Abstract
The actions of glucocorticoids are mediated by the glucocorticoid receptor (GR), which is activated upon ligand binding, and can alter the expression of target genes either by transrepression or transactivation. We have applied FRAP (fluorescence recovery after photobleaching) to quantitatively assess the mobility of the yellow fluorescent protein (YFP)-tagged human GR α-isoform (hGRα) in the nucleus of transiently transfected COS-1 cells and to elucidate determinants of its mobility. Addition of the high-affinity agonist dexamethasone markedly decreases the mobility of the receptor in a concentration-dependent manner, whereas low-affinity ligands like corticosterone decrease the mobility to a much lesser extent. Analysis of other hGRα ligands differing in affinity suggests that it is the affinity of the ligand that is a major determinant of the decrease in mobility. Similar results were observed for two hGRα antagonists, the low-affinity antagonist ZK98299 and the high-affinity antagonist RU486. The effect of ligand affinity on mobility was confirmed with the hGRα mutant Q642V, which has an altered affinity for triamcinolone acetonide, dexamethasone, and corticosterone. Analysis of hGRα deletion mutants indicates that both the DNA-binding domain and the ligand-binding domain of the receptor are required for a maximal ligand-induced decrease in receptor mobility. Interestingly, the mobility of transfected hGRα differs among cell types. Finally, the proteasome inhibitor MG132 immobilizes a subpopulation of unliganded receptors, via a mechanism requiring the DNA-binding domain and the N-terminal part of the ligand-binding domain. Ligand binding makes the GR resistant to the immobilizing effect of MG132, and this effect depends on the affinity of the ligand. Our data suggest that ligand binding induces a conformational change of the receptor which is dependent on the affinity of the ligand. This altered conformation decreases the mobility of the receptor, probably by targeting the receptor to relatively immobile nuclear domains with which it transiently associates. In addition, this conformational change blocks immobilization of the receptor by MG132.
The effects of glucocorticoid hormones are mediated by an intracellular receptor, the glucocorticoid receptor (GR), which is a member of the nuclear receptor family that includes the steroid receptors. In the absence of ligand, GR is located in the cytoplasm, where it forms a complex with heat shock proteins and immunophilins (45). Upon ligand-induced activation, the receptor dissociates from this complex and translocates to the nucleus (44), where it can initiate transcription of specific target genes by binding to glucocorticoid-responsive elements (GREs) in the promoter region of target genes and interact with transcriptional coactivators (4, 12, 20, 31, 36). Alternatively, the receptor can bind to negative GREs, on which GR binding results in inhibition of transcriptional activation (14, 38). In the absence of DNA binding, GR can alter transcription by inhibiting the action of other transcription factors such as NF-κB (9, 47, 50) or AP-1 (33, 51, 61).
Using green fluorescent protein (GFP)-tagged proteins, it is now possible to study intracellular trafficking of nuclear receptors in living cells. Complete nuclear translocation of a GFP-GR chimera has been observed within 30 min after addition of ligand (28). Similar data on nuclear translocation of GFP-tagged receptors have been obtained for the adrogen receptor (AR) (49, 57, 58), the mineralocorticoid receptor (MR) (18), and the vitamin D receptor (46). In the nucleus, the agonist-bound GFP-GR has been reported to organize into discrete foci (28), which is consistent with earlier results from immunofluorescence studies of endogenous GRs (59). A similar punctate distribution in the nucleus has also been found for the agonist-bound GFP-tagged estrogen receptor alpha (ERα) (29, 52), AR (49, 57, 58), MR (18), vitamin D receptor (46), and thyroid hormone receptor β (3). Treatment with an antagonist does not result in foci formation of GR (28), AR (49, 57, 58), and MR (18), and ERα antagonists induced a less pronounced punctate receptor distribution after antagonist binding (29, 52).
