Abstract
In breast cancer cells, estrogens activate the Src/Erk pathway through an interaction of the estrogen receptor alpha (ERα) with the SH2 domain of c-Src. Progestins have been reported to activate also this pathway either via an interaction of the progesterone receptor isoform B (PRB) with ERα, which itself activates c-Src, or by direct interaction of PRB with the SH3 domain of c-Src. Here we identify two domains of PRB, ERID-I and -II, mediating a direct interaction with the ligand-binding domain of ERα. ERID-I and ERID-II flank a proline cluster responsible for binding of PRB to c-Src. In mammalian cells, the interaction of PRB with ERα and the progestin activation of the Src/Erk cascade are abolished by deletion of either ERID-I or ERID-II. These regions are not required for transactivation of a progesterone-responsive reporter gene. Mutations in the proline cluster of PRB that prevent a direct interaction with c-Src do not affect the strong activation of c-Src by progestins in the presence of ERα. Thus, in cells with ERα, ERID-I and ERID-II are necessary and sufficient for progestin activation of the endogenous Src/Erk pathway.
Steroid hormones influence a plethora of cellular functions, depending on the nature of the target cell and the constellation of signals impinging on the cell at a given time. To achieve the necessary coordination with other signaling pathways in the complex intracellular space, steroid hormones likely use a variety of mechanisms. Until very recently, attention has mainly been focused on the transcriptional effects of steroid hormones. These responses are mediated by the intracellular hormone receptors, which participate in multiple interactions with DNA, other sequence-specific transcription factors, transcriptional coregulators, and the general transcriptional machinery (3). In the last few years, a great effort has been devoted to understanding the nature of the transcriptional coregulators and how they mediate the interaction of the hormone receptors with chromatin remodelling complexes and the transcriptional apparatus (18). Considerable progress has been achieved, leading to the recognition of covalent and conformational chromatin changes as key steps in transcriptional regulation by steroid hormone receptors and other transcription factors (5).
In addition to their direct transcriptional effects, steroid hormones have been found to influence the activity of many other signaling pathways by so-called “nongenomic mechanisms” (25, 34, 38). These effects are mediated by interactions at the membrane or cytoplasmic level and offer a possibility for integration of the steroid hormone signals at the entry site of many other physiological signals acting via membrane receptors (39). Very often, these nongenomic effects have been attributed to poorly characterized receptors, whose relationship with the conventional nuclear receptors remains unclear (38). In the case of the ovarian hormones estrogens and progestins, cross talk with a number of other signaling pathways has been described, including cyclic AMP (1, 16), Ca-calmodulin (15), the G protein-coupled receptors (20), and the mitogen-activated protein (MAP) kinase pathway (25, 26). In breast cancer cells, estrogens stimulate cell proliferation, and this effect can be blocked by inhibitors of the MAP kinase signaling pathway (30) or by intracellular calcium chelators (22). This pathway is activated by estrogens through an interaction of the classical estrogen receptor alpha (ERα) with c-Src, which can be detected by coimmunoprecipitation (30). c-Src activity is enhanced 2 min after addition of 17β-estradiol, reaches a peak after 5 min, and returns to basal levels after 15 to 30 min. Activation of c-Src can be inhibited by steroidal and nonsteroidal antiestrogens and is followed by transient activation of Ras, Raf, and Erk1/2 (7, 31). The participation of the classical ERα in activation of the mitogenic pathway was demonstrated in gene transfection studies in COS-7 cells (31). Activation of the cascade in these cells by estrogens was strictly dependent on transfection of ERα, was inhibited by antiestrogens, and led to the formation of a complex of ERα and c-Src. Similar results have been obtained with androgens and the androgen receptor (AR) in LNCaP prostate cancer cells (29, 32). ERα interacts with the SH2 domain of c-Src, whereas AR interacts with the SH3 domain, and a ternary complex containing ERα, AR, and c-Src can be immunoprecipitated from LNCaP cells treated with estrogens and androgens (29).
Because progestins are also able to stimulate proliferation of breast cancer cells in culture (13), we tested whether the progesterone receptor (PR) could activate the mitogenic cascade in a similar fashion to ERα and AR. Following treatment of the breast cancer cell line T47D with either synthetic progestin R5020 or 17β-estradiol, there was a similar rapid and transient activation of the Src/Ras/Erk cascade (31). Unexpectedly, however, activation by progestins was inhibited not only by the antiprogestin RU486, but also by steroidal and nonsteroidal antiestrogens (31). In transfection experiments with COS-7 cells, activation of the MAP kinase cascade by progestins required expression not only of isoform B of the progesterone receptor (PRB) but also of ERα (31). Most intriguingly, both in T47D cells and in transfected COS-7 cells, PRB was precipitated with antibodies to ERα and vice versa, demonstrating the existence of an intracellular complex between the two hormone receptors (31). However, contrary to what was found for ERα and AR, antibodies to PRB did not precipitate c-Src, suggesting that the activation of the Src/Ras/Erk cascade by progestins was indirect and mediated by an interaction of PRB with the unliganded ERα, which itself activated c-Src.
Recently this view has been challenged by a study showing a direct interaction of a proline-rich region of PRB with c-Src, which leads to activation of the Src/Erk pathway (4). Although this interaction may be relevant for progesterone-dependent maturation of Xenopus oocytes, its physiological significance in breast cancer cells remains unclear. In COS-7 cells, the activation of the Src/Erk pathway by progestins was dependent on the presence of ER, and c-Src had to be cotransfected (4).
To clarify this issue and to investigate further the novel cross talk between progestins and estrogens, we have analyzed the interaction between PR and either ERα or c-Src in yeast two-hybrid assays, glutathione S-transferase (GST) pull-down experiments, and immunoprecipitation experiments with mammalian cells. Here we show that two domains of the N-terminal half of PRB (ERID-I and -II) interact with ERα directly and independently and are both required for the activation by progestins of the Src/Erk cascade in COS-7 cells cotransfected with PR and ERα. We confirm the existence in PRB of a proline-rich region, located between ERID-I and -II, which directly interacts with c-Src in vitro. However, this region was responsible for only a very weak activation of the endogenous Src without activation of Erk and could be deleted without influencing the strong activation of the Src/Erk pathway seen in the presence of ERα. Moreover, ERID-I and -II are not relevant for the transcriptional activation of a progesterone-responsive reporter gene. These findings open the way for a dissociation of the progesterone signaling by cross talk with the MAP kinase pathways and by transcriptional mechanism, which will allow the identification of the gene networks regulated by these different pathways.
MATERIALS AND METHODS
Plasmids.
The yeast vectors pGADT7 and pGBKT7, encoding the Gal4 activation domain (Gal4AD) and the DNA binding domain (Gal4DBD), respectively, were from Clontech. To generate the pGADT7:PRB plasmid (encoding the Gal4AD-PRB fusion protein), the human PRB cDNA obtained from pBK-PR (19) was inserted into pGADT7, downstream and in frame with the Gal4AD. Vectors encoding fusion proteins of Gal4AD plus PRA or different PR deletion mutants were constructed by inserting the cDNA fragments encoding the corresponding amino acids of PR into the pGADT7 plasmid, downstream of Gal4AD. The cDNAs of the various PR fragments were obtained by restriction of full-length hPRB cDNA. pGADT7:PRmPro (encoding the Gal4AD-PRmPro fusion protein) was constructed by inserting the PRmPro cDNA obtained from pSG5:PRmPro (described below) into pGADT7.
pGBKT7:ERα (encoding Gal4DBD-ERα) was constructed by inserting the human ERα cDNA obtained from pSG5-ERα into pGBKT7, downstream of Gal4DBD. Plasmids encoding fusion proteins of Gal4DBD plus different ERα deletion mutants were generated by inserting cDNA fragments of the corresponding amino acids of ERα into the pGBKT7 plasmid, downstream and in frame with Gal4DBD. The ER fragment cDNAs were obtained by enzymatic restriction or PCR amplification from the full-length ERα cDNA by standard procedures. To generate pGBKT7:HEG537F, the EcoRI fragment excised from pSG5-HEG537 (9) was cloned into the EcoRI site of pGBKT7.
pGADT7:Gal4AD:PR ERID-II was constructed from pGADT7:PR 456-546 by inserting a new Gal4AD sequence after the T7 RNA polymerase promoter, upstream and in frame with PR 456-546 (ERID-II). The resulting plasmid expresses a Gal4AD-PR ERID-II fusion protein from the T7 promoter. The Gal4AD sequence was generated by PCR amplification with the appropriate plasmids. To generate pGADT7:Gal4AD:PR ERID-I (expressing a Gal4AD-PR ERID-I fusion protein from the T7 promoter), PR ERID-II sequence of pGADT7:Gal4AD:PR ERID-II was removed and replaced by the PR ERID-I fragment obtained from pGADT7:PR ERID-I. To obtain pGADT7:Gal4AD (expressing Gal4AD from the T7 promoter), the PR ERID-II sequence was removed from pGADT7:GAl4AD:PR ERID-II, and the plasmid was religated.
pGEX-2TK:ERαLBD (encoding GST-ERαLBD fusion protein) was obtained by inserting the ERαLBD cDNA derived from pGBKT7:ERαLBD into pGEX-2TK (Amersham Pharmacia Biotech). pGEX-2TK:SrcSH3 (GST-Src SH3) was a generous gift of Gulio Superti-Furga.
pSG5-ERα (HEG0) and pSG5-PRB were kind gifts of Pierre Chambon and have been described previously (23, 36). pSG5-PR ΔERID-I, pSG5-PR ΔERID-II, pSG5-PR ΔERID-I+II, and pSG5-PRΔPro were constructed by subcloning the inserts of the corresponding pGADT7:PR mutants into the pSG5 vector (Stratagene). pSG5-PRmPro corresponds to the pSG5 plasmid carrying a PR mutant in which three prolines (positions 422, 423, and 426) were replaced by alanines (4). PRmPro was generated by PCR-mediated site-directed mutagenesis (2) with two subsequent PCR amplifications. All constructs were verified by sequencing.
The pAGEMMTVLu (MMTV-Luc) plasmid, carrying the mouse mammary tumor virus (MMTV) promoter linked to a luciferase (Luc) reporter gene, was described previously (24).
Cells.
T47D human breast cancer cells were grown in RPMI 1640 medium supplement with 10% fetal bovine serum, 2 mM L-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and insulin (0.2 U/ml). COS-7 cells were grown routinely in Dulbecco's modified Eagle medium supplemented with 5% fetal bovine serum, 1 mM L-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Coimmunoprecipitation of ERα and PR.
Cell lysates were prepared as described previously (30) and incubated overnight at 4°C with 0.5 μg of anti-ERα per ml (Santa Cruz sc543), 1 μg of anti-PR per ml (Stressgene SR-1110), or 1 μg of nonspecific rabbit immunoglobulin G (IgG) per ml. Protein A/G Plus agarose (Santa Cruz) was added, and the mixture was incubated for an additional hour at 4°C. At the end of the incubation, samples were centrifuged, supernatants (SNs) were collected, and pellets were washed four times with lysis buffer. The immunoprecipitated proteins (IPs) were eluted from the beads by being boiled in sodium dodecyl sulfate (SDS) sample buffer. Cell lysate (input), SNs, and IPs were probed by Western blots with either anti-PR or anti-ERα antibodies. The band intensities (integrated density [ID]) were measured by NIH Image 1.61 software. The ID of each band was corrected for the total volume of the fraction. The proportion of ERα associated with PRB was estimated from the samples immunoprecipitated with the anti-PR antibody by calculating the ratio ER in the IP fraction/(ER in the IP fraction + ER in the SN fraction). The proportion of PRB associated with ERα was estimated from the samples immunoprecipitated with the anti-ERα antibody, by calculating the ratio PRB in the IP fraction/(PRB in the IP fraction + PRB in the SN fraction).
Yeast two-hybrid assay.
For the yeast two-hybrid assay, we used the Matchmaker yeast two-hybrid system 3 (Clontech). The yeast strain AH109, which contained the ADE2, HIS3, and lacZ reporters under the control of three different Gal4-responsive promoters, was cotransformed with pGADT7:PR (PRB, PRA, or the different mutants) and pGBKT7:ERα (ERα or the mutants). Alternatively, empty pGADT7 and pGBKT7 plasmids were used for negative controls. Two individual clones were isolated on SD medium deficient in leucine and tryptophan (SD/−leu/−trp), expanded, and used for further analysis of the ER-PR interaction. Cotransformants were selected for their ability to grow in medium lacking histidine and adenine. 17β-Estradiol was added to the plates at a final concentration of 1 μM.
GST pull-down assay.
35S-labeled human PRB and different mutants of PR were generated by using the TNT coupled rabbit reticulocyte lysate system (Promega), according to the manufacturer's protocol. cDNAs encoding human PRB (wild type and mutants) cloned into pGADT7 vectors were used as templates. Expression of fusion proteins in Escherichia coli strain BL21 was induced with 0.1 mM isopropyl-β-thiogalactopyranoside (IPTG) for 6 h at 20°C, and bacteria were harvested. For the generation of GST-SrcSH3 and GST proteins, lysates were prepared as described previously (28). The GST-ERαLBD fusion protein (and GST protein as control) was recovered as previously described (17), with minor modifications. GST and GST fusion proteins were purified from the lysates on glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) by incubating 5 ml of cellular lysates (derived from 100 ml of culture) with 133 μl of beads (which had been previously washed three times and resuspended in 100 μl of Tris-buffered saline [TBS]) on a rotating wheel at 4°C for 30 min. The beads were then washed twice with TBS (containing 1 mM dithiothreitol [DTT], 1 mg of bovine serum albumin per ml, and protease inhibitors) and once with binding buffer (25 mM HEPES [pH 7.5], 12.5 mM MgCl2, 150 mM KCl, 20% glycerol, 0.1% NP40 plus 1 mM DTT, 1 mg of BSA per ml, protease inhibitors). After resuspension of the beads in binding buffer, an appropriate amount of 35S-labeled PRB (wild type or mutants) translation products was added, as well as, when required, 1 μM hormone ligand. The beads were incubated on a rotating wheel at 4°C for 1 h and washed three times with binding buffer. The bound proteins were eluted from the beads by boiling in SDS loading buffer, separated by SDS-polyacrylamide gel electrophoresis (PAGE), and visualized by fluorography.
Transient transfection of COS-7 cells.
COS-7 cells were transfected with the Lipofectamine Plus reagent (Life Technologies), according to the manufacturer's instructions. For immunoprecipitation experiments and Src or Erk activity assays, the expression vectors for ERα and PRB, pSG5-ERα (36) and pSG5-PR (23), respectively, were cotransfected. Alternatively, pSG5-PR was replaced for different PR mutants as indicated in each experiment. For luciferase assays, the MMTV-Luc reporter plasmid (37) carrying the MMTV promoter linked to the firefly luciferase gene was cotransfected together with ERα and PRB (wild type or mutants) expression vectors. pRSV-βGal was also included in each transfection to normalize transfection efficiency.
Erk immunoprecipitation and activity assay.
The Erk immunoprecipitation and activity assay was performed as previously described (30). Briefly, cell lysates were prepared and incubated overnight with rabbit polyclonal anti-Erk antibodies (Santa Cruz). Protein A/G Plus agarose (Santa Cruz) was added, and the mixture was incubated for additional 45 min. After washing, the samples were centrifuged, and the pellets were assayed for myelin basic protein (MBP) phosphorylation with [γ-32P]ATP. Reactions were stopped by addition of 2× SDS sample buffer and analyzed by SDS-PAGE followed by autoradiography.
Src immunoprecipitation and activity assay.
Cell lysates (1 mg of protein per ml) were immunoprecipitated with anti-Src mouse monoclonal antibodies (clone 327 from Calbiochem) as previously described (31). Immunoprecipitates were assayed for Src activity with acidified enolase as a substrate in the presence of [γ-32P]ATP. The reaction was carried out for 15 min at 30°C and stopped by addition of 2× SDS sample buffer. Enolase phosphorylation was analyzed by SDS-PAGE followed by autoradiography.
Luciferase assay.
Cells were treated with 10 nM R5020 or ethanol during 24 h. Cell lysates were prepared and luciferase and β-galactosidase activities were determined with assay kits from Promega according to the manufacturer's instructions. To correct for differences in transfection efficiencies, luciferase units were normalized for β-galactosidase activities in the same cell lysate.
RESULTS
A fraction of PRB interacts with ERα in vivo.
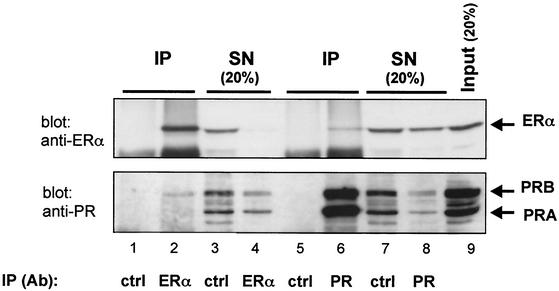
We have previously reported a ligand-independent interaction between PRB and ERα in T47D cells and in COS-7 cells transfected with both receptors (31). In order to estimate the proportion of ERα and PRB participating in this complex, we have performed coimmunoprecipitation experiments with T47D cells. Cell lysates were prepared and immunoprecipitated separately with anti-ERα and anti-PR antibodies. The SNs were collected for further analyis, and the IPs were eluted from the beads in Laemmli sample buffer. Cell lysate (input), SNs, and IPs were probed by Western blots with either anti-PR or anti-ERα antibodies The band intensities were measured, and the percentage of associated proteins was calculated as described in Materials and Methods. The results presented in Fig. 1 show that 6% of the total PRB present in the cells is bound to ERα, and 5% of the total ERα is associated with PRB. Thus, at a given time, only a small fraction of total cellular progesterone receptor population is part of a complex including the ER and vice versa.
FIG. 1.
Interaction between ERα and PRB in T47D cells. Cell lysates were immunoprecipitated with anti-ERα or anti-PR antibodies or nonspecific IgG (control [ctrl]). SNs were collected, and IPs were removed from the beads in SDS sample buffer. IPs (lanes 1, 2, 5, and 6), SNs (lanes 3, 4, 7, and 8), and cell lysate (input) (lane 9) were analyzed by Western blotting with anti-ERα (upper panel) and anti-PR (lower panel) antibodies. ERα and PR bands are indicated by arrows. Lanes 3, 4, 7, and 8 correspond to 20% of the amount of proteins present in the supernatants. Lane 9 represents 20% of the amount of lysate used in the immunoprecipitation.
Defining the interaction between PRB and ERα in yeast.
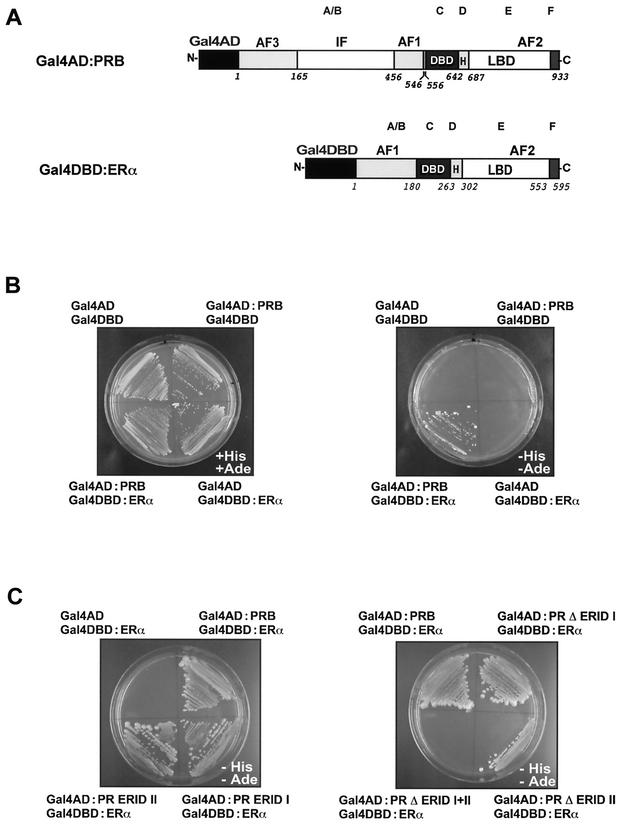
To investigate this PRB-ERα interaction in more detail, we used a yeast two-hybrid assay. ERα was fused to the C-terminal end of the DBD of Gal4 to yield Gal4DBD-ERα, and PRB was fused to the C-terminal end of the activation domain of Gal4 to yield the Gal4AD-PRB fusion protein (Fig. 2 A). The yeast reporter strain AH109 was transformed with these expression vectors or with the corresponding empty plasmid pGADT7 (Gal4AD) or pGBKT7 (Gal4DBD) as a negative control. Cotransformed colonies were streaked onto medium with or without histidine and adenine. In this assay, the formation of a complex between Gal4DBD-ERα and Gal4AD-PRB confers histidine and adenine auxotrophy (see Materials and Methods). All cotransformants grew similarly in nonselective medium (Fig. 2B, left plate). When plated on selective medium (in the absence of histidine and adenine), only yeast cells expressing Gal4DBD-ERα and Gal4AD-PRB grew (Fig. 2B, right plate). This interaction was observed only when yeast cells were grown in the presence of estradiol. Yeast cotransformed with one or both empty plasmids did not grow in the selective medium, indicating that histidine-adenine auxotrophy is the result of the interaction between ERα and PRB. Similar results were observed when the auxotrophies (histidine or adenine) were checked separately (data not shown).
FIG. 2.
Interaction between PRB and ERα in yeast. (A) Schematic representation of the fusion proteins used in this study. PRB was fused to the AD of Gal4 (Gal4AD) and ERα was fused to the DBD of Gal4 (Gal4DBD) to yield Gal4AD-PRB and Gal4DBD-ERα, respectively. The domain structure of human PRB and ERα is indicated. The letters at the top refer to the domain designation accepted for all nuclear receptors. The activation functions (AF1, AF2, and AF3), the DBD, the hinge region (H), and the LBD are indicated. An inhibitory domain in the N-terminalhalf of PR is also indicated (IF). The numbers refer to the amino acid residues. (B) PRB interacts with ERα in yeast. The yeast reporter strain AH109 was cotransformed with pGADT7 (Gal4AD), pGBKT7 (Gal4DBD), Gal4AD-PRB, and Gal4DBD-ERα plasmids in the indicated four combinations. Cotransformants were isolated on SD medium deficient in leucine and tryptophan (SD/−leu/−trp). A cotransformant colony from each plate was expanded and streaked on SD/−leu/−trp (nonselective medium, left plate) or on the same medium lacking histidine and adenine (selective medium, right plate). 17β-Estradiol was added to the plates at a final concentration of 1 μM. (C) The ERID-I and ERID-II regions of PRB mediate the interaction with ERα. The yeast strain AH109 was cotransformed with Gal4DBD-ERα plus the indicated fusions of Gal4AD with wild-type and mutant PRs. The empty Gal4AD plasmid was used in a control experiment. Selection was performed as described in panel B. 17β-Estradiol (1 μM) was added to the plates.
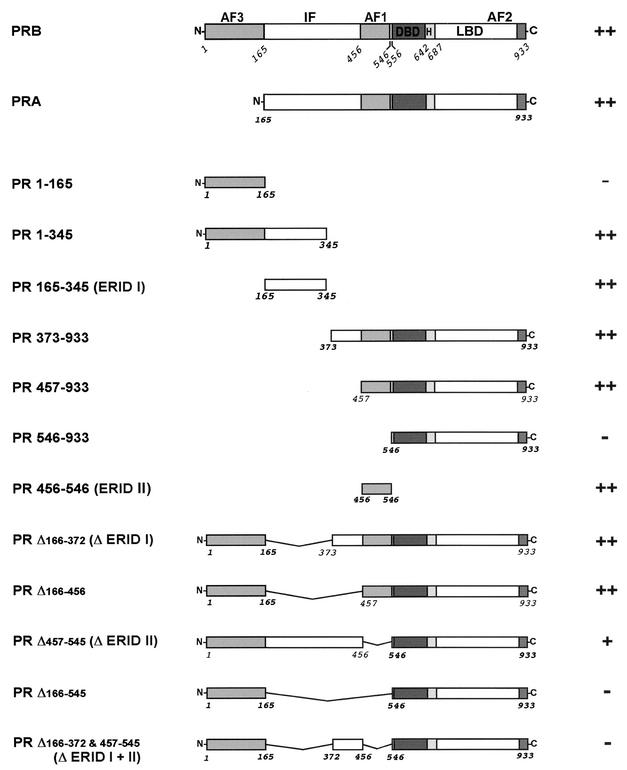
In order to define the domains of PRB involved in the interaction with ERα, we generated a series of PR deletion mutants as fusion proteins with Gal4AD and tested them with the yeast two-hybrid assay (Fig. 3). All mutants were expressed at similar levels in yeast, as was demonstrated by Western blotting (data not shown). Two PR domains were identified as interacting independently with ERα (Fig. 2C and 3): a domain called ERID-I (for ER-interacting domain I), extending from amino acids 165 to 345; and ERID-II, comprising amino acids 456 to 546. Each receptor construct containing one of these two domains interacted with ERα, and the simultaneous deletion of both domains completely abolished the interaction. None of the Gal4AD-PR mutants promoted growth when cotransformed with the empty Gal4DBD plasmid (data not shown).
FIG. 3.
Involvement of PRB domains in the interaction with ERα in yeast. The wild-type forms of human PRB and PRA as well as the various deletion constructs are shown schematically. The interaction (++ or −) between ERα and different regions of PR was examined with the yeast two-hybrid assay. The yeast strain AH109 was cotransformed with Gal4DBD-ERα plus the different Gal4AD-PR constructs (wild-type PR and mutants), and selection was performed as described in the legend to Fig. 2B.
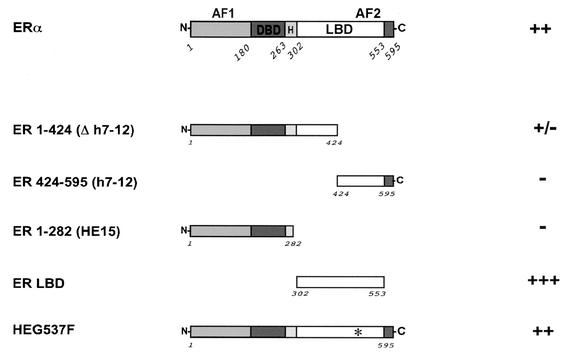
To investigate the region of ERα necessary for the interaction with PRB, several ERα mutants were fused to Gal4DBD and tested in the yeast two-hybrid assay (Fig. 4). We found that the ERαLBD alone mediated the interaction with PRB; ERα mutants in which the LBD was absent or interrupted could not interact with PRB. The mutation of Tyr 537 (HEG537F) (9) had no effect on the interaction. We also tested all PR mutants with the ERαLBD and found that the interactions were similar if not identical to those observed with the complete ERα (data not shown). In particular, both ERID-I and ERID-II interacted independently with the ERαLBD. Thus, the ERαLBD is necessary and sufficient to mediate the interaction with PRB.
FIG. 4.
Involvement of ERα domains in the interaction with PRB in yeast. Wild-type ERα and the various deletion constructs are shown schematically. The interaction (++, +++, or −) between PRB and different regions of ERα was examined with the yeast two-hybrid assay.
ERαLBD interacts with PRB in vitro.
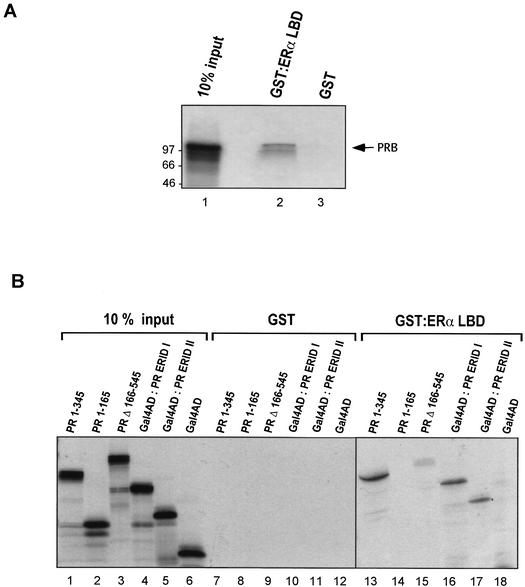
Since the ERαLBD was sufficient for the interaction with PRB in yeast, we concentrated our characterization of the interaction on this region of ERα. To determine if this interaction is direct, we used GST pull-down experiments. GST and the GST-ERαLBD fusion protein were expressed in E. coli BL21, immobilized onto glutathione-Sepharose, and incubated with 35S-labeled PRB produced by in vitro transcription and translation in a rabbit reticulocyte lysate. The bound material was analyzed by SDS-PAGE and autoradiography. The ERαLBD expressed in E. coli was functional, as demonstrated by its estrogen-dependent interaction with the coactivator RIP140 (10) in a pull-down assay (data not shown). The wild-type PRB was specifically retained by GST-ERαLBD, whereas binding to the matrix containing GST alone was negligible (Fig. 5 A). We conclude that the interaction between the two hormone receptors is direct and does not depend on additional proteins present in the yeast cells. This in vitro interaction between ERαLBD and PRB occurred in the absence of hormones and was not affected by the addition of estradiol or progesterone (data not shown).
FIG. 5.
Interaction between PRB and ERα in vitro. GST and the GST-ERαLBD fusion protein were expressed in E. coli BL21, bound to glutathione-Sepharose, and incubated with 35S-labeled PRB (wild type or mutants) produced by in vitro transcription and translation in a rabbit reticulocyte lysate. After extensive washing, retained proteins were eluted with SDS loading buffer, separated by SDS-PAGE, and visualized by fluorography. (A) The wild-type PRB interacts with the LBD of ERα in vitro. Lane 1 represents 10% of the amount of 35S-labeled PRB used in the binding assay. The amount of PRB retained by GST-ERαLBD is shown in lane 2. Addition of 1 μM 17β-estradiol, 1 μM R5020, or both together did not alter the amount of PRB retained (data not shown). GST alone did not retain any PRB (lane 3). The positions of molecular mass markers are indicated to the left in kilodaltons. (B) Interaction between mutants of PRB and GST-ERαLBD. The input lanes (lanes 1 to 6) represent 10% of the amount of 35S-labeled PR mutants used in the binding assay. PRB mutants were tested for binding to GST (lanes 7 to 12) and to GST-ERαLBD (lanes 13 to 18).
We next tested whether the individual regions of PRB identified in the yeast two-hybrid assays, ERID-I and ERID-II, are capable of interacting with ERαLBD in vitro. Figure 5B shows the results of the pull-down assay with the recombinant PR mutants transcribed and translated in vitro. None of the proteins was retained by the matrix containing GST alone (Fig. 5B, lanes 7 to 12), whereas the N-terminal fragment PR 1-345 containing ERID-I showed specific interaction with ERαLBD (Fig. 5B, lane 13). Two subfragments of this construct (PR 1-165 and PR 165-345) were independently evaluated. As observed in the two-hybrid assay (Fig. 3), PR 1-165 displayed no interaction with ERαLBD in vitro (Fig. 5B, lane 14). The PR 165-345 fragment corresponding to ERID-I could not be tested, since it was not expressed in sufficient amounts in the reticulocyte lysate system. However, construction with the Gal4AD fused upstream of PR 165-345, yielded a well-expressed fusion protein, Gal4AD-PR ERID-I, which specifically interacted with GST-ERαLBD in the pull-down assay (Fig. 5B, lane 16). As a control, no binding of Gal4AD alone to GST-ERαLBD was observed (lane 18). As with the ERID-I fragment, the PR fragment corresponding to ERID-II was not properly expressed in vitro. Therefore, we again fused the N-terminal end of ERID-II to the C-terminal end of Gal4AD and obtained efficient expression in the reticulocyte lysate system. The fusion protein Gal4AD-PR ERID-II was specifically retained by the GST-ERα matrix (lane 17). Finally, a PR construct from which both ERID-I and ERID-II were deleted (PR Δ166-545) exhibited only a faint interaction with ERαLBD (lane 15). We conclude that the two interacting regions identified in the yeast two-hybrid assays directly interact with the ERαLBD in vitro.
PRB interacts with c-Src via a proline cluster region in vitro.
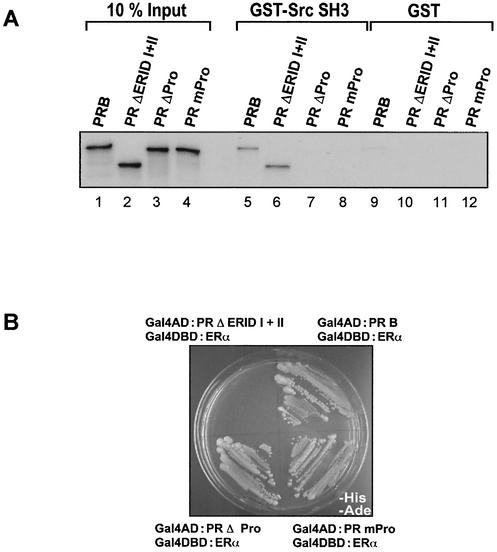
In a previous report, we did not find an association of PRB with Src by coimmunoprecipitation experiments with T47D and COS-7 cells cotransfected with ERα and PRB, independent of the hormone treatment (31). However, a direct interaction of PR with Src was recently reported (4). Since the coimmunoprecipitation could be not sensitive enough to detect this association, the possibility of a direct interaction between PRB and c-Src was tested in vitro. We focused our study on the N-terminal half of PRB, which has been shown to interact with the SH3 domain of c-Src (4). We confirmed the existence of an interaction between c-Src and PR by a yeast two-hybrid assay (data not shown) and detected a direct interaction of PR with the SH3 domain of c-Src in GST pull-down experiments (Fig. 6 A, lane 5). Neither ERID-I nor ERID-II is involved in this interaction (lane 6). However, deletion of the proline cluster region (amino acids 396 to 456) located between ERID-I and ERID-II (PRΔPro) eliminated the interaction of PRB with the SH3 domain of c-Src (lane 7). Point mutations of a cluster of three prolines in this region, PRmPro, were sufficient to disrupt the interaction with c-Src (lane 8) as previously reported (4). Contrary to the deletion of the ERID-I and -II regions (PR ΔERID-I+II), neither the deletion of the proline cluster region (PRΔPro) nor the point mutation of the three proline residues (PRmPro) had any influence on the interaction of PRB with ERα (Fig. 6B). Therefore, the N-terminal half of PRB contains two regions for interaction with ERα and an independent region for interaction with c-Src.
FIG. 6.
Direct interaction of PRB and the c-Src SH3 domain. (A) In vitro-translated 35S-labeled PRs (wild type and mutants) were incubated with GST or GST-SrcSH3 fusion protein immobilized on glutathione-Sepharose. Bound proteins were analyzed by SDS-PAGE and fluorography. (B) Yeast two-hybrid assays with wild-type or mutant PRB and ERα. The yeast strain AH109 was cotransformed with Gal4DBD-ERα plus the indicated fusions of Gal4AD with PR (wild type and mutants). Selection was performed as described in the legend to Fig. 2B.
The two domains of PRB, ERID-I and -II, are needed for interaction with ERα in vivo.
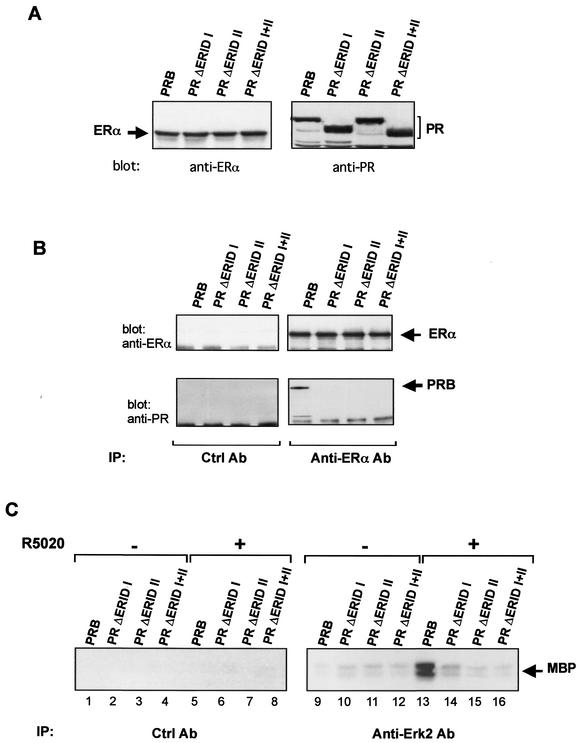
To verify whether the regions of PRB that interact with ERα in yeast and in vitro also mediate the interaction between both receptors in mammalian cells, coimmunoprecipitation experiments were carried out with transfected COS-7 cells. Wild-type PRB and PR mutants carrying deletions of ERID-I, ERID-II, or both regions were transfected in COS-7 cells together with ERα. Cell lysates were prepared, immunoprecipitated with anti-ERα antibody, and blotted with anti-ERα and anti-PRB antibodies. ERα, PRB, and the different PR mutants were expressed at similar levels in COS-7 cells (Fig. 7 A). Only the wild-type PRB coimmunoprecipitated with ERα, and no association was observed for PR ΔERID-I, PR ΔERID-II, and PR ΔERID-I+II (Fig. 7B). These results confirm that the interaction between PRB and ERα in mammalian cells is mediated through the domains ERID-I and ERID-II. However, whereas one of these domains alone is sufficient for interaction with ERα in yeast and in vitro, both domains are required and have to act together in mammalian cells.
FIG. 7.
Interaction between ERα and PRB in COS-7 cells. COS-7 cells were transiently cotransfected with ERα and various PR expression vectors (PRB wild type, PR ΔERID-I, PR ΔERID-II, or PR ΔERID-I+II). (A) The expression of ERα and the different PR constructs was verified by immunoblotting of total cell lysates with anti-ERα or anti-PR antibodies. (B) Coimmunoprecipitation of ERα and PR. Cell lysates were immunoprecipitated with anti-ERα antibody or nonspecific IgG (control antibody [Ctrl Ab]). Each immunoprecipitate was analyzed with anti-ERα or anti-PR antibodies. ERα and PRB bands are indicated by arrows. (C) Effect of progestins on Erk activity. Cells were treated for 5 min with 10 nM R5020 (lanes 5 to 8 and 13 to 16) or with the ethanol (lanes 1 to 4 and 9 to 12). Cell lysates were prepared, immunoprecipitated with anti-Erk1/2 antibodies, and assayed for MAP kinase activity with MBP as a substrate as described previously (30). The left panel (lanes 1 to 8) shows the results obtained with preimmune serum as a control.
Both ERα-interacting domains of PRB are required for activation of the MAP kinase cascade by progestins.
To investigate the functional significance of the ER-interacting regions, we next tested the various deletion mutants of PRB for their ability to activate the MAP kinase pathway in response to added progestins. To this end, COS-7 cells were cotransfected with ERα-expressing plasmids along with vectors expressing wild-type or mutant PRB. Forty-eight hours after transfection, the cells were treated with the synthetic progestin R5020, and cell extracts were prepared 5 min thereafter. The Erk activity in the extracts was determined by immunoprecipitation with anti-Erk antibodies followed by a kinase assay with MBP as a substrate. The results clearly show that whereas the wild-type PRB activated MAP kinase as previously reported (31), neither the individual deletion mutants PR ΔERID-I and PR ΔERID-II nor the double mutant PR ΔERID-I+II showed activity in this assay (Fig. 7C). It should be pointed out that, although these mutants contain the intact proline cluster region and their ability to interact with Src is not affected (Fig. 6A), none of them mediates activation of Erk. Thus, like in the immunoprecipitation assay, both ER-interacting regions of PRB are required for activation of the MAP kinase pathway in response to progestins.
The interaction of PRB with c-Src is not essential for progestin activation of the endogenous Src/Erk cascade in the presence of ERα.
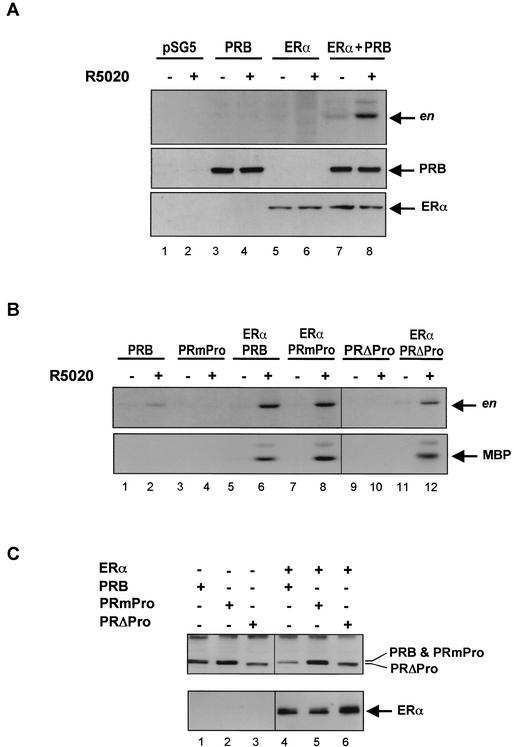
Next we tested whether the direct interaction between PR and c-Src contributes to the activation of the Src/Erk signaling pathway in response to progestins as previously proposed (4). COS-7 cells were cotransfected with ERα alone or together with PRB-expressing plasmids and treated for 5 min with R5020. Lysates were prepared and immunoprecipitated with anti-Src or anti-Erk antibodies. Src or Erk activity was assayed with acidified enolase or MBP as the substrates, respectively. In COS-7 cells transfected with PR, treatment with progestins led to nondetectable (Fig. 8 A, upper panel, lanes 3 and 4) or weak (Fig. 8B, upper panel, lanes 1 and 2) activation of c-Src, and did not mediate activation of Erk1/2 (Fig. 8B, lower panel, lanes 1 and 2). This effect on the c-Src activity was eliminated by deletion of the proline cluster region of PR (Fig. 8B, upper panel, lanes 9 and 10) or by point mutation of the three relevant proline residues (Fig. 8B, upper panel, lanes 3 and 4). Therefore, this weak and hardly detectable effect on the endogenous Src is mediated by the proline cluster region of PRB, which interacts with the SH3 domain of c-Src in yeast and in vitro. Deletion of ERID-I, ERID-II, or both domains had no effect on the activation of Src in the absence of ERα (data not shown). We confirm previous results (4) showing that a more significant activation can be observed when COS-7 cells are cotransfected with an expression vector for c-Src (data not shown), but the physiological significance of this interaction with overexpressed c-Src remains questionable.
FIG. 8.
Effect of progestins on Src and Erk activity in COS-7 cells transfected with PR (wild type and mutants) and ERα expression vectors. (A) COS-7 cells were transfected with pSG5 control plasmid (lanes 1 and 2), wild-type PRB (lanes 3 and 4), ERα (lanes 5 and 6), or both PRB and ERα (lanes 7 and 8) expression vectors and treated for 5 min with 10 nM R5020 or with ethanol. Cell lysates were immunoprecipitated with anti-Src antibodies. The immunoprecipitates were assayed for Src activity with acidified enolase (en) as a substrate (upper panel). The expression of PRB and ERα was verified by immunoblotting of total cell lysates with anti-PR (middle panel) or anti-ERα (lower panel) antibodies. (B) COS-7 cells were transfected with PRB (wild type or mutant PRmPro or PRΔPro) expression vectors alone or together with ERα cDNA. Cells were treated for 5 min with 10 nM R5020 or with ethanol. Lysates were prepared, divided into two aliquots, and immunoprecipitated with anti-Src or anti-Erk antibodies. The immunoprecipitates were assayed for Src and Erk activity with acidified enolase (en) or MBP as substrates, respectively. (C) Expression of PRB (wild type and mutants) and ERα was verified by immunoblotting of total cell lysates with anti-PR (upper panel) or anti-ERα (lower panel) antibodies.
A much more pronounced progestin activation of endogenous c-Src was observed in COS-7 cells cotransfected with expression vectors for PRB and ERα (Fig. 8A, upper panel, lanes 7 and 8, and B, upper panel, lanes 5 and 6). Cells transfected only with the expression vector for ERα did not respond to progestins in terms of c-Src activation (Fig. 8A upper panel, lanes 5 and 6). Progestins also mediated a strong activation of Erk in COS-7 cells coexpressing PRB and ERα (Fig. 8B, lower panel, lanes 5 and 6). These strong effects of progestins on endogenous c-Src and Erk1/2 were not affected by deletion of the proline cluster region of PR (Fig. 8B, lanes 11 and 12) nor by point mutation of the three relevant proline residues (Fig. 8B, lanes 7 and 8). We have shown above that the deletion of ERID-I or ERID-II eliminates the effect of progestins on Erk1/2 activation in the presence of ERα (Fig. 7C). The different activities of the various PR constructions were not due to differences in expression levels of the corresponding proteins, as demonstrated by Western blotting (Fig. 8C). We conclude that the relevant pathway leading to activation of the Src/Erk signaling pathway in cells equipped with PRB and ERα is independent of the interaction between the proline cluster region of PR and the SH3 domain of c-Src and requires an interaction of PRB with ERα.
Neither ERID-I nor ERID-II is necessary for transactivation of a progesterone reporter gene.
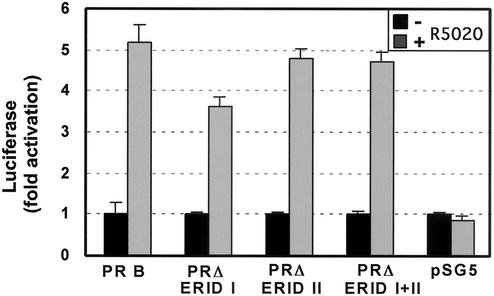
Finally, we wanted to know whether the two ERα-interacting regions of PRB play a role in transactivation of progesterone-responsive reporter genes. In order to compare these results with those from the MAP kinase activation assays, we used COS-7 cells and cotransfected an expression vector for ERα and PRB or PR mutants. As reporter gene, we used the hormone-responsive region and the promoter of the MMTV fused to the luciferase gene, which is known to respond to progesterone in various cell lines (6, 12). After treatment with R5020, we observed a fivefold induction of luciferase activity in cells expressing wild-type PRB (Fig. 9). All of the PR deletion mutants tested were also active in this assay, although the values obtained with PR ΔERID-I were slightly lower than those found with the wild-type receptor. Therefore, neither ERID-I nor ERID-II of PRB is essential for transcriptional activation of reporter genes in response to progestins. These results demonstrate that the mutant PR proteins are functional and that the activation of the c-Src/Erk pathway is independent of transcriptional effects of PRB.
FIG. 9.
Transactivation of an MMTV-Luc reporter gene by progestins in COS-7 cells. COS-7 cells were transiently cotransfected with ERα and PR (PRB wild type, PR ΔERID-I, PR ΔERID-II, or PR ΔERID I+II) expression vectors together with a reporter gene containing the MMTV promoter linked to the luciferase gene (37). Cells were incubated with (shaded bars) or without (black bars) 10 nM R5020 for 24 h, and the luciferase activity was determined in whole-cell extracts (37). The average and standard deviation of two experiments performed in duplicate are shown.
DISCUSSION
Identification of ERID-I and ERID-II and their role in progestin activation of the Src/Erk cascade.
In previous work, we have identified an interaction between PRB and ERα in breast cancer cell lines, which appeared to be important for activation of the Src/Ras/Erk signaling pathway and for cell proliferation in response to progestins (31). The main evidence derives from the inhibitory effect of antiestrogens on progestin activation of the Src/Erk pathway in breast cancer cells (31). In addition, we have shown that a point mutant of ER that contains a phenylalanine instead of a tyrosine at position 537 (HEGY537F) cannot activate the Src/Erk pathway in COS-7 cells in response to estrogen and cannot mediate progestin activation of this pathway in cells coexpressing PRB (29). These previous results underline the importance of the cross talk between PRB and ER for the effects of progestins on the Src/Ras/Erk signaling cascade. Here we show that approximately 5% of the total ERα and 6% of the PRB present in the cells are involved in this interaction in the absence of hormones. We present evidence of a direct association between both hormone receptors in GST pull-down experiments, which again does not require the presence of hormones. The requirement for estrogen in the yeast two-hybrid assay might be due to the need for the Gal4DBD-ERα fusion protein to translocate into the nucleus.
We have characterized the relevant protein surfaces for this PRB-ERα interaction by using yeast two-hybrid and GST pull-down assays. In both assays, PRB interacts with ERα through two independent domains located in the N-terminal half of PRB, ERID-I, and ERID-II. Each of these domains is sufficient to mediate an interaction with the LBD of ERα in yeast or in vitro. In mammalian cells, both ERID-I and ERID-II are required for the interaction of PRB with ERα and for efficient activation of the Src/Erk cascade. Deletion of one of the two domains is sufficient to disrupt the interaction with ERα, as demonstrated by coimmunoprecipitation, and almost completely eliminates progestin activation of c-Src or Erk. The reason for the requirement of both domains in vivo (as opposed to in vitro) remains to be established.
In addition to PRB, most progesterone-responsive cells contain a shorter isoform of PR, PRA, which lacks the first 164 amino acids containing the activation function 3 (AF3; Fig. 3). In transfected mammalian cells, PRA does not activate the Src/Erk cascade in response to progestins and does not interact with ERα (31), although it contains ERID-I and ERID-II and interacts with ERα in yeast. This suggests that in the context of PRA, ERID-I, ERID-II, or both are masked in mammalian cells. Masking could result from intramolecular interactions or from interactions of PRA with other cellular proteins. There are indications for the existence of an inhibitory region in PR, called IF, which prevents the activity of the activation function AF1 in the context of PRA (21). Because ERID-II coincides with AF1, it is possible that IF also masks access to ERID-II and in this way prevents the interaction of PRA with ERα in cells. It is also possible that the two isoforms of PR exhibit different intracellular distributions and that PRA does not reach the cytoplasmic side of the cell membrane, where PRB interacts with ERα. The observation that PRA is preferentially nuclear would favor this interpretation (27). Alternatively, differences in the pattern of phosphorylation of the two isoforms could influence the interaction with ERα, because one phosphorylation site, Ser 294, is located within ERID-I and is phosphorylated in PRB but not in PRA (14). It is possible that some of the functional differences between PRB and PRA (11) relate to the inability of PRA to activate the endogenous c-Src/Erk signaling pathway in response to progestins rather than to the lack of the activation function AF3 (33).
The proline cluster of PRB is not essential for efficient activation of the Src/Erk cascade in cells containing ERα.
In addition to its interaction with ERα, PRB can also directly interact with the SH3 domain of c-Src, and this interaction is mediated by a proline cluster located between ERID-I and ERID-II. This interaction is independent of ERID-I or ERID-II and can be disrupted by deletion of the proline cluster region or by mutation of three proline residues in the cluster (Fig. 8) (4). These mutations, however, do not interfere with the interaction of PRB with ERα nor with the ERα-mediated activation of the endogenous Src/Erk cascade by progestins. In fact, in COS-7 cells transfected only with PRB, progestins exhibit a weak or nondetectable activation of endogenous c-Src, which is eliminated by deletion or mutation of the proline cluster region of PRB. A more pronounced activation can be observed in cells cotransfected with PRB and c-Src, which is also inhibited by mutations in the proline-rich region (data not shown). The physiological relevance of this direct interaction of PR with Src remains unclear. In COS-7 cells, the activation of Src by progestins in the absence of ERα does not lead to an activation of the Erk pathway. A significant activation of the Src/Erk cascade in response to progestins depends on the coexpression of ERα and is inhibited by deletion of the ERID-I or ERID-II regions. Moreover, in T47D breast cancer cells, which contain endogenous ERα and PRB, treatment with antiestrogens completely blocks the activation of the Src/Erk pathway by progestins (31).
The irrelevance of this direct interaction of PRB with c-Src for the progestin activation of the Src/Erk cascade is also supported by the observation that PRA, although it interacts with c-Src in vitro and in cells (4), does not mediate activation of the endogenous Src/Erk signaling pathway in mammalian cells (31). In the previous study (4), it was not conclusively shown that progestins activate this pathway via the endogenous PR independently of ERα. In fact, in COS-7 cells, the response to progestins depended on cotransfection of expression vectors for ERα (4). Results with the mammary cell line MCF-12A are not convincing in the absence of a demonstration that this cell line is actually ER negative in terms of estrogen activation of the Src/Erk pathway, because MCF-12A cells have been reported to respond to estrogens (40). Alternatively, one has to show that progestin activation of the Src/Erk cascade is not inhibited by antiestrogens in breast cancer cells.
The reason why the interaction between PRB and c-Src observed in vitro does not seem to play a significant role in activation of the endogenous Src/Erk cascade in mammalian cells may reside in the nature of the multiprotein receptor complex sensing steroid hormones near the cell membrane. We have shown that prior to the addition of any hormone, there is a preformed complex between PRB and ERα (31). It is possible that only the population of PRB complexed with ERα responds to progestins in terms of activation of the endogenous Src/Erk cascade, and this may depend on the organization of the various components of the multiprotein receptor. Biochemical elucidation of the nature of this receptor complex and its response to ligands may help our understanding of the mechanism of cross talk between steroid hormone receptors and the Src/Erk signaling pathway. The composition of the receptor complex could be different in the cytoplasm of Xenopus laevis oocytes, where the interaction of the proline cluster region of PR with c-Src may play an important role in progesterone-induced maturation (4).
Our findings with PRB are clearly different from those reported for prostate cells with the AR, which has also been shown to interact directly with the SH3 domain of c-Src (29). In this case, there is a functional synergism between androgens and estrogens, and a ternary complex between ERα, AR, and c-Src can be detected by coimmunoprecipitation. These findings suggest that the two receptors can interact simultaneously with two different domains of c-Src, ERα with the SH2 domain and AR with the SH3 domain. In the case of breast cancer cells, there is no synergism between progestins and estrogens in terms of Erk activation, and no ternary complex between PRB, ERα, and c-Src can be detected (31).
Genetic dissociation of progestin responses mediated by cross talk with signaling pathways and by transcriptional regulation.
One important question concerning the various signaling mechanisms involving PR relates to the nature of their target genes. We know that cross talk with the Src/Erk cascade is essential for cell proliferation in response to estrogens and progestins (31), but we ignore the genes mediating this response. We also know that direct transcriptional activation is not required for the progestin cross talk with the Src/Erk pathway, because a point mutation in AF2 that completely inactivates reporter gene transactivation (19) does not abolish the activation of Src/Erk (31). It will be interesting to use PR mutants defective in specific signaling functions for studying with DNA microarrays the populations of genes regulated by direct transcriptional activation, by cross talk with ERα, or by direct interaction with c-Src. It is also possible that two or more of these signaling pathways are required for controlling the expression of particular subsets of genes. The identification of the relevant protein regions involved in the various interactions and the construction of point mutations abolishing selectively each of the individual signaling mechanisms would allow this type of study. The results of these investigations could identify additional targets for selective pharmacological interference with specific hormonal responses.
Although this work focused on the cross talk between steroid hormone receptors and the Src/Erk pathway, it is obvious that understanding cell regulation by steroid hormones will have to take into consideration their cross talk with other signaling pathways. The recently reported interaction of ER with the p85 subunit of phosphoinositol-3-kinase (8, 35) and the subsequent activation of protein kinase B (PKB/Akt), as well as the cross talk with signaling via small G protein-coupled receptors (20), are of particular importance to understand the role of steroid hormones in the physiology of their target cells through the integrative function of their receptors.
Acknowledgments
We thank Vincent Cavaillés (Montpellier), Giulio Superti-Furga (EMBL Heidelberg), and Pierre Chambon (Strasbourg) for various plasmids and Jörg Klug (Marburg) for help in preparing the manuscript.
This work was supported by grants from the Deutsche Krebshilfe, the European Union Biomed Program, the Kempkes-Stiftung, and the Associazione Italiana per la Ricerca sul Cancro.
REFERENCES
- 1.Aronica, S. M., and B. S. Katzenellenbogen. 1993. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-1. Mol. Endocrinol. 7:743-752. [DOI] [PubMed] [Google Scholar]
- 2.Barettino, D., M. D. M. V. Ruiz, and H. G. Stunnenberg. 1994. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 13:3039-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beato, M., P. Herrlich, and G. Schütz. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851-857. [DOI] [PubMed] [Google Scholar]
- 4.Boonyaratanakornkit, V., M. P. Scott, V. Ribon, L. Sherman, S. M. Anderson, J. L. Maller, W. T. Miller, and D. P. Edwards. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell 8:269-280. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. E., T. Lechner, L. Howe, and J. L. Workman. 2000. The many HATs of transcription coactivators. Trends Biochem. Sci. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 6.Brüggemeier, U., L. Rogge, E. L. Winnacker, and M. Beato. 1990. Nuclear factor I acts as a transcription factor on the MMTV promoter but competes with steroid hormone receptors for DNA binding. EMBO J. 9:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castoria, G., M. V. Barone, M. Di Domenico, A. Bilancio, D. Ametrano, A. Migliaccio, and F. Auricchio. 1999. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J. 18:2500-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castoria, G., A. Migliaccio, A. Bilancio, M. Di Domenico, A. de Falco, M. Lombardi, R. Fiorentino, L. Varricchio, M. V. Barone, and F. Auricchio. 2001. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 20:6050-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castoria, G., A. Migliaccio, S. Green, M. Di Domenico, P. Chambon, and F. Auricchio. 1993. Properties of a purified estradiol-dependent calf uterus tyrosine kinase. Biochemistry 32:1740-1750. [DOI] [PubMed] [Google Scholar]
- 10.Cavaillès, V., S. Dauvois, P. S. Danielian, and M. G. Parker. 1994. Interaction of proteins with transcriptionally active estrogen receptors. Proc. Natl. Acad. Sci. USA 91:10009-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalbos, D., and F. Galtier. 1994. Differential effect of forms A and B of human progesterone receptor on estradiol-dependent transcription. J. Biol. Chem. 269:23007-23012. [PubMed] [Google Scholar]
- 12.Chalepakis, G., J. Arnemann, E. P. Slater, H. Brüller, B. Gross, and M. Beato. 1988. Differential gene activation by glucocorticoids and progestins through them hormone regulatory element of mouse mammary tumor virus. Cell 53:371-382. [DOI] [PubMed] [Google Scholar]
- 13.Clarke, C. L., and R. L. Sutherland. 1990. Progestin regulation of cellular proliferation. Endocr. Rev. 11:266-301. [DOI] [PubMed] [Google Scholar]
- 14.Clemm, D. L., L. Sherman, V. Boonyaratanakornkit, W. T. Schrader, N. L. Weigel, and D. P. Edwards. 2000. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol. Endocrinol. 14:52-65. [DOI] [PubMed] [Google Scholar]
- 15.Collins, P., and C. Webb. 1999. Estrogen hits the surface. Nat. Med. 5:1130-1131. [DOI] [PubMed] [Google Scholar]
- 16.Edwards, D. P., N. L. Weigel, S. K. Nordeen, and C. A. Beck. 1993. Modulators of cellular protein phosphorylation alter the trans-activation function of human progesterone receptor and the biological activity of progesterone antagonists. Breast Cancer Res. Treat. 27:41-56. [DOI] [PubMed] [Google Scholar]
- 17.Frangioni, J. V., and B. G. Neel. 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 210:179-187. [DOI] [PubMed] [Google Scholar]
- 18.Freedman, L. P. 1999. Increasing the complexity of coactivation in nuclear receptor signaling. Cell 97:5-8. [DOI] [PubMed] [Google Scholar]
- 19.Gong, W., S. Chávez, and M. Beato. 1997. Point mutation in the ligand binding domain of the progesterone receptor generates a transdominant negative phenotype. Mol. Endocrinol. 11:1476-1485. [DOI] [PubMed] [Google Scholar]
- 20.Grazzini, E., G. Guillon, B. Mouillac, and H. H. Zingg. 1998. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature 392:509-512. [DOI] [PubMed] [Google Scholar]
- 21.Hovland, A. R., R. L. Powell, G. S. Takimoto, L. Tung, and K. B. Horwitz. 1998. An N-terminal inhibitory function, IF, suppresses transcription by the A-isoform but not the B-isoform of human progesterone receptors. J. Biol. Chem. 273:5455-5460. [DOI] [PubMed] [Google Scholar]
- 22.Improta-Brears, T., A. R. Whorton, F. Codazzi, J. D. York, T. Meyer, and D. P. McDonnell. 1999. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc. Natl. Acad. Sci. USA 96:4686-4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kastner, P., A. Krust, B. Turcotte, U. Stropp, L. Tora, H. Gronemeyer, and P. Chambon. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9:1603-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klehr, D., K. Maass, and J. Bode. 1991. Scaffold-attached regions from the human interferon β domain can be used to enhance the stable expression of genes under the control of various promoters. Biochemistry 30:1264-1270. [DOI] [PubMed] [Google Scholar]
- 25.Kousteni, S., T. Bellido, L. I. Plotkin, C. A. O'Brien, D. L. Bodenner, L. Han, K. Han, G. B. DiGregorio, J. A. Katzenellenbogen, B. S. Katzenellenbogen, P. K. Roberson, R. S. Weinstein, R. L. Jilka, and S. C. Manolagas. 2001. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719-730. [PubMed] [Google Scholar]
- 26.Lange, C. A., J. K. Richer, and K. B. Horwitz. 1999. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol. Endocrinol. 13:829-836. [DOI] [PubMed] [Google Scholar]
- 27.Lim, C. S., C. T. Baumann, H. Htun, W. Xian, M. Irie, C. L. Smith, and G. L. Hager. 1999. Differential localization and activity of the A- and B-forms of the human progesterone receptor using green fluorescent protein chimeras. Mol. Endocrinol. 13:366-375. [DOI] [PubMed] [Google Scholar]
- 28.Luttrell, D. K., A. Lee, T. J. Lansing, R. M. Crosby, K. D. Jung, D. Willard, M. Luther, M. Rodriguez, J. Berman, and T. M. Gilmer. 1994. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc. Natl. Acad. Sci. USA 91:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migliaccio, A., G. Castoria, M. Di Domenico, A. de Falco, A. Bilancio, M. Lombardi, M. V. Barone, D. Ametrano, M. S. Zannini, C. Abbondanza, and F. Auricchio. 2000. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 19:5406-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migliaccio, A., M. Di Domenico, G. Castoria, A. de Falco, P. Bontempo, E. Nola, and F. Auricchio. 1996. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 15:1292-1300. [PMC free article] [PubMed] [Google Scholar]
- 31.Migliaccio, A., D. Piccolo, G. Castoria, M. Di Domenico, A. Bilancio, M. Lombardi, W. Gong, M. Beato, and F. Auricchio. 1998. Activation of the src/p21ras/erk pathway by progesterone receptor via a crosstalk with estrogen receptor. EMBO J. 17:2008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterziel, H., S. Mink, A. Schonert, M. Becker, H. Klocker, and A. C. Cato. 1999. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 18:6322-6329. [DOI] [PubMed] [Google Scholar]
- 33.Sartorius, C. A., M. Y. Melville, A. R. Hovland, L. Tung, G. S. Takimoto, and K. B. Horwitz. 1994. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol. Endocrinol. 8:1347-1360. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt, B. M., D. Gerdes, M. Feuring, E. Falkenstein, M. Christ, and M. Wehling. 2000. Rapid, nongenomic steroid actions: a new age? Front. Neuroendocrinol. 21:57-94. [DOI] [PubMed] [Google Scholar]
- 35.Simoncini, T., A. Hafezi-Moghadam, D. P. Brazil, K. Ley, W. W. Chin, and J. K. Liao. 2000. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tora, L., A. Mullick, D. Metzger, M. Ponglikitmongkol, I. Park, and P. Chambon. 1989. The cloned human estrogen receptor contains a mutation which alters its hormone binding properties. EMBO J. 8:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truss, M., J. Bartsch, and M. Beato. 1994. Antiprogestins prevent progesterone receptor binding to hormone responsive elements in vivo. Proc. Natl. Acad. Sci. USA 91:11333-11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehling, M. 1997. Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol. 59:365-393. [DOI] [PubMed] [Google Scholar]
- 39.Weigel, N. L., and Y. Zhang. 1998. Ligand-independent activation of steroid hormone receptors. J. Mol. Med. 76:469-479. [DOI] [PubMed] [Google Scholar]
- 40.Xie, D., C. W. Miller, J. O'Kelly, K. Nakachi, A. Sakashita, J. W. Said, J. Gornbein, and H. P. Koeffler. 2001. Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease. J. Biol. Chem. 276:14187-14194. [DOI] [PubMed] [Google Scholar]