventricle, the slow component of IKr is intact and IKs is upregulated, possibly compensating for the loss of the fast component of IKr. We are presently investigating this possibility by breeding ERG1 B−/− mice with MinK−/− mice which lack IKs.
In contrast to its role in fetal mice, ERG1 appears to play only a minor role in ventricular repolarization in adult mice. By using a transgenic strategy, Babij et al. (2) overexpressed a dominant-negative human long QT2 mutation, G628S, in an ERG1 A transcript. This transgenic adult mouse model did not manifest prolongation of the QT interval or prolongation of the action potential duration, despite reducing IKr expression. It is not surprising that even though IKr was eliminated in adult ERG1 B−/− ventricular myocytes, no prolongation of the QT interval was observed in the present study. This is also in keeping with previous studies that indicate that components of the transient outward current (Ito) are the dominant repolarizing current in adult mice (39, 40). While ERG1 plays only a minor role in ventricular repolarization in normal mouse ventricle, this current may play an important role when disease downregulates the dominant Ito repolarizing currents. For example, overexpression of calcineurin in mice leads to sudden cardiac death relating to hypertrophy, spontaneous abrupt bradycardia, and pleomorphic ventricular tachycardia (8, 21). Treatment of these mice with the selective IKr channel blocker, dofetilide, precipitates bradycardia associated with the development of an arrhythmia similar to torsade de pointes ventricular tachycardia (8). In review, ERG1 plays an important role in ventricular repolarization during normal development and in disease but appears to have only a minor role in normal adult mouse ventricle.
Role of ERG1 in sinus node and bradycardias.
The major phenotype observed in the ERG1 B−/− mice is the development of abrupt and episodic bradycardias. Episodic bradycardias occur in some families with long QT2 syndrome and can trigger spontaneous arrhythmias (30, 44). These episodes of bradycardia may relate to a role for the ERG1 potassium channel in sinus node and/or in neural tissue regulating sinus node function. Previous studies provide evidence that ERG1 is extensively expressed in the sinus node of most species (3, 33), but unfortunately this has not been explored with mice. However, pharmacological blockade of IKr slows the sinus heart rate in a number of species (9), including mice (41). Previous studies have also confirmed that ERG1 is extensively expressed in peripheral sympathetic ganglia (43). Data from the present study cannot discern whether the abrupt bradycardias observed in ERG1 B−/− mice relate to abnormalities in expression of ERG1 B in the sinus node or autonomic ganglia. In review, the phenotype of the ERG1 B−/− mice recapitulates the abrupt bradycardia phenotype seen in some families with long QT2 syndrome but does not result in prolonged repolarization.
ERG1 B is a biological determinant of ventricular IKr expression.
The most distinctive feature of the ERG1 potassium channel is its ultrarapid C-type inactivation kinetics (28, 32). During repolarization, recovery from inactivation occurs at a rate faster than that of deactivation, leading to a large outward tail current that is thought to be antiarrhythmic by opposing early after-depolarizations (32). Since the rate of deactivation affects the size and duration of the tail current, isoform differences may play a role in arrhythmia prevention. When expressed heterologously, both ERG1 A and ERG1 B deactivate with fast and slow components (12, 15). The fast component of ERG1 B deactivates 10-fold faster than that of ERG1 A. In fetal myocytes, we were able to distinguish two components of deactivation, the more rapid of which was absent in ERG1 B−/− myocytes. For +/+ adults we found rapid and slow deactivating components, both of which were abolished in −/− mice. This study demonstrates that ERG1 B is necessary for plasma membrane expression of IKr in ventricular cardiac myocytes of the adult mouse. These conclusions contrast with those of another study, which demonstrated that ERG1 B was not detectable by Western blotting in adult mouse ventricle despite the use of a variety of antibody preparations (25). A possible explanation of this discrepancy is the low abundance of ERG1 B, which contributes to the difficulty in its detection by Western blotting. This is consistent with the low current density of IKr in adult ventricle (0.2 pA/pF). This is approximately 100-fold less than Ito expression in adult ventricular myocytes. Importantly, the previous study demonstrated that the ERG1 antibodies to the common region stained both the surface and internal structures of cardiac myocytes while the ERG1 A-specific antibody stained only internally. This observation is consistent with our finding that ERG1 B is necessary for IKr expression in the surface membranes of adult cardiac myocytes.
Is it possible that ERG1 B also plays an important role in the human heart? The primary evidence for this possibility relates to a human long QT mutation, an exon 4 duplication that frameshifts ERG1 A at amino acid 200 (200fs), but it is not predicted to have any direct effect on ERG1 B expression (10). This is the sole recessive mutation that has been identified in long QT2 patients. The parents of the affected offspring, missing only one genomic copy of ERG1 A, have borderline QT intervals and T-wave abnormalities, but neither they nor anyone else in a large extended family has ever presented with symptoms associated with long QT2 syndrome. The first affected child of this consanguineous marriage died at 36 weeks in utero, and the second was rescued only with intervention at birth. The surviving child at 1.5 years was paced but was otherwise healthy. These findings are consistent with our results with mice, where ERG1 A plays an important physiological role in the late fetal stage but plays little or no role in the adult mouse.
Caution is required in interpreting these results. Many point mutations and frameshifts in human ERG1 directly affect only ERG1 A and are dominant (4, 34). Therefore, unless there is something unique about 200fs, its lack of dominance may simply reflect low penetrance, which has been demonstrated for several human ERG1 mutations (26). A possibly unique feature of the 200fs could involve an ability to preserve PAS domain function. It has been demonstrated that a 135-amino-acid PAS domain fragment can restore slow deactivation to NH2-terminal-truncated ERG1 A when the mRNAs were coinjected into Xenopus oocytes (22). When one considers the greater levels of ERG1 A mRNA relative to that of ERG1 B in the human heart (13), it would not be surprising if the 200fs PAS domain were to interact with ERG1 B. This interaction might assist the passage of ERG1 B to the plasma membrane and/or slow deactivation of ERG1 B. In either case, outward current would be increased during repolarization, resulting in shortening of action potential duration and potentially preventing early after-depolarizations. COOH-terminally truncated fragments of ERG1 A are present in heart preparations despite all efforts to block protease activity (25). It is possible that these PAS domain-containing fragments interact with ERG1 B in normal cardiac cells. Another reason to think that ERG1 B plays an important role in the human heart is its relatively low mRNA abundance. Several studies have suggested that the dominant nature of many ERG1 mutations results from a gene-dosage effect (27). Since IKr is a relatively rare surface protein and ERG1 A is a moderately abundant mRNA species, it is hard to imagine how reducing its mRNA level would significantly lower protein levels. Alternatively, reducing the amount of already-rare ERG1 B mRNA is expected to have direct effects on the quantity of surface protein.
In conclusion, selective deletion of ERG1 B eliminates the rapidly deactivating component of IKr in fetal myocytes and eliminates all IKr in adult ventricular myocytes. The reduction in repolarizing current does not result in a long QT phenotype but does predispose the mouse heart to episodic abrupt sinus bradycardias.
REFERENCES
- 1.Ackerman, M. J. 1998. The long QT syndrome: ion channel diseases of the heart. Mayo Clin. Proc. 73:250-269. [DOI] [PubMed] [Google Scholar]
- 2.Babij, P., G. R. Askew, B. Nieuwenhuijsen, C. M. Su, T. R. Bridal, B. Jow, T. M. Argentieri, J. Kulik, L. J. DeGennaro, W. Spinelli, and T. J. Colatsky. 1998. Inhibition of cardiac delayed rectifier K+ current by overexpression of the long QT syndrome HERG G628S mutation in transgenic mice. Circ. Res. 83:668-678. [DOI] [PubMed] [Google Scholar]
- 3.Brahmajothi, M. V., M. J. Morales, K. A. Reimer, and H. C. Strauss. 1997. Regional localization of ERG, the channel protein responsible for the rapid component of the delayed rectifier, K+ current in the ferret heart. Circ. Res. 81:128-135. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J., A. Zou, I. Splawski, M. T. Keating, and M. C. Sanguinetti. 1999. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J. Biol. Chem. 274:10113-10118. [DOI] [PubMed] [Google Scholar]