In addition to the analysis of trafficking and distribution, GFP-tagged proteins have recently been used to study protein mobility by fluorescence recovery after photobleaching (FRAP). Studies of the mobility of the endonuclease ERCC1/XPF (27), the nucleosomal binding protein HMG-17, the pre-mRNA splicing factor SF2/ASF, and the rRNA processing protein fibrillarin (43) revealed that proteins involved in diverse nuclear processes move rapidly throughout the entire nucleus. The mobility of ERα was recently investigated by Stenoien et al. (54), who demonstrated that ERα and the coactivator SRC-1 move rapidly through the nucleus in the absence of ligand and that their mobility decreases upon activation by ligand. This decreased mobility was ligand dependent. McNally et al. (37) studied a subpopulation of GRs that is bound to DNA and found a high mobility of these GRs, suggesting a rapid exchange between DNA-bound GRs and the remainder of the GR pool in the nucleus.
We have investigated the average mobility of the entire GR pool in the nucleus, using FRAP analysis of a yellow fluorescent protein (YFP)-tagged human GRα (hGRα, the transcriptionally active isoform of the human glucocorticoid receptor). Our studies reveal that hGRα's mobility is decreased by ligand binding and that this decrease in mobility is dependent on the affinity of the ligand for its cognate receptor. Furthermore, the involvement of the proteasome in altering the mobility of hGRα is investigated.
MATERIALS AND METHODS
Plasmids.
Plasmid pEYFP-C1 was purchased from BD Biosciences Clontech (Palo Alto, Calif.). Plasmid pEYFP-hGRα was constructed in the laboratory of M. Negishi (National Institute of Environmental Health Sciences, Research Triangle Park, N.C.) using PCR amplification of hGRα cDNA with Pfu polymerase (Stratagene), the plasmid pCMVhGRα as the template (40), and a specific set of primers to generate XhoI and BamHI sites at the 5′ and 3′ end, respectively (62), with primer sequences CCGCTCGAGCAATGGACTCCAAAGAATCATT and CCGGGATCCTCACTTTTGATGAAACAGAAG. The amplification product was digested with these enzymes and cloned into pEYFP-C1, resulting in a vector encoding an in-frame N-terminal fusion of hGRα with YFP, separated by seven random amino acids.
Mutated versions of this vector were made in our laboratory. Mutant Q642V was created by site-directed mutagenesis of pEYFP-hGRα using the QuikChange kit (Stratagene, La Jolla, Calif.) according to the manufacturer's instructions (primers CTCTACCCTGCATGTATGACGUATGTAAACACATGCTGTTTATC and GATAAACAGCATGTGTTTACATACGTCATACATGCAGGGTAGAG). Deletion mutants Δ77-262, Δ9-385, and Δ428-490 and truncation mutants I550 and I582 were generated like pEYFP-hGRα. PCR products were generated using the respective mutants of pRShGRα (24, 25) obtained from R. Evans (Salk Institute, San Diego, Calif.) and subsequently digested and ligated into pEYFP-C1 as described above.
Compounds.
Cortexolone, corticosterone, cortisol, cortisone, dexamethasone, and RU486 were purchased from Steraloids Inc. (Wilton, N.H.). Triamcinolone, triamcinolone acetonide, and MG132 were obtained from CalBiochem (San Diego, Calif.).
Cell culture and transfection.
COS-1, HEK293, and HeLa cells were grown as described previously (1, 7). One day before transfection, cells were transferred to 78.5-cm2 dishes (7.5 · 105 cells per dish). Cells were transfected using TransIt reagent (PanVera) with either the YFP-hGRα expression vector pEYFP-hGRα or a mutated version of this vector. Per dish, 0.4 μg of plasmid was used. After a 5-h incubation with the TransIt reagent-DNA mixture, cells were refed with supplemented Dulbecco's modified Eagle's medium. One day after transfection, cells were transferred to 9.6-cm2 dishes containing glass bottoms (MatTek Corp.) (1.5 · 105 cells per dish). The next day cells were subjected to FRAP analysis.
FRAP.
Between 3 and 6 h after addition of the indicated steroid, medium was replaced by HEPES buffer (37°C) and immediately FRAP analysis was performed. Cells were observed by using a Zeiss LSM 510 confocal laser scanning microscope, using a Plan-Apochromat 100× oil immersion objective (1.4 numeric aperture). Cells were excited with an argon laser at 514 nm, and emission was collected using a 530-nm long-pass filter. Images were taken every 196.6 ms at a resolution of 128 by 128 pixels (pixel size, 0.29 by 0.29 μm; pixel time, 3.52 μs). After the first image, a selected rectangular region of fixed size (50 by 10 pixels) in the nