Abstract
Axenfeld-Rieger syndrome is an autosomal-dominant disorder caused by mutations in the PITX2 homeodomain protein. We have studied the mechanism underlying the dominant negative K88E mutation, which occurs at position 50 of the homeodomain. By using yeast two-hybrid and in vitro pulldown assays, we have documented that PITX2a can form homodimers in the absence of DNA. Moreover, the K88E mutant had even stronger dimerization ability, primarily due to interactions involving the C-terminal region. Dimerization allowed cooperative binding of wild-type (WT) PITX2a to DNA containing tandem bicoid sites in a head-to-tail orientation (Hill coefficient, 1.73). In contrast, the WT-K88E heterodimer bound the tandem sites with greatly reduced cooperativity and decreased transactivation activity. To further explore the role of position 50 in PITX2a dimerization, we introduced a charge-conservative mutation of lysine to arginine (K88R). The K88R protein had greatly reduced binding to a TAATCC element and did not specifically bind any other TAATNN motif. Like K88E, K88R formed relatively stronger dimers with WT. As predicted by our model, the K88R protein acted in a dominant negative manner to suppress WT PITX2a activity. These results suggest that the position 50 residue in the PITX2 homeodomain plays an important role in both DNA binding and dimerization activities.
The PITX2 gene was cloned based on its linkage in Axenfeld-Rieger syndrome (ARS) (30). ARS is an autosomal-dominant human disorder characterized by ocular anterior chamber anomalies causing glaucoma in more than 50% of affected individuals as well as dental hypoplasia, mild craniofacial dysmorphism, and umbilical stump abnormalities. Other features associated with this syndrome include abnormal cardiac, limb, and pituitary development (28, 30). Three isoforms of PITX2 (designated a, b, and c) that differ only in their amino termini have been identified (9, 15). PITX2a and the related PITX1 protein have been shown to interact with Pit-1, a transcription factor that regulates pituitary cell differentiation, including the expression of the thyroid-stimulating hormone, growth hormone, and prolactin genes (4, 16, 31). We have used the prolactin promoter as a model target for studying PITX2a activities. Transient transfection assays with the prolactin promoter, which contains both Pit-1 and PITX2a binding sites, have shown a strong synergistic effect of PITX2a and Pit-1 on transactivation (4). Consistent with these data, knockout mice lacking Pitx2 show arrested pituitary gland development (10).
PITX2a is a 33-kDa homeodomain protein that is a functional member of the bicoid homeodomain subfamily, as defined by a lysine at residue 50 of the homeodomain (4). It has been demonstrated that this residue in the bicoid protein interacts with base pairs 5 and 6 of the hexanucleotide consensus 5′-TAATCC-3′ (12, 32). PITX2a has been shown to specifically bind to this consensus sequence and transactivate corresponding reporter genes (4). We have previously reported an ARS mutation that changes the lysine (K) at position 50 to a glutamic acid (E) (29). The mutation is referred to as K88E (position 88 is the location of K50 in PITX2a). The K88E protein is defective in DNA binding, transactivation, and Pit-1 synergism. Unexpectedly, K88E was also able to suppress the synergism of wild-type (WT) PITX2a and Pit-1 (29). Based on these results, we proposed a model in which the dominant negative ability of the mutant protein was due to dimerization with WT PITX2a. Yet the inability to bind DNA alone did not seem sufficient to account for the dominant negative activity, since a different ARS mutant (PITX2a-T68P) also had reduced DNA binding yet was not a dominant-negative mutant. This suggested that the inhibitory property was a specific consequence of the position 50 mutation.
In this study, we have tested the hypothesis that the dominant-negative property of K88E is due to the formation of nonfunctional heterodimers with WT PITX2a. We first document the ability of WT PITX2a and K88E to form dimers. We then characterized the effect of WT and K88E dimerization on DNA binding and transactivation activity. Based on the stronger interactions seen with the K88E mutant, we tested the contribution of the position 50 amino acid by making a charge-conservative mutation from lysine to arginine (K88R). Analogous to the K88E mutant, K88R could not bind DNA, formed strong dimers with WT PITX2a, and was able to suppress WT PITX2a activity in a dominant-negative manner. The results support our prediction that position 50 of the homeodomain is key for both PITX2 DNA binding and dimerization activities.
MATERIALS AND METHODS
Yeast two-hybrid analysis.
The bait and prey cDNAs were cloned by standard PCR techniques into the GAL4 DNA binding domain (DBD) fusion vector (pGBKT7) (Clontech) and the GAL4 activation domain (AD) fusion vector (pGADT7) (Clontech), respectively. pGBKT7 and pGADT7 vectors allow growth under tryptophan (−Trp) and leucine (−Leu) auxotrophic selection, respectively. For selection, yeast (Saccharomyces cerevisiae) was grown in SD minimal medium with the necessary drop-out supplements (Clontech) for tryptophan, leucine, histidine, and/or adenine auxotrophic selection. The MATa reporter strain AH109 was used as a mating partner for the MATα Y187 strain. The AH109 strain contains the ADE2, HIS3, and lacZ markers, which are under the control of different upstream GAL4 activating sequences and TATA boxes. Mating was carried out in accordance with the protocol provided with the Clontech MatchMaker3 yeast two-hybrid system between Y187 cells carrying the prey vector and AH109 cells expressing the bait vector. Yeast containing the bait and prey constructs was grown on the selection plates. For the matings, one colony of each was picked and placed in 0.5 ml of 2× YPDA medium (Clontech) without auxotrophic selection. The cultures were grown overnight at 30°C. One hundred microliters of 1:10 dilutions of mated cultures were spread on SD−Leu/−Trp plates. The plates were then incubated at 30°C for 3 to 5 days. The resulting mated yeast was replica plated onto SD−Leu/−Trp/−His, and/or SD−Leu/−Trp/−His/−Ade plates with various concentrations (0, 2.5, 5, and 10 mM) of 3-amino triazole (3-AT) to test for interaction. Expression of the bait fusion proteins was determined by Western blotting, as described below, by using the Gal4 DBD antibody (Santa Cruz).
β-Galactosidase activity assay.
Individual colonies were taken from the mated yeast and grown in 10 ml of −Leu/−Trp medium to an optical density at 600 nm of 0.4 to 0.6. The yeast were collected by centrifugation and resuspended in 1 ml of buffer Z (Clontech). Resuspended yeast (100 μl) was lysed by freezing in liquid nitrogen (30 to 60 s) followed by thawing at 37°C (30 to 60 s) four times. The β-galactosidase activity was assayed by measuring chemiluminescence from the lysate (25 μl) in a 96-well plate luminometer (Dynex) with the Galacto-Light Plus kit (Tropix).
Glutathione S-transferase (GST) pulldown assay.
For preparing total lysates, transfections were done by using 10 μl of Lipofectamine 2000 (Invitrogen) in 60-mm dishes with 5 × 105 Chinese hamster ovary (CHO) cells and 5 μg of Myc-PITX2a or the pCMV5 empty vector containing the cytomegalovirus (CMV) promoter as a control. Cells were incubated for 24 to 48 h after transfection, washed with phosphate-buffered saline, and lysed with 1× lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris [pH 7.4], 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, 0.1% NP-40, and 2% protease inhibitor cocktail [Sigma]) for 30 min at 4°C. Following lysis, cells were scraped and centrifuged, and the supernatant was collected. Protein was measured by Bradford assays (Bio-Rad) with a bovine serum albumin standard.
GST fusion proteins (GST-PITX2a, GST-K88E, or GST-K88R) were immobilized on glutathione Sepharose beads (Amersham Pharmacia Biotech) and incubated with various amounts of total lysate from CHO cells that had been transfected with Myc-tagged PITX2a or the CMV5 control plasmid in a 100-μl volume. The lysates and beads were incubated in phosphate-buffered saline with 300 ng of ethidium bromide/μl (if indicated) or 15 pmol of Bcd 2 × 5n double-stranded DNA (if indicated) for 15 min. Following incubation, the beads were centrifuged and washed three times with buffer containing 50 mM Tris [pH 7.0] and 140 mM NaCl. The beads were resuspended in 5× sodium dodecyl sulfate (SDS) sample buffer to a final 1× concentration, boiled for 5 min, and loaded on a 12.5% SDS-polyacrylamide gel for electrophoresis. Proteins were transferred to Immobilon P polyvinylidene difluoride filters (Millipore). Western blotting was done by using a c-Myc antibody (9E10, Santa Cruz Labs) and ECL reagents (Amersham Pharmacia Biotech).
Autoradiographs from Western blots were scanned as TIFF files by using Adobe Photoshop, and band intensities were calculated with NIH Image software. The percentage of bound protein was determined by comparison to the band intensity of an aliquot of the input protein from the same blot.
Electrophoretic mobility shift assay (EMSA).
The PITX2a K88R cDNA was cloned into the SalI and NotI sites of pGex6p-2 (Amersham Pharmacia Biotech). The K88R point mutation was created by PCR-based site-directed mutagenesis (13) by using Pfu DNA polymerase (Stratagene). WT and K88E fusion proteins have been described previously (29). Protein preparations were done in a manner similar to that described previously (4). The GST moiety was removed by PreScission protease (Amersham Pharmacia Biotech). Proteins were quantitated by Bradford assays and visualized on SDS-polyacrylamide gels by either Coomassie or silver staining.
The 32P-labeled probe for EMSAs was generated by annealing complementary oligonucleotides containing the sequences listed in Fig. 4A and end filled by using Klenow polymerase with [32P]dATP as described previously (33). Standard binding assays were carried out by incubating the oligonucleotide probe (1.0 or 0.2 pmol, as indicated) in a 20-μl reaction containing binding buffer (20 mM HEPES [pH 7.5], 5% glycerol, 50 mM NaCl, and 1 mM dithiothreitol), 0.1 μg of poly(dI.dC), and 100 to 300 ng of protein on ice for 15 min. The samples were electrophoresed for 2.5 h at 280 V on 8% polyacrylamide gels, as described previously (4).
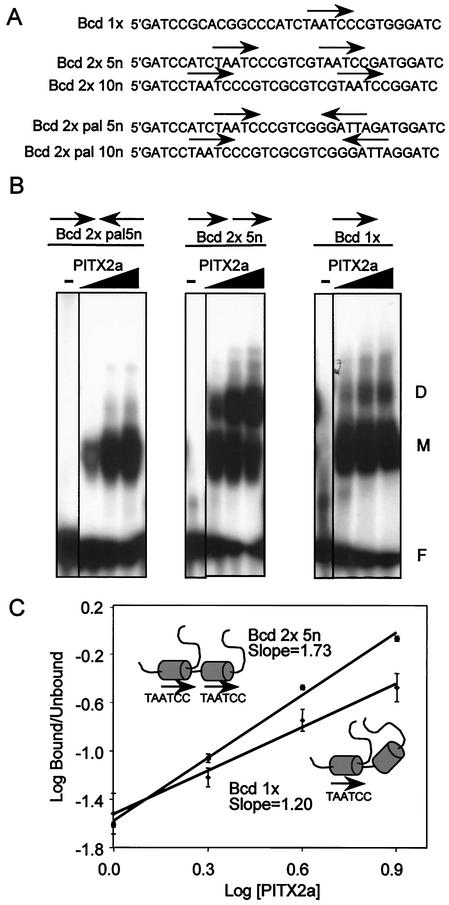
FIG. 4.
Cooperative binding of PITX2a to tandem head-to-tail bicoid sites. (A) Sequences of oligonucleotides containing a single bicoid consensus site (Bcd 1x) or two tandem sites in head-to-tail (Bcd 2x) or head-to-head (Bcd 2x pal) orientation separated by 5 or 10 nucleotides (5n and 10n, respectively). (B) Increasing amounts of bacterially expressed PITX2a (250, 500, and 1000 ng) were incubated with the indicated probes in an EMSA. The free probe, monomer, and dimer complexes are indicated by the letters F, M, and D, respectively. (C) Plot to estimate the Hill coefficient for the single site of Bcd 1x (lower line, diamonds) and the head-to-tail sites of Bcd 2 × 5n (upper line, circles). The amounts of free probe, monomer, and dimer complexes were determined by using a PhosphorImager system. The concentrations of PITX2 are expressed in arbitrary units of 1, 2, 4, and 8, representing ∼0.2, 0.4, 0.8, and 1.6 μM PITX2. The data represent the mean log (bound/unbound) ± standard error of the mean from three separate experiments. The PITX2a protein, with homeodomains depicted as shaded cylinders, is schematically represented on the two DNA substrates.
The Hill plot was generated by using measurements of free probe, monomer, and dimer complexes obtained from PhosphorImager (Molecular Dynamics) quantitation of the EMSA gels. The bound and unbound fractions (B and U, respectively) of Bcd binding sites were calculated by using the formulas [B] = [dimer]+1/2[monomer] and [U] = [free probe]+1/2[monomer]. The slope representing the Hill coefficient was calculated by linear regression. The concentrations of PITX2 for the x axis were arbitrarily assigned values of 1 (125 ng), 2 (250 ng), 3 (375 ng), etc.
SAABs.
PCR-based selection and amplification binding assays (SAABs) were performed as described previously (3, 5). Briefly, a degenerate oligonucleotide that contained TAAT followed by two random nucleotides (i.e., TAATNN) was used and was flanked by known primer sequences A (5′-TGAATTCATGATGCACGGCC-3′) and B (5′-GGAATTCATCGATACAGTTC-3′). Double-stranded degenerate oligonucleotides were generated by annealing primer B (7 μg) to the single-stranded degenerate oligonucleotide (14 μg) followed by incubation with Klenow polymerase. In vitro binding of 1 μg of GST fusion proteins (PITX2a and K88R) on glutathione Sepharose beads to 50 ng of double-stranded degenerate oligonucleotide was carried out at 4°C for 1 h in the presence of 10 volumes of binding buffer (20 mM HEPES [pH 7.5], 5% glycerol, 50 mM NaCl, and 1 mM dithiothreitol), 0.02 μg of poly(dI.dC), and 0.2 μg of bovine serum albumin. The beads were then concentrated, washed twice with binding buffer, and resuspended in 30 μl of distilled H2O. Finally, 10 μl of beads was used with primers A and B to PCR amplify bound sequences in a 25-μl reaction mixture for 15 cycles of 94 (45 s), 55 (1 min), and 72°C (30 s). Subsequent rounds were carried out by using 1 μl (approximately 50 ng) of PCR products. Products were cloned into pGEM-T vector (Promega) and sequenced.
Cell culture and reporter gene assays.
The WT PITX2a, K88E, T68P, and K88R cDNAs were cloned into pcDNA3.1 MycHisC expression vector (Invitrogen) containing the CMV promoter and an in-frame C-terminal c-Myc epitope at BamHI and HindIII sites. The single (Bcd 1x) and tandem (Bcd 2 × 5n) bicoid elements shown in Fig. 4A were cloned into the BamHI site of the thymidine kinase luciferase reporter (4). The insertions were sequenced and confirmed as single copies, with the Bcd 1x in the antisense orientation and the Bcd 2 × 5n in the sense orientation. The prolactin-luciferase reporter contains a 2.5-kb fragment of the rat prolactin enhancer-promoter (23). CHO cells were cultured in 60-mm dishes in alpha-modified Eagle's medium, 10% fetal bovine serum, 300 μg of glutamine/ml, 100 units of penicillin/ml, and 100 μg of streptomycin/ml. The mouse oral epithelial cell line LS8 was kindly provided by Malcolm Snead (University of Southern California) and grown in Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 100 units of penicillin/ml, and 100 μg of streptomycin/ml. Unless otherwise indicated, all transfections were done by lipofection. Approximately 1 × 105 CHO or LS8 cells were cultured in 12-well plates and transfected with a total of 2 μg of DNA (including 0.25 to 1.0 μg luciferase reporter and 0.01 to 0.5 μg of each expression vector, as indicated) by using 4 μl of Lipofectamine 2000 (Invitrogen). CMV5 DNA was used to bring the total amount of DNA to 2 μg. A simian virus 40 β-galactosidase plasmid (0.1 μg of pSV-βgal) (Clontech) was used as a control. The DNA was incubated with Lipofectamine 2000 in the absence of serum in accordance with the manufacturer's protocol and was then added to cells in antibiotic-free medium in the presence of serum and incubated for 16 to 20 h. Following incubation, the cells were scraped, lysed, and assayed by using luciferase reagents (Promega) and β-galactosidase reagents (Tropix Inc.). Luciferase activities were corrected for transfection efficiency. For electroporation, CHO cells were electroporated as described previously (17), with 5 to 10 μg of expression vectors with the Myc-tag, 5 μg of prolactin-luciferase, and pSV-βgal (1 μg) at 340mV and 960μF. Statistical significance was calculated by using a simple t test for means of two samples.
RESULTS
WT PITX2a forms a dimer.
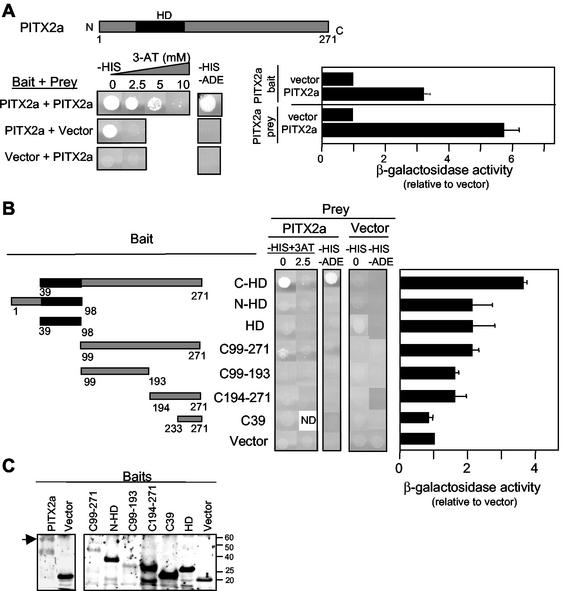
We tested the ability of PITX2a to dimerize by using the yeast two-hybrid system. PITX2a was fused with the Gal4 DBD or Gal4 AD for use as bait and prey, respectively. Full-length PITX2a was able to interact with itself according to results of three independent Gal4-dependent measurements (Fig. 1A). Growth was detected under histidine and adenine auxotrophic selection. In the absence of histidine, growth was seen in the presence of up to 5 mM 3-AT, a competitive inhibitor of the His3 enzyme. As a control, there was no growth above background when the PITX2a prey was matched with the bait vector containing only the Gal4 DBD. Likewise, there was no growth when the PITX2a bait was matched with the Gal4 AD prey vector in the presence of 2.5 mM 3-AT or the absence of both adenine and histidine. However, in the absence of 3-AT from the histidine selection medium, there was growth in yeast containing the full-length PITX2a bait alone or with the Gal4 AD plasmid (Fig. 1A). This background growth was not seen with any of the PITX2a fragments (Fig. 1B). This suggests that the transactivation activity of WT PITX2a requires contributions from homeodomain and N- and C-terminal regions. The third measure of interaction was increased β-galactosidase activity. There was sixfold greater activity over the background of PITX2a prey with the Gal4 DBD bait vector and threefold greater activity over the background of PITX2a bait with the Gal4 AD prey vector (Fig. 1A).
FIG. 1.
WT PITX2a can form dimers. (A) The amino acid numbering for the schematic corresponds to the PITX2a isoform. The black region represents the homeodomain (HD). Mated yeast culture (5 μl) was spotted on SD/−His/−Leu/−Trp plates with 0 to 10 mM 3-AT (−HIS) and SD/−Ade/−His/−Leu/−Trp plates (−HIS −ADE) and incubated at 30°C for 5 days. The SD/−Leu/−Trp auxotrophic medium was used in all plates to maintain bait and prey selection. The β-galactosidase activity was assayed from mated yeast colonies by using chemiluminescent detection. The vector interaction with the PITX2a bait or prey was set to 1. The data represent results from four to six separate colonies obtained from two to three separate matings and represent the mean β-galactosidase activity (relative to that of vector) ± the standard error of the mean. (B) Schematic representation of the PITX2 constructs used as baits in the yeast-two hybrid analysis. The mated yeast cultures were spotted on −HIS plates with 0 to 2.5 mM 3-AT and −HIS −ADE plates; β-galactosidase activity was measured as described in the legend for panel A. The data represent results from four to six colonies from two to three separate matings. ND, not determined. (C) The expression of bait constructs used in panels A and B were confirmed by Western blotting by using the anti-GAL4 DBD antibody. Full-length PITX2a bait and molecular weight markers are indicated.
A series of baits containing fragments of the PITX2a protein was used to identify the dimerization domain. The C-HD fragment, which contains the C-terminal region and the homeodomain, was able to interact with full-length PITX2a (Fig. 1B). This demonstrates that the N terminus, which varies among the different PITX2 isoforms, is not required for dimerization. The fragment containing the amino-terminal region and homeodomain yielded no growth under auxotrophic selection, and β-galactosidase activity was only twofold above background. The homeodomain alone or C-terminal fragments without the homeodomain also revealed little or no interaction with full-length PITX2a (Fig. 1B). We also tested whether the homeodomain alone would interact with itself (as prey) or with the C-terminal fragments. There was no detectable interaction (data not shown). As a control, expression of the bait fusion proteins was confirmed by Western blot analyses by using an antibody against the Gal4 DBD (Fig. 1C). The C-terminal fusion proteins were readily detected. By comparison, the larger full-length PITX2a and C-HD fragments were consistently weaker signals and were often difficult to detect. Thus, WT PITX2a dimerization appears to require both the homeodomain and C-terminal tail.
K88E forms stronger dimers via C-terminal-region interactions.
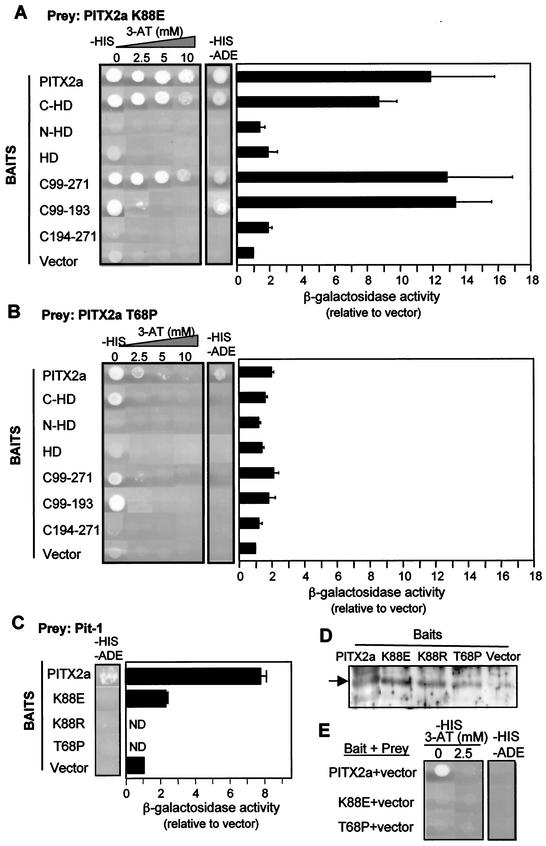
To test our prediction that the dominant-negative nature of the K88E mutant is due to the formation of nonfunctional heterodimers with WT PITX2a (29), we evaluated K88E dimerization activity. The K88E mutant interacted with full-length PITX2a (Fig. 2A). The relative strength of the interaction was measured by growth in the presence of increasing concentrations of 3-AT and β-galactosidase reporter gene activity. Unexpectedly, we found that K88E interaction with WT PITX2a resulted in growth under more stringent conditions and approximately twofold greater β-galactosidase activity than seen with WT homodimerization (Fig. 1A versus Fig. 2A). Another difference from WT was the absence of background growth with K88E as the bait (Fig. 2E). As a control, expression of K88E and other bait proteins was confirmed by Western blots (Fig. 2D).
FIG.2.
K88E mutant interacts more strongly with WT PITX2a. The indicated baits and the preys PITX2a K88E (A) or PITX2a T68P (B) or Pit-1 (C) were mated as described in the legend to Fig. 1 and plated on SD/−His/−Leu/−Trp plates with 0 to 10 mM 3-AT (−HIS) and/or SD/−Ade/−His/−Leu/−Trp plates (−HIS −ADE) to measure the strength of interaction. As in Fig. 1, β-galactosidase activity was also measured. The data represent results from four to six separate colonies obtained from two to three separate matings and represent the mean β-galactosidase activity (relative to that of the vector) ± the standard error of the mean. (D) Western blot of the bait constructs shown in panel C with anti-GAL4 DBD antibody. Full-length PITX2a is indicated. (E) Background activity of PITX2a, K88E, and T68P baits with the prey vector was determined as described in the legend to Fig. 1A. ND, not determined.
As seen with PITX2a homodimerization, the fragment containing the C-terminal tail and homeodomain (C-HD) was sufficient for dimerization. Interestingly, the C-terminal tail alone (residues 99 to 271) was sufficient to interact with K88E even under the most stringent conditions tested (Fig. 2A). We further narrowed down this region to the C-terminal 94 residues (C99-193) proximal to the homeodomain (Fig. 2A). This contrasts with the WT protein (Fig. 1B). The K88E interaction with the two C-terminal tail fragments showed a sixfold greater β-galactosidase activity than the WT interaction (Fig. 2A). We then tested whether the K88E homeodomain alone would interact with WT PITX2a homeodomain or with the C-terminal fragments. There was no detectable interaction (data not shown). These findings indicate that full-length K88E can form strong dimers with PITX2a, primarily by increased interactions with the C-terminal region.
For comparison, we assessed the dimerization of the T68P mutant (Fig. 2B). This mutant was also defective in DNA binding, transactivation, and Pit-1 synergism but was not dominant negative (29). Our results show that T68P is able to only weakly interact with WT PITX2a, based on the auxotrophic selection data. The β-galactosidase activities were barely above the vector background (Fig. 2B). Importantly, there is no increased interaction with the C-terminal domain. These findings may explain why T68P does not show the same dominant-negative behavior as K88E.
ARS mutants do not interact with Pit-1.
We have previously shown that the dominant negative effect of K88E is more pronounced in the presence of its synergistic partner, the POU homeodomain protein Pit-1 (29). In order to investigate the basis of this effect, we compared the interactions of Pit-1 with WT PITX2a and K88E in the yeast two-hybrid assay. WT PITX2a interacted with Pit-1 as measured by both growth and β-galactosidase assays (Fig. 2C). In contrast, the matching of Pit-1 with K88E, T68P, and K88R (discussed later) did not yield growth under selection (Fig. 2C). There appears to be some interaction, since Pit-1 with K88E resulted in β-galactosidase activity that was 2.5-fold greater than the vector background (Fig. 2C). The reduced interactions of Pit-1 with ARS mutants K88E and T68P may help explain why these mutants are unable to synergize with Pit-1. However, while reduced interaction may contribute to the inhibition of PITX2a activity, it alone is not sufficient to explain the dominant-negative effect, since T68P, which is not dominant negative, also failed to show interaction with Pit-1. As a control, expression of PITX2a WT and mutant bait fusion proteins was confirmed by Western blot (Fig. 2D).
Increased dimerization of K88E in vitro.
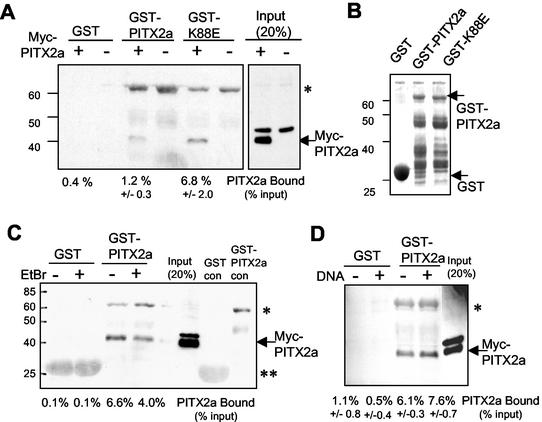
To independently confirm the dimerization of PITX2a and the increased interaction between PITX2a and K88E seen in the two-hybrid assay, we performed in vitro GST pulldown assays (Fig. 3A). As a source of PITX2a for the binding assay, we used total lysates prepared from CHO cells that had been transfected with an expression vector encoding PITX2a with a Myc epitope tag. Lysates from cells transfected with either the control vector or the Myc-PITX2a vector were incubated with glutathione-Sepharose beads containing GST, GST-PITX2a, or GST-K88E in the presence of ethidium bromide. Ethidium bromide was added to disrupt any effect of contaminating bacterial DNA that can potentially result in low-affinity retention of DNA binding proteins. A Western blot with anti-Myc antibody showed approximately fivefold increased binding of Myc-PITX2a to GST-K88E compared with that to GST-PITX2a. As controls, the GST protein and lysates from CHO cells transfected with the control vector showed no binding (Fig. 3A). There were similar amounts of GST-PITX2a and GST-K88E proteins used in the binding assays (Fig. 3B). The band around 60 kDa is due to nonspecific binding of anti-Myc antibody to GST-PITX2 (Fig. 3C, control lanes).
FIG. 3.
Detection of the stronger K88E interaction by GST pulldown assays. (A) Total lysate (90 μg) from CHO cells transfected with (+) or without (−) Myc-tagged PITX2a was incubated with glutathione-Sepharose beads containing GST alone, GST-PITX2a, or GST-K88E. Following incubation, washes, and SDS-polyacrylamide gel electrophoresis, Western blotting was performed by using anti-Myc antibody. Eighteen micrograms of total lysate (20% of the input) was used as a positive control. The Myc-tagged PITX2a protein and molecular weight markers are indicated. A nonspecific band that is present in the lysates from cells transfected with either the Myc-PITX2a or control plasmid is seen just above the Myc-PITX2a protein. The band intensities were calculated by using NIH Image software, and the amount of bound protein is indicated as the percentage of the input signal from two to four separate experiments (mean ± standard error of the mean). (B) Coomassie-stained gel of GST, GST-PITX2a, and GST-K88E protein preparations. GST, GST-PITX2a, and molecular weight standards are indicated. (C and D) The GST pulldown assay shown in panel A with GST and GST-PITX2a was repeated (C) in the presence (+) or absence (−) of 300 μg of ethidium bromide/ml or Bcd 2x 5n (D) DNA (see Fig. 4A). The Myc-PITX2a and molecular weight markers are indicated. The band intensities were calculated as described above. The data in panel D represent results of three separate experiments and represent the mean percentage bound ± standard error of the mean. The anti-Myc antibody appears to nonspecifically recognize the GST-PITX2a protein (*), and to a lesser extent, the GST protein (**).
PITX2a dimerization is DNA independent.
We then asked whether PITX2a dimerization was increased in the presence of bicoid DNA binding sites. For this test, we performed the GST pulldown assay in the absence of ethidium bromide. For comparison, we found that addition of ethidium bromide reduced PITX2a binding by about twofold, with little or no effect on background (Fig. 3C). Addition of oligonucleotides with tandem copies of the bicoid site that is recognized by PITX2a showed no effect on the amount of bound PITX2a (Fig. 3D). Thus, in vitro dimerization appears to be DNA independent.
Dimerization allows cooperative binding of PITX2a to DNA.
To further understand the mechanism of WT and mutant PITX2a dimerization, we developed a series of oligonucleotides containing tandem bicoid (Bcd) sites (TAATCC). PITX2a has been shown to bind the Bcd element in vitro and in vivo (4). The Bcd element was arranged as a single site (Bcd 1x) or in tandem head-to-tail (Bcd 2x) and head-to-head (Bcd 2x pal) orientations separated by 5 or 10 nucleotides (5n or 10n) (Fig. 4A). These elements were first used to test the effect of PITX2a dimerization in an in vitro DNA binding assay (EMSA). In all experiments with bacterially expressed GST-PITX2a protein, the GST moiety was removed by PreScission protease cleavage. PITX2a formed a dimer preferentially with the Bcd 2 × 5n probe over Bcd 1x or Bcd 2x pal 5n (Fig. 4B). The results seen with the Bcd 2 × 10n and Bcd 2x pal 10n substrates were similar to those seen with the 5-bp insertions (data not shown). The greatest binding was seen with the Bcd 2x element, followed by binding to the single Bcd element (Bcd 1x). The weak binding of both monomers and dimers to the Bcd 2x pal DNA suggests that either the flanking DNA sequence or the orientation of the sites is critical for PITX2a binding to DNA.
We then used the Bcd 1x and Bcd 2x oligonucleotides to assess the cooperativity of PITX2a DNA binding (Fig. 4C). The amount of dimer on the palindromic element was too small for reliable measurements. Cooperativity was determined by measuring monomer and dimer complexes as a function of PITX2a concentration and calculating the Hill coefficients. The Hill coefficients are represented by the slopes in Fig. 4C, with a slope of greater than 1 indicating cooperative DNA binding. With the tandem head-to-tail element (Bcd 2 × 5n), there was a Hill coefficient of 1.73, which indicates cooperative binding. The weaker dimer formation on the single bicoid element (Bcd 1x) showed a lesser degree of cooperativity, with a Hill coefficient of 1.20. These results demonstrate that there is cooperative binding of PITX2a to tandem bicoid sites.
The PITX2a dimer is more stable than the monomer on DNA.
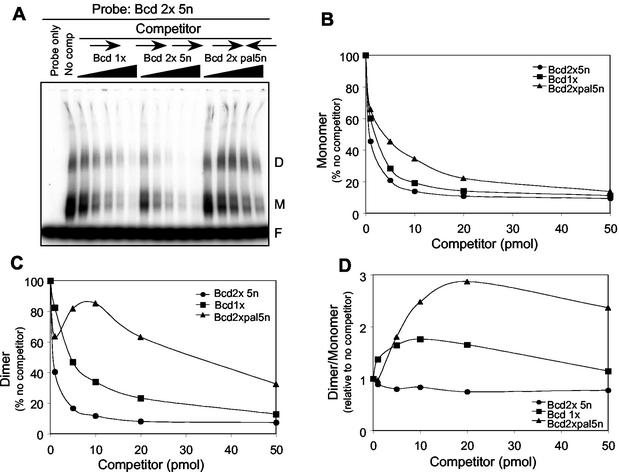
To measure the relative affinities of PITX2a bound as a monomer and dimer, we set up a competition EMSA to measure complex stabilities. The relative degrees of competition of the monomer and dimer complexes on 1 pmol of labeled Bcd 2x (head-to-tail) DNA substrate was measured by using up to 50 pmol of Bcd 1x, Bcd 2 × 5n, and Bcd 2x pal 5n unlabeled DNA as competitors (Fig. 5A). The monomer complex was competed almost equally well by Bcd 1x and Bcd 2 × 5n DNA, and to a lesser degree by Bcd 2x pal 5n (Fig. 5B). In contrast, the dimer complex was not competed as well by Bcd1x and only poorly by Bcd 2x pal 5n (Fig. 5C). This demonstrates that the DNAs that primarily form only monomers (Bcd 1x and Bcd 2x pal) cannot easily disrupt the PITX2a dimer complex relative to the monomer complex. The relative competition of the complexes is graphically represented as the ratio of dimer to monomer at each amount of competitor DNA (Fig. 5D). The dimer-to-monomer ratio stays relatively constant for self-competition with Bcd 2 × 5n but increases twofold for the Bcd 1x competitor. This shows that the single Bcd site preferentially competes the monomer but not the dimer complex. The relative competition of the monomer over the dimer complex is even more pronounced with the Bcd 2x pal 5n competitor (Fig. 5D). These results indicate that the PITX2a molecules are not just occupying the two binding sites on the Bcd 2x probe as independent monomers but rather that there is a greater apparent affinity for PITX2a bound to the tandem DNA elements as a dimer.
FIG. 5.
The dimer is competed poorly by the single site and head-to-head binding sites. (A) Bacterially expressed PITX2a (500 ng) was incubated with the head-to-tail Bcd 2 × 5n 32P-labeled probe in an EMSA. The protein and labeled DNA were incubated in the absence (no comp) or presence of 1-, 5-, 10-, 20-, or 50-molar excesses of unlabeled competitor DNA, as indicated. The free probe, monomer, and dimer complexes are indicated by the letters F, M, and D, respectively. (B and C) The amounts of monomer (B) and dimer (C) complexes in the presence of the various amounts of different competitors were measured by using a PhosphorImager system. The signal in the absence of competitor was set at 100%. (D) Plot of the dimer/monomer ratio calculated from the results shown in panels B and C, with the ratio in the absence of competitor set at 1. The upward curve seen with the Bcd 2x pal 5n and Bcd 1x competitors indicates that these DNAs primarily compete for the monomer complex rather than the dimer complex.
K88E prevents cooperative binding of WT PITX2a to DNA.
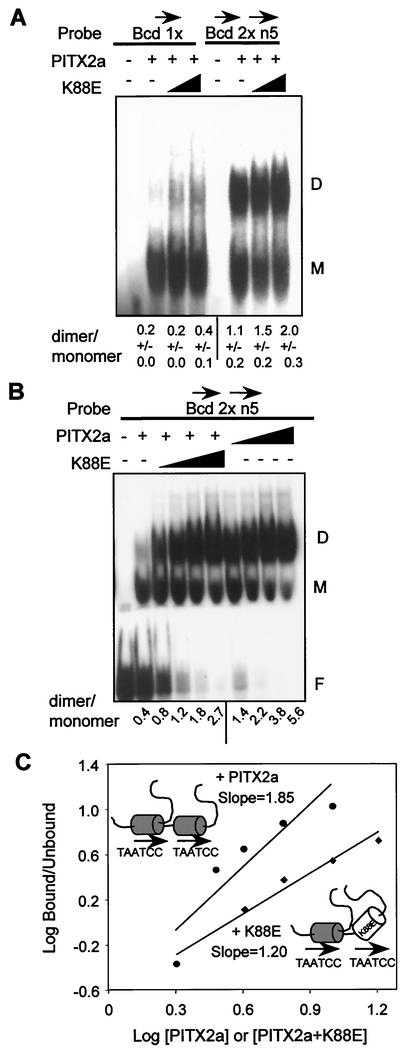
Saadi et al. have previously shown that K88E does not bind DNA (29). However, the question of whether a heterodimer of WT PITX2a and K88E could bind DNA was left open. WT-K88E heterodimer formation on DNA was measured by addition of K88E with the WT protein in EMSAs (Fig. 6). For comparison, additional WT PITX2a was tested in parallel reactions. To facilitate dimer formation, we used the Bcd 2 × 5n DNA substrate and lower amounts of probe (0.2 pmol in Fig. 6B versus 1 pmol in Fig. 6A). Addition of K88E increased the formation of dimers relative to monomers to about the same degree (twofold) on the Bcd 1x and Bcd 2 × 5n DNA substrates. However, addition of WT PITX2a caused a much more robust increase in the dimer-to-monomer ratio. The ratio increased from 0.4 to 1.8 and 5.6, respectively, at comparable amounts of K88E and WT PITX2a (Fig. 6B). This suggests that the K88E-WT heterodimer can bind DNA on a suitable dimer substrate but that the mutant heterodimer forms a weaker complex with DNA than the WT homodimer.
FIG. 6.
The WT-K88E heterodimer binds DNA without cooperativity. (A) EMSA with 500 ng of bacterially expressed PITX2a incubated with 1 pmol of the Bcd 1x or the head-to-tail Bcd 2 × 5n probe in the presence of increasing amounts of K88E (0, 1,000, and 2,000 ng). The monomer and dimer complexes are indicated by the letters M and D, respectively. The amounts of monomer and dimer complexes were determined by using a PhosphorImager system. The data represent mean dimer/monomer ratios ± standard errors of the means from three separate experiments. (B) EMSA with 250 ng of PITX2a incubated with 0.2 pmol of Bcd 2 × 5n probe in the presence of increasing amounts of WT (125, 250, 500, and 1,000 ng) or K88E (250, 500, 1,000, and 2,000 ng) (note that more K88E protein was used). The free probe, monomer, and dimer complexes are indicated. The ratios of dimer to monomer complexes from this representative experiment with both WT and K88E on the same gel are shown. (C) The Hill coefficient was estimated as described in the legend to Fig. 4C for the WT (upper line,circles) and WT plus K88E (lower line, diamonds). The concentrations for WT are expressed in arbitrary units of 2, 3, 4, 6, and 9, representing ∼0.4, 0.6, 0.8, 1.2, and 1.8 μM, respectively, while those for WT-K88E are 2, 4, 6, 9, and 18, representing ∼0.4, 0.8, 1.2, 1.8, 3. and 6 μM, respectively. The WT (shaded homeodomain) and K88E (open homeodomain) protein-DNA complexes are schematically represented.
To test whether the WT-K88E heterodimer bound DNA in a cooperative manner, we used the Bcd 2 × 5n DNA substrate at limiting concentrations to drive dimer formation. There was relatively little cooperativity (Hill coefficient, 1.20) compared to the WT homodimer binding in parallel reactions (Hill coefficient, 1.85) (Fig. 6C). Thus, the WT-K88E heterodimer appears to be able to bind DNA, although to a lesser degree and with much less cooperativity than the WT homodimer.
K88E preferentially represses the tandem bicoid element.
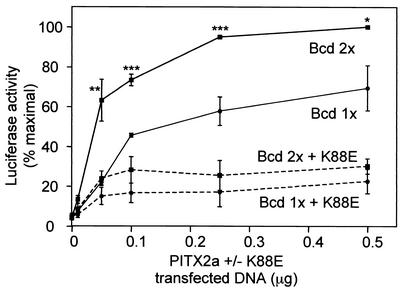
We next asked if PITX2a cooperativity also results in better transactivation of a reporter containing a single copy of the Bcd 2 × 5n tandem sites than a reporter with a single copy of the Bcd 1x single site. The CHO cells were used as a heterologous cell line that does not express the endogenous Pitx2 gene. We proposed that transfections with high amounts of reporter relative to the PITX2a WT and K88E expression vectors would allow us to assay cooperativity on tandem bicoid sites. Indeed, low amounts (0.01, 0.05, 0.1 μg) of transfected PITX2a (Fig. 7, solid lines) showed a steeper increase in activation of Bcd 2 × 5n than Bcd 1x reporter, indicating cooperativity. We then tested the effect of K88E on PITX2a activation of the Bcd 2 × 5n and Bcd 1x reporter genes (Fig. 7, dashed lines). Saadi et al. had previously found that K88E only weakly repressed PITX2a activation of a reporter gene containing multiple Bcd elements in COS7 cells (29). We have subsequently found that in the CHO and LS8 cell lines, K88E can repress activation of that reporter to a much greater degree than observed in COS7 cells. The reason for this cell specificity is not known (see Discussion). In CHO cells, expression of K88E suppressed WT PITX2a activation of luciferase reporters containing the Bcd 1x monomer and Bcd 2 × 5n dimer substrates (Fig. 7, dashed lines). At all points, there were equivalent amounts of WT and K88E expression vectors. Importantly, the repression of the dimer substrate Bcd 2 × 5n relative to the monomer substrate Bcd 1x was greatest at the lowest concentration of expression vectors. At 0.05 μg of expression vectors, there was 62% inhibition of the dimer substrate versus 32% inhibition of the monomer substrate. At higher concentrations of PITX2a and K88E expression vectors, there was comparable repression of both reporters by K88E, with 70 and 67% inhibition of the dimer and monomer reporters, respectively.
FIG. 7.
Preferential K88E repression of PITX2a activation of the tandem bicoid element. CHO cells were transfected by lipofection with 1 μg of Bcd 1x (circles) or Bcd 2 × 5n (squares) reporter plasmids and increasing amounts of WT PITX2a (0.01, 0.05, 0.1, 0.25, and 0.5 μg, as indicated) in the absence (solid line) or presence (dotted line) of equal amounts of K88E. The activity was normalized to that of Bcd 2 × 5n with 0.5 μg of WT PITX2a, which was set at 100%. The means ± standard errors of the means from three to four separate experiments are shown. WT PITX2a shows significantly higher activation of the Bcd 2 × 5n reporter than the Bcd 1x reporter (*, P < 0.04; **, P < 0.01; ***, P < 0.005).
Mutation of homeodomain position 50 to an arginine (PITX2a-K88R) prevents DNA binding.
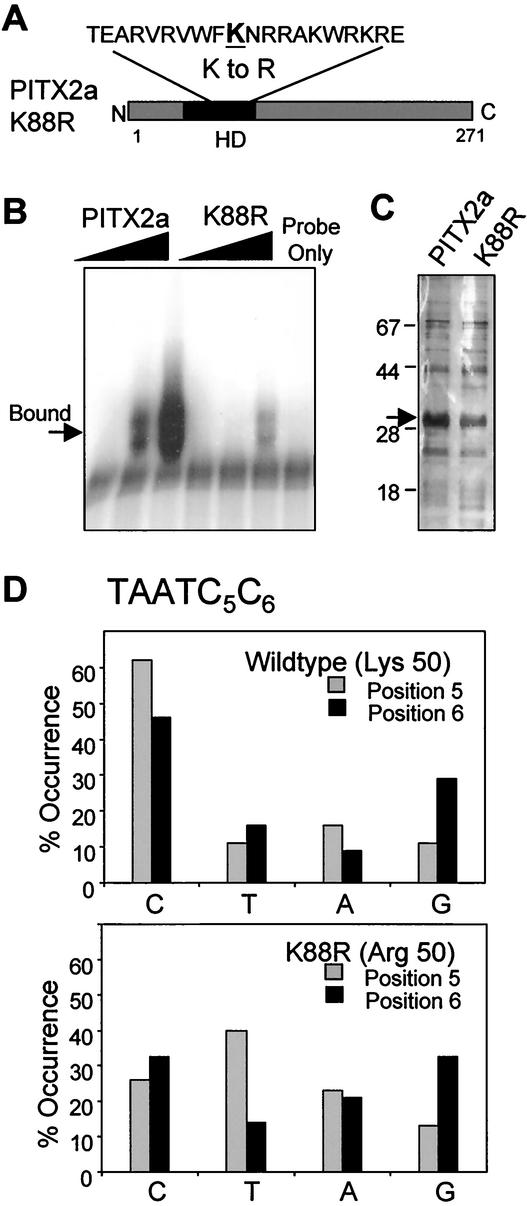
We then asked whether the increased dimerization of the K88E mutant was specific for the glutamic acid mutation at position 50. As a starting point, we wanted a residue at position 50 that would minimize disruption of secondary structure yet not allow DNA binding, analogous to the K88E mutant. We also wanted a residue that would be most similar to the parental lysine. Based on alignments of known homeodomain proteins, we reasoned that an arginine would be the best choice (see Discussion). We made a charge-conservative change of the lysine to an arginine to generate the PITX2a-K88R (K88R) protein (Fig. 8A). Computer modeling of the K88R homeodomain-DNA complex suggested that it would not bind DNA due to steric hindrance of the 3′ dinucleotide, as previously reported for the K88E mutant (29). These models were extrapolated from the Antennapedia (Antp) homeodomain-DNA structural data. The analysis shows that the WT position 50 lysine does not clash with 3′ dinucleotide base pairs of the TAATCC consensus sequence but that the arginine at position 50 does create an observable steric clash with these residues (data not shown). The overall energy requirement for the K88R mutant homeodomain-DNA complex was calculated to be 13.24 kcal/mol higher than that of the WT PITX2 complex. The inability of K88R to bind DNA was demonstrated by EMSA by using the TAATCC bicoid consensus sequence (Fig. 8B). Only weak binding of the K88R mutant was detected at relatively high amounts of protein generated from GST fusion proteins (Fig. 8C).
FIG. 8.
K88R mutant protein has decreased DNA binding to the bicoid consensus site. (A) Schematic indicating the lysine-to-arginine change in PITX2a K88R. (B) Increasing amounts (100, 200, and 300 ng) of WT PITX2a and K88R proteins were incubated with the Bcd 1x probe (containing the bicoid consensus sequence TAATCC) in an EMSA. The bound complex is indicated. The lower band seen with the probe alone is most likely a multimer of the free probe that has been run off this gel. (C) Silver-stained gel of the PITX2a and K88R protein preparations following removal of the GST moiety, with WT and K88R proteins (arrow) and molecular weight markers indicated. (D) SAAB results for GST-PITX2a or GST-K88R proteins with a probe containing a degenerate (TAATNN) binding sequence are summarized. The overall frequency of each nucleotide occurring at either position 5 or position 6 is shown. For WT PITX2a, a total of 44 sequences were analyzed. For K88R, a total of 43 sequences were analyzed.
To complete the DNA binding analysis of K88R, we wanted to be sure that we had not generated a protein with a new binding specificity. The most likely change in specificity would have been a 3′ dinucleotide other than the cytosine pair. This possibility was addressed by a SAAB assay by using a probe degenerate for positions 5 and 6 (TAATNN) (Fig. 8D). WT PITX2a preferred a cytosine at both positions 5 and 6, as expected (62 and 46%, respectively). In contrast, the K88R mutant did not show a strong preference for any nucleotide at either position 5 or 6, indicating that the K88R protein has not acquired a new binding specificity.
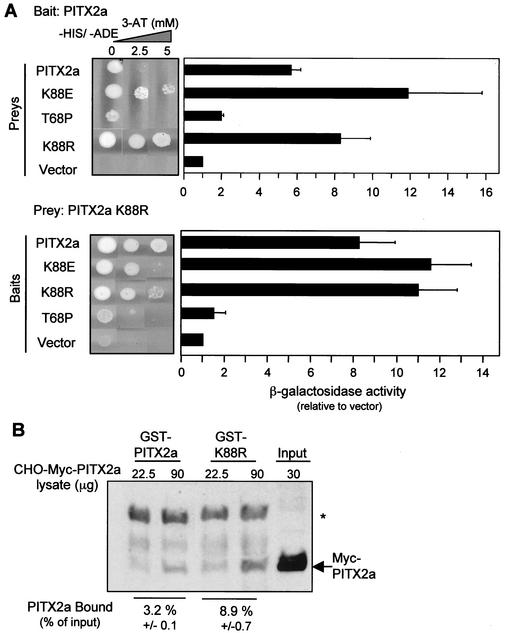
Increased dimerization of K88R.
The ability of K88R to dimerize with WT PITX2a was tested in the yeast two-hybrid assay (Fig. 9). The strength of interaction was measured by growth under double auxotrophic selection conditions (−His−Ade) with increasing amounts of 3-AT. Note that the increased stringency of double auxotrophic selection precludes direct comparison with the 3-AT growth results of Fig. 1 and 2. K88R interacted more strongly than the WT homodimers (Fig. 9A, upper panel). K88R also strongly interacted with K88E and with itself but not with T68P (Fig. 9A, lower panel). The degree of increased interaction measured by β-galactosidase activity was comparable to that of the K88E mutant interactions.
FIG. 9.
K88R mutant forms stronger dimers with WT PITX2a. (A) The indicated baits and preys were mated as described in the legend to Fig. 1 and plated on SD/−Ade/−His/−Leu/−Trp plates with 0 to 5 mM 3-AT (−HIS, −ADE) to show the strength of interaction. As in Fig. 1, β-galactosidase activity is also shown. The data represent results from four to six separate colonies obtained from two to three separate matings and represent the mean β-galactosidase activities (relative to that of the vector) ± standard errors of the means. (B) Lysate (22.5 and 90 μg) from CHO cells transfected with the Myc-PITX2a vector was incubated with GST-PITX2a or GST-K88R and Western blotted as described in the legend for Fig. 3A. For comparison, 30 μg of the input lysate is shown. The intensities of the bands were quantitated by using NIH Image software. The amount of bound protein is indicated as the mean percent of the input signal ± standard deviation of the two samples from the experiment shown. The anti-Myc antibody appears to nonspecifically recognize the GST-PITX2a protein (*).
The increased two-hybrid interaction was confirmed by a GST pulldown assay (Fig. 9B). Lysates of CHO cells that had been transfected with the Myc-PITX2a vector were used as a source of PITX2a for the binding assay. There was approximately threefold increased binding to GST-K88R over WT, which is comparable to the increased binding to GST-K88E.
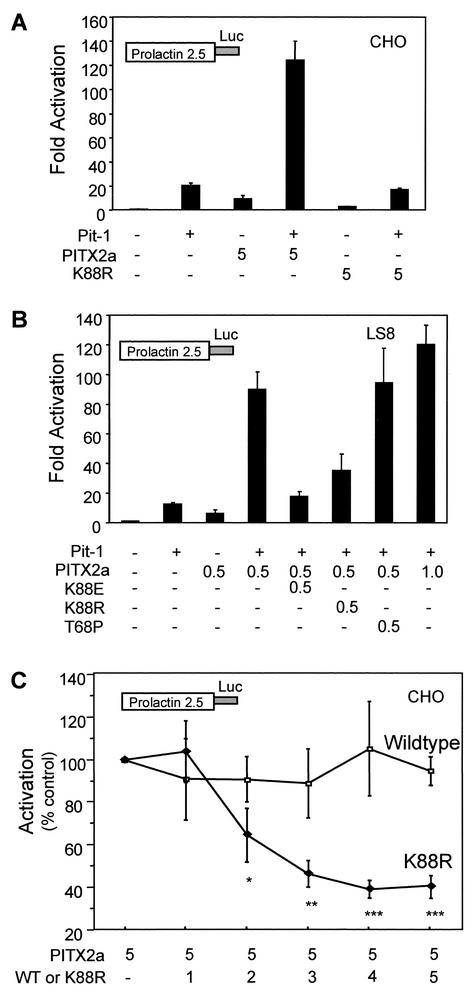
K88R is dominant negative, similar to K88E.
Given the decreased DNA binding activity, we predicted that the K88R mutant protein would also have reduced transactivation activity in transient cotransfection assays. For these studies, we used a reporter gene containing the prolactin promoter, which is believed to be a natural target of the PITX2a protein. WT PITX2a activated the prolactin promoter approximately ninefold in CHO cells (Fig. 10A), while K88R only weakly activated the prolactin promoter (approximatley threefold). Similar results were seen in the LS8 cells (not shown). We also tested activity in the presence and absence of the POU homeodomain protein Pit-1. The K88R mutant failed to show synergism with Pit-1 in CHO (Fig. 10A) and LS8 cells (data not shown). These results demonstrate that the K88R mutant protein has defective transactivation activity that is similar to its reduced binding activity.
FIG. 10.
K88R mutant prevents WT PITX2a synergism with Pit-1. (A) CHO cells were transfected by electroporation with the prolactin-luciferase reporter alone or with 5 μg of PITX2a and/or K88R in the presence and absence of Pit-1 (10 μg), as indicated. (B) LS8 cells were transfected by lipofection with PITX2a (0.5 or 1 μg, as indicated) and Pit-1 (0.5 μg) alone or in combination with 0.5 μg of K88E, K88R, or T68P, as indicated. The activity is shown as the mean activation (fold) relative to results for reporter alone, ± standard errors of the means, from four separate experiments. (C) CHO cells were cotransfected by electroporation with the prolactin-luciferase reporter, Pit-1 (10 μg), PITX2a (5 μg), and the indicated amounts (μg) of additional PITX2a or K88R. Cotransfection of increasing amounts of K88R with 5 μg of WT PITX2a shows a significant decrease in activation relative to that seen with 5 μg of WT PITX2a alone (*, P < 0.03; **, P < 0.002; ***, P < 0.001). The data represent results from four separate experiments and represent the mean activations (fold) ± standard errors of the means.
We then tested whether K88R would repress WT PITX2a activity. Cotransfections of equal amounts of WT PITX2a and K88R mutant DNA were done in the presence of Pit-1 for comparison with the repression of activation by K88E reported previously (29). The K88R mutant suppressed the synergistic effect of WT PITX2a significantly in both LS8 (Fig. 10B) and CHO cells (Fig. 10C), similar to K88E (Fig. 10B). In contrast, T68P was not able to suppress the WT activity (Fig. 10B). As expected, increased amounts of K88R resulted in an increased dominant-negative effect (Fig. 10C). At equivalent amounts of DNA encoding WT and K88R, transactivation activity was repressed to 40% of WT activity. As a control for the increased amount of transfected DNA, addition of twice as much WT PITX2a did not reduce activity (Fig. 10B,C). These results demonstrate that K88R can also repress WT PITX2a transactivation activity.
DISCUSSION
We have tested the hypothesis that the ARS K88E mutant protein has dominant-negative activity due to the formation of nonfunctional heterodimers with WT PITX2a. PITX2a dimerization was documented by using a combination of yeast two-hybrid and in vitro binding assays. PITX2a thus joins the growing ranks of homeodomain proteins, especially within the Paired, POU, Bicoid, and HOM-C subfamilies, that can form homodimers (21, 24, 34, 35, 39). Unexpectedly, we found that not only can the K88E mutant dimerize with WT PITX2a, but that this interaction is approximately two- to fivefold stronger than that with the WT homodimer. This stronger dimerization appears to be due to interactions with the C-terminal tail that are not seen to the same extent with WT homodimers. In contrast, another ARS mutant, T68P, which does not show dominant-negative activity, did not have increased dimerization activity. To further test the link between increased dimerization ability and the dominant-negative phenotype, we generated the K88R mutant, which contains an arginine at position 50 of the homeodomain. The K88R protein had increased dimerization activity, did not bind DNA, and acted in a dominant-negative manner to repress WT PITX2a activity, similar to the K88E mutant. These results support the conclusion that the ability of K88E to form relatively strong dimers with WT PITX2a underlies its dominant-negative phenotype.
When choosing the amino acid substitution that resulted in the K88R mutant, we had two criteria. First, we decided to make a charge-conservative change from a lysine to an arginine, since the lysine-to-glutamic acid mutation changed both the size and the charge of the residue. Second, we wanted to generate a mutant that could not bind DNA, similar to the K88E mutant. We reasoned that an arginine mutant might not bind DNA, since among the more than 350 homeodomain proteins in the database, the vast majority of residues at position 50 are glutamine, with a smaller number of cysteine, serine, lysine, or histidine residues. An arginine is reported in only two homeodomains, namely, ATBF (AT-binding fetal protein) and Zfh2 (Zinc finger homeodomain protein 2) (7, 11). ATBF has 4 homeodomains and 17 zinc finger domains, and Zfh2 has 3 homeodomains and 16 zinc finger domains (7). In both cases, only one homeodomain has an arginine at position 50, while the rest have the common glutamine residue. It is not known if the arginine-containing homeodomains can bind DNA. Computer modeling predicted that the PITX2a arginine mutant would be defective in DNA binding due to steric hindrance from the guanidinyl group of arginine, analogous to the glutamic acid mutation (29). This predicted binding defect was confirmed by empirical data showing that K88R could not bind the bicoid or other TAATNN DNA elements in vitro. We suggest that the arginine-containing homeodomains of ATBF and Xfh2 also do not bind DNA, but rather might be involved in dimerization.
Position 50 of the homeodomain appears to be involved in dimerization of PITX2a in at least two other homeodomain proteins in addition to its well-studied important role in DNA binding (1, 12, 22, 32). The Drosophila Paired homeodomains dimerize with resultant cooperative binding to DNA, and mutation of a serine to a glutamine at position 50 results in fourfold greater cooperativity than WT (35, 36). Yuan et al. have reported qualitatively increased dimerization of Drosophila Bicoid following mutation of the lysine at position 50 to either an alanine or glutamine (38). The PITX2 homeodomain has 60% identity to the Paired homeodomain and 38% identity to the Bicoid homeodomain. While speculative, it is possible that these shared residues may play important roles in dimerization. Together with our data on the K88E and K88R mutants, these data support the conclusion that position 50 can play an important role in both DNA binding and dimerization.
We have mapped PITX2a dimerization to the homeodomain and the adjacent C-terminal stretch. Dimerization of homeodomain proteins can occur with only the homeodomain or may require flanking regions of the protein outside of the homeodomain (14, 25, 34-36, 38, 39). We have shown that the WT or K88E homeodomain alone is not sufficient for binding to the C-terminal region or to each other in the yeast two-hybrid assay. These results indicate that the increased binding of K88E to the WT C-terminal region requires the context of the full-length protein. By using an in vitro DNA binding assay, we had previously reported that the homeodomain alone can form a dimer on DNA (2). The reason for this difference may be due to high levels of DNA and/or protein driving the binding to DNA, which would not occur in the yeast assay, but in either case, it is apparent that the PITX2a homeodomain is involved in dimerization. Burz and Hanes have used a genetic screen to identify mutations that affect Bicoid cooperativity but not DNA binding or transactivation (6). Mutations were found both in and outside of the homeodomain. Likewise, Bicoid dimerization requires both the homeodomain and either the flanking N-terminal or C-terminal region (38). In another well-characterized example, the yeast MATα2 homeodomain protein determines mating type specificity by forming heterodimers with MATa1 and/or MCM1. The crystal structure of the MATα2/MATa1/DNA ternary complex has revealed that the α2/a1 heterodimer is dependent on a 21-residue C-terminal α-helical tail region of α2 immediately following the homeodomain. These C-terminal residues partly form a three-helix bundle by packing between helices 1 and 2 of the a1 homeodomain (18). Computer analysis of the PITX2a C99-194 residues also suggests the presence of an α-helical region. We propose that this region of PITX2a may interact with the homeodomain to allow dimerization. This model might account for the increased binding to the C-terminal region by the K88E homeodomain mutant. In further support of this model, we have previously reported that removal of regions of the C-terminal portion of PITX2a results in increased DNA binding activity (2).
What is the consequence of dimerization? The data indicate that dimerization allows cooperative binding of WT PITX2 to DNA containing tandem bicoid elements (Hill coefficient, 1.7). A head-to-tail arrangement is preferred over the monomer site or a head-to-head arrangement. Yuan et al. have shown that Bicoid protein cooperatively binds to head-to-tail and tail-to-tail double sites that are separated by long distances (7 to 36 bp) but not to head-to-tail or tail-to-tail sites separated by 3 bp. In contrast, they found that Bicoid protein formed a dimer on head-to-head sites only if separated by 3 bp, but with weak cooperativity (Hill coefficient, 1.13) (37). Our data with PITX2a are consistent with these observations. Dimerization can occur independently of DNA and is not increased by the addition of DNA containing tandem binding sites. However, because PITX2a binding was decreased in the presence of ethidium bromide, this leaves the possibility of some DNA contribution to dimerization; alternatively, there could be partial denaturation of PITX2a by ethidium bromide.
Interestingly, the K88E-WT heterodimer bound DNA with decreased cooperativity. Cooperativity was reduced in the presence of K88E to the same value seen with WT protein on a monomer site (Hill coefficient, 1.2). Similarly, K88E had the same effect on the proportion of dimers formed on the monomer and dimer sites and was overall less effective than WT in forming dimers on DNA. Combined with our evidence that K88E does not bind DNA on its own, these data are all consistent with the schematic in Fig. 6 showing K88E attached only by protein interactions. The biological significance of this decreased cooperative binding is manifested as a twofold preferential inhibition of the dimer substrate relative to the monomer substrate at low levels of the PITX2a expression vector in transactivation assays. Cooperative binding would most likely have the greatest effect on the dimer substrate under these conditions. At higher levels of expression vector, there was comparable inhibition of both the dimer and monomer substrates. However, decreased cooperativity alone cannot be the only mechanism of repression, since otherwise we should not have seen any repression of the single-bicoid element. This suggests that in addition to decreasing cooperative binding to DNA, the K88E mutant must also decrease WT transactivation activity.
How does K88E decrease transactivation by WT PITX2a? We suggest that the PITX2a homeodomain is required for relieving inhibition from the C-terminal tail to allow transactivation. There is precedence for homeodomain mutations preventing transactivation. Furukawa et al. have recently reported that mutation of R57 and R59 of the homeodomain to glycine led to disruption of dimerization and transactivation in Cart1, a paired-like homeodomain protein (8). Interestingly, they found that the C-terminal region of Cart1 inhibits transactivation and that this region contains the 14-bp OAR/Aristaless domain that is also found in the C terminus of PITX2a. With the PITX2a WT-K88E heterodimer, the C-terminal tails would be maintained in their inhibitory state. This prediction is supported by our observations that (i) the K88E mutant has increased dimerization with the C-terminal region of WT PITX2a, which has been shown to interact with Pit-1 and presumably other factors, (ii) K88E does not have transactivation ability when fused to the Gal4 DBD in yeast, while WT PITX2a does, and (iii) in COS7 cells, the dominant-negative activity of K88E was much more pronounced on the prolactin promoter in the presence of Pit1, suggesting an inhibition of synergism with Pit1 (29). With respect to this last point, in contrast to COS7 cells, K88E is able to repress PITX2a in the absence of Pit-1 synergism in CHO and LS8 cells. Since LS8 cells are a mouse oral epithelia cell line that expresses the endogenous Pitx2a gene (11a), we have chosen to use those cells along with CHO cells as a heterologous companion. While the basis for the apparent cell specificity is completely speculative, we note that in CHO cells, we routinely see 15- to 30-fold activation of the synthetic bicoid reporter, while in COS7 cells, the magnitude of PITX2a activation was at best three- to fivefold. This raises the possibility that K88E may be repressing a functional interaction between PITX2a and a protein in CHO cells, analogous to Pit-1. In this regard, the finding that K88E, K88R, and T68P mutants all have reduced binding to Pit-1 is consistent with the observations that K88E has a more pronounced effect in the presence of Pit-1 (29). However, reduced Pit-1 binding cannot be the only mechanism, since K88E repression can occur in the absence of a physical interaction with Pit-1 and T68P is not dominant negative (29).
Taken together, these results indicate that the dominant-negative phenotype of the K88E mutant is due to an increased interaction with the C-terminal region of WT PITX2a. This results in reduced cooperative DNA binding and inhibition of transactivation by preventing Pit-1 and other factors from neutralizing the inhibitory C-terminal region. This prediction is consistent with a previous model in which the C-terminal tail inhibits PITX2 until relieved by interaction with Pit-1 or other partners (2). Our model predicts that the K88E protein interaction with WT PITX2 maintains the C-terminal region in an inactive state.
Finally, what are the implications of these findings on ARS? ARS mutations range from deletions to missense changes that are commonly viewed as haploinsufficient. Yet there is considerable heterogeneity in ARS phenotypes, and heterozygous Pitx2 knockout mice have only a mild phenotype (10, 19, 20). Therefore, it could be hypothesized that some missense mutations in ARS patients do not simply knock out PITX2 function, but rather, they may possess dominant-negative characteristics. Quentien et al. have recently reported that the R91P PITX2a mutant is dominant negative but can still bind DNA (27). Interestingly, Priston et al. have reported an ARS mutation (V83L) in PITX2a that shows decreased DNA binding but increased transactivation, implying that the DNA binding domain of PITX2 can independently affect transactivation (26). The degree and nature of dimerization of these and other missense mutants might contribute to the range of phenotypes and pathogenesis in ARS patients.
Acknowledgments
We thank Rafael Toro, Elena Semina, Jun Ma, and Jeff Murray for discussions.
This work was supported by a predoctoral fellowship from the American Heart Association (I.S.) and by a grant from the National Institutes of Health (DE 13076).
REFERENCES
- 1.Ades, S. E., and R. T. Sauer. 1994. Differential DNA-binding specificity of the engrailed homeodomain: the role of residue 50. Biochemistry 33:9187-9194. [DOI] [PubMed] [Google Scholar]
- 2.Amendt, B. A., L. B. Sutherland, and A. F. Russo. 1999. Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Mol. Cell. Biol. 19:7001-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amendt, B. A., L. B. Sutherland, and A. F. Russo. 1999. Transcriptional antagonism between Hmx1 and Nkx2.5 for a shared DNA-binding site. J. Biol. Chem. 274:11635-11642. [DOI] [PubMed] [Google Scholar]
- 4.Amendt, B. A., L. B. Sutherland, E. V. Semina, and A. F. Russo. 1998. The molecular basis of Rieger syndrome. Analysis of Pitx2 homeodomain protein activities. J. Biol. Chem. 273:20066-20072. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell, T. K., and H. Weintraub. 1990. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science 250:1104-1110. [DOI] [PubMed] [Google Scholar]
- 6.Burz, D. S., and S. D. Hanes. 2001. Isolation of mutations that disrupt cooperative DNA binding by the Drosophila bicoid protein. J. Mol. Biol. 305:219-230. [DOI] [PubMed] [Google Scholar]
- 7.Duboule, D. 1994. Guidebook to the homeobox genes. Oxford University Press, Oxford, United Kingdom.
- 8.Furukawa, K., T. Iioka, M. Morishita, A. Yamaguchi, H. Shindo, H. Namba, S. Yamashita, and T. Tsukazaki. 2002. Functional domains of paired-like homeoprotein Cart1 and the relationship between dimerization and transcription activity. Genes Cells 7:1135-1147. [DOI] [PubMed] [Google Scholar]
- 9.Gage, P. J., and S. A. Camper. 1997. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum. Mol. Genet. 6:457-464. [DOI] [PubMed] [Google Scholar]
- 10.Gage, P. J., H. Suh, and S. A. Camper. 1999. Dosage requirement of Pitx2 for development of multiple organs. Development 126:4643-4651. [DOI] [PubMed] [Google Scholar]
- 11.Gehring, W. J., M. Affolter, and T. Burglin. 1994. Homeodomain proteins. Annu. Rev. Biochem. 63:487-526. [DOI] [PubMed] [Google Scholar]
- 11a.Green, P. D., T. A. Hjalt, D. E. Kirk, L. B. Sutherland, B. L. Thomas, P. T. Sharpe, M. L. Snead, J. C. Murray, A. F. Russo, and B. A. Amendt. 2001. Antagonistic regulation of Dlx2 expression by PITX2 and Msx2: implications for tooth development. Gene Expr. 9:265-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanes, S. D., and R. Brent. 1989. DNA specificity of the bicoid activator protein is determined by homeodomain recognition helix residue 9. Cell 57:1275-1283. [DOI] [PubMed] [Google Scholar]
- 13.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 14.Kasahara, H., A. Usheva, T. Ueyama, H. Aoki, N. Horikoshi, and S. Izumo. 2001. Characterization of homo- and heterodimerization of cardiac Csx/Nkx2.5 homeoprotein. J. Biol. Chem. 276:4570-4580. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura, K., H. Miura, M. Yanazawa, T. Miyashita, and K. Kato. 1997. Expression patterns of Brx1 (Rieg gene), Sonic hedgehog, Nkx2.2, Dlx1 and Arx during zona limitans intrathalamica and embryonic ventral lateral geniculate nuclear formation. Mech. Dev. 67:83-96. [DOI] [PubMed] [Google Scholar]
- 16.Lamonerie, T., J. J. Tremblay, C. Lanctot, M. Therrien, Y. Gauthier, and J. Drouin. 1996. Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev. 10:1284-1295. [DOI] [PubMed] [Google Scholar]
- 17.Lanigan, T. M., and A. F. Russo. 1997. Binding of upstream stimulatory factor and a cell-specific activator to the calcitonin/calcitonin gene-related peptide enhancer. J. Biol. Chem. 272:18316-18324. [DOI] [PubMed] [Google Scholar]
- 18.Li, T., M. R. Stark, A. D. Johnson, and C. Wolberger. 1995. Crystal structure of the MATa1/MAT alpha 2 homeodomain heterodimer bound to DNA. Science 270:262-269. [DOI] [PubMed] [Google Scholar]
- 19.Lin, C. R., C. Kioussi, S. O'Connell, P. Briata, D. Szeto, F. Liu, J. C. Izpisua-Belmonte, and M. G. Rosenfeld. 1999. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401:279-282. [DOI] [PubMed] [Google Scholar]
- 20.Lu, M. F., C. Pressman, R. Dyer, R. L. Johnson, and J. F. Martin. 1999. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature 401:276-278. [DOI] [PubMed] [Google Scholar]
- 21.Ma, X., D. Yuan, K. Diepold, T. Scarborough, and J. Ma. 1996. The Drosophila morphogenetic protein Bicoid binds DNA cooperatively. Development 122:1195-1206. [DOI] [PubMed] [Google Scholar]
- 22.Mathias, J. R., H. Zhong, Y. Jin, and A. K. Vershon. 2001. Altering the DNA-binding specificity of the yeast Mat alpha 2 homeodomain protein. J. Biol. Chem. 276:32696-32703. [DOI] [PubMed] [Google Scholar]
- 23.Maurer, R. A., and A. C. Notides. 1987. Identification of an estrogen-responsive element from the 5′-flanking region of the rat prolactin gene. Mol. Cell. Biol. 7:4247-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phelan, M. L., I. Rambaldi, and M. S. Featherstone. 1995. Cooperative interactions between HOX and PBX proteins mediated by a conserved peptide motif. Mol. Cell. Biol. 15:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomerantz, J. L., T. M. Kristie, and P. A. Sharp. 1992. Recognition of the surface of a homeo domain protein. Genes Dev. 6:2047-2057. [DOI] [PubMed] [Google Scholar]
- 26.Priston, M., K. Kozlowski, D. Gill, K. Letwin, Y. Buys, A. V. Levin, M. A. Walter, and E. Heon. 2001. Functional analyses of two newly identified PITX2 mutants reveal a novel molecular mechanism for Axenfeld-Rieger syndrome. Hum. Mol. Genet. 10:1631-1638. [DOI] [PubMed] [Google Scholar]
- 27.Quentien, M. H., F. Pitoia, G. Gunz, M. P. Guillet, A. Enjalbert, and I. Pellegrini. 2002. Regulation of prolactin, GH, and Pit-1 gene expression in anterior pituitary by Pitx2: an approach using Pitx2 mutants. Endocrinology 143:2839-2851. [DOI] [PubMed] [Google Scholar]
- 28.Rieger, H. 1935. Dysgenesis mesodermalis coreneal et iridis. Z. Augenheilk 86:333. [Google Scholar]
- 29.Saadi, I., E. V. Semina, B. A. Amendt, D. J. Harris, K. P. Murphy, J. C. Murray, and A. F. Russo. 2001. Identification of a dominant negative homeodomain mutation in Rieger syndrome. J. Biol. Chem. 276:23034-23041. [DOI] [PubMed] [Google Scholar]
- 30.Semina, E. V., R. Reiter, N. J. Leysens, W. L. Alward, K. W. Small, N. A. Datson, J. Siegel-Bartelt, D. Bierke-Nelson, P. Bitoun, B. U. Zabel, J. C. Carey, and J. C. Murray. 1996. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat. Genet. 14:392-399. [DOI] [PubMed] [Google Scholar]
- 31.Szeto, D. P., A. K. Ryan, S. M. O'Connell, and M. G. Rosenfeld. 1996. P-OTX: a PIT-1-interacting homeodomain factor expressed during anterior pituitary gland development. Proc. Natl. Acad. Sci. USA 93:7706-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treisman, J., P. Gonczy, M. Vashishtha, E. Harris, and C. Desplan. 1989. A single amino acid can determine the DNA binding specificity of homeodomain proteins. Cell 59:553-562. [DOI] [PubMed] [Google Scholar]
- 33.Tverberg, L. A., and A. F. Russo. 1993. Regulation of the calcitonin/calcitonin gene-related peptide gene by cell-specific synergy between helix-loop-helix and octamer-binding transcription factors. J. Biol. Chem. 268:15965-15973. [PubMed] [Google Scholar]
- 34.Voss, J. W., L. Wilson, and M. G. Rosenfeld. 1991. POU-domain proteins Pit-1 and Oct-1 interact to form a heteromeric complex and can cooperate to induce expression of the prolactin promoter. Genes Dev. 5:1309-1320. [DOI] [PubMed] [Google Scholar]
- 35.Wilson, D., G. Sheng, T. Lecuit, N. Dostatni, and C. Desplan. 1993. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 7:2120-2134. [DOI] [PubMed] [Google Scholar]
- 36.Wolberger, C. 1996. Homeodomain interactions. Curr. Opin. Struct. Biol. 6:62-68. [DOI] [PubMed] [Google Scholar]
- 37.Yuan, D., X. Ma, and J. Ma. 1999. Recognition of multiple patterns of DNA sites by Drosophila homeodomain protein Bicoid. J. Biochem (Tokyo) 125:809-817. [DOI] [PubMed] [Google Scholar]
- 38.Yuan, D., X. Ma, and J. Ma. 1996. Sequences outside the homeodomain of bicoid are required for protein-protein interaction. J. Biol. Chem. 271:21660-21665. [PubMed] [Google Scholar]
- 39.Zhang, H., G. Hu, H. Wang, P. Sciavolino, N. Iler, M. M. Shen, and C. Abate-Shen. 1997. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol. Cell. Biol. 17:2920-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]