- 5.Curran, M. E., I. Splawski, K. W. Timothy, G. M. Vincent, E. D. Green, and M. T. Keating. 1995. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80:795-803. [DOI] [PubMed] [Google Scholar]
- 6.Danielsson, B. R., A. C. Skold, and F. Azarbayjani. 2001. Class III antiarrhythmics and phenytoin: teratogenicity due to embryonic cardiac dysrhythmia and reoxygenation damage. Curr. Pharm. Des. 7:787-802. [DOI] [PubMed] [Google Scholar]
- 7.Deng, C., and M. R. Capecchi. 1992. Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol. Cell. Biol. 12:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, D., Y. Duan, D. Roach, S. L. Swirp, L. Wang, J. P. Lees-Miller, R. S. Sheldon, J. D. Molkentin, and H. J. Duff. Overexpression of calcineurin in mouse causes sudden cardiac death associated with decreased density of K+ channels. Cardiovasc. Res., in press. [DOI] [PubMed]
- 9.Furukawa, Y., Y. Miyashita, K. Nakajima, M. Hirose, F. Kurogouchi, and S. Chiba. 1999. Effects of verapamil, zatebradine, and E-4031 on the pacemaker location and rate in response to sympathetic stimulation in dog hearts. J. Pharmacol. Exp. Ther. 289:1334-1342. [PubMed] [Google Scholar]
- 10.Hoorntje, T., M. Alders, P. van Tintelen, K. van der Lip, N. Sreeram, A. van der Wal, M. Mannens, and A. Wilde. 1999. Homozygous premature truncation of the HERG protein: the human HERG knockout. Circulation 100:1264-1267. [DOI] [PubMed] [Google Scholar]
- 11.Kato, H., and K. Moriwaki. 1972. Factors involved in the production of banded structures in mammalian chromosomes. Chromosoma 38:105-120. [DOI] [PubMed] [Google Scholar]
- 12.Lees-Miller, J. P., C. Kondo, L. Wang, and H. J. Duff. 1997. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ. Res. 81:719-726. [DOI] [PubMed] [Google Scholar]
- 13.London, B., E. Aydar, C. M. Lewarchik, J. S. Seibel, C. T. January, and G. A. Robertson. 1998. N- and C-terminal isoforms of HERG in the human heart. Biophys. J. 74:A26. [Google Scholar]
- 14.London, B., and X. Pan. 1998. QT interval prolongation and arrhythmias in heterozygous MERG1-targeted mice. Circulation 98:279. [Google Scholar]
- 15.London, B., M. C. Trudeau, K. P. Newton, A. K. Beyer, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, C. A. Satler, and G. A. Robertson. 1997. Two isoforms of the mouse ether-a-go-go-related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ. Res. 81:870-878. [DOI] [PubMed] [Google Scholar]
- 16.Manley, N. R., J. R. Barrow, T. Zhang, and M. R. Capecchi. 2001. Hoxb2 and Hoxb4 act together to specify ventral body wall formation. Dev. Biol. 237:130-144. [DOI] [PubMed] [Google Scholar]
- 17.Marban, E. 1999. Heart failure: the electrophysiologic connection. J. Cardiovasc. Electrophysiol. 10:1425-1428. [DOI] [PubMed] [Google Scholar]
- 18.Marban, E. 2002. Cardiac channelopathies. Nature 415:213-218. [DOI] [PubMed] [Google Scholar]
- 19.McLenachan, J. M., E. Henderson, K. I. Morris, and H. J. Dargie. 1987. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N. Engl. J. Med. 317:787-792. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell, G. F., A. Jeron, and G. Koren. 1998. Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. 274:H747-H751. [DOI] [PubMed] [Google Scholar]
- 21.Molkenton, J. D., J. R. Lu, C. L. Antos, B. Markham, J. Richardson, J. Robbins, S. R. Grant, and E. N. Olson. 1998. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morais Cabral, J. H., A. Lee, S. L. Cohen, B. T. Chait, M. Li, and R. Mackinnon. 1998. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell 95:649-655. [DOI] [PubMed] [Google Scholar]
- 23.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nerbonne, J. M., C. G. Nichols, T. L. Schwarz, and D. Escande. 2001. Genetic manipulation of cardiac K(+) channel function in mice: what have we learned, and where do we go from here? Circ. Res. 89:944-956. [DOI] [PubMed] [Google Scholar]
- 25.Pond, A. L., B. K. Scheve, A. T. Benedict, K. Petrecca, D. R. Van Wagoner, A. Shrier, and J. M. Nerbonne. 2000. Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional I(Kr) channels. J. Biol. Chem. 275:5997-6006. [DOI] [PubMed] [Google Scholar]
- 26.Priori, S. G., C. Napolitano, and P. J. Schwartz. 1999. Low penetrance in the long QT syndrome: clinical impact. Circulation 99:529-533. [DOI] [PubMed] [Google Scholar]
- 27.Sanguinetti, M. C., M. E. Curran, P. S. Spector, and M. T. Keating. 1996. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc. Natl. Acad. Sci. USA 93:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanguinetti, M. C., C. Jiang, M. E. Curran, and M. T. Keating. 1995. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81:299-307. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz, P. J., S. G. Priori, C. Spazzolini, A. J. Moss, G. M. Vincent, C. Napolitano, I. Denjoy, P. Guicheney, G. Breithardt, M. T. Keating, J. A. Towbin, A. H. Beggs, P. Brink, A. A. Wilde, L. Toivonen, W. Zareba, J. L. Robinson, K. W. Timothy, V. Corfield, D. Wattanasirichaigoon, C. Corbett, W. Haverkamp, E. Schulze-Bahr, M. H. Lehmann, K. Schwartz, P. Coumel, and R. Bloise. 2001. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103:89-95. [DOI] [PubMed] [Google Scholar]
- 30.Shen, C. T., Y. C. Wu, S. S. Yu, and N. K. Wang. 1997. Multi-undulant T-U-wave, sinus bradycardia and long QT syndrome: a possible phenotype of mutant genes controlling the inward potassium rectifiers. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 38:267-275. [PubMed] [Google Scholar]
- 31.Skold, A. C., and B. R. Danielsson. 2000. Developmental toxicity of the class III antiarrhythmic agent almokalant in mice. Adverse effects mediated via induction of embryonic heart rhythm abnormalities. Arzneimittelforschung 50:520-525. [DOI] [PubMed] [Google Scholar]
- 32.Smith, P. L., T. Baukrowitz, and G. Yellen. 1996. The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379:833-836. [DOI] [PubMed] [Google Scholar]
- 33.Song, D. K., Y. E. Earm, and W. K. Ho. 1999. Blockade of the delayed rectifier K+ currents, IKr, in rabbit sinoatrial node cells by external divalent cations. Pflugers Arch. 438:147-153. [DOI] [PubMed] [Google Scholar]
- 34.Splawski, I., J. Shen, K. W. Timothy, M. H. Lehmann, S. Priori, J. L. Robinson, A. J. Moss, P. J. Schwartz, J. A. Towbin, G. M. Vincent, and M. T. Keating. 2000. Protein spectrum of mutations in long QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102:1178-1185. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson, W. G., L. W. Stevenson, H. R. Middlekauff, and L. A. Saxon. 1993. Sudden death prevention in patients with advanced ventricular dysfunction. Circulation 88:2953-2961. [DOI] [PubMed] [Google Scholar]
- 36.Tsuzuki, T., and D. E. Rancourt. 1998. ES cell gene targeting using phage vectors generated by plasmid-phage recombination. Nucleic Acids Res. 26:988-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandenberg, J. I., B. D. Walker, and T. J. Campbell. 2001. HERG K+ channels: friend and foe. Trends Pharmacol. Sci. 22:240-246. [DOI] [PubMed] [Google Scholar]
- 38.Vincent, G. M. 1998. The molecular genetics of the long QT syndrome: genes causing fainting and sudden death. Annu. Rev. Med. 49:263-274. [DOI] [PubMed] [Google Scholar]
- 39.Wang, L., and H. J. Duff. 1997. Developmental changes in transient outward current in mouse ventricle. Circ. Res. 81:120-127. [DOI] [PubMed] [Google Scholar]
- 40.Wang, L., Z. P. Feng, C. S. Kondo, R. S. Sheldon, and H. J. Duff. 1996. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circ. Res. 79:79-85. [DOI] [PubMed] [Google Scholar]
- 41.Wang, L., S. Swirp, and H. J. Duff. 2000. Age-dependent response of the electrocardiogram to K(+) channel blockers in mice. Am. J. Physiol. 278:C73-C80. [DOI] [PubMed] [Google Scholar]
- 42.Wang, Z., B. Fermini, and S. Nattel. 1994. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc. Res. 28:1540-1546. [DOI] [PubMed] [Google Scholar]
- 43.Wymore, R. S., G. A. Gintant, R. T. Wymore, J. E. Dixon, D. McKinnon, and I. S. Cohen. 1997. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ. Res. 80:261-268. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida, H., M. Horie, H. Otani, T. Kawashima, Y. Onishi, and S. Sasayama. 2001. Bradycardia-induced long QT syndrome caused by a de novo missense mutation in the S2-S3 inner loop of HERG. Am. J. Med. Genet. 98:348-352. [DOI] [PubMed] [Google Scholar]
- 45.Zhou, Z., Q. Gong, B. Ye, Z. Fan, J. C. Makielski, G. A. Robertson, and C. T. January. 1998. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys. J. 74:230-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, Z., Q. Gong, and C. T. January. 1999. Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J. Biol. Chem. 274:31123-31126. [DOI] [PubMed] [Google Scholar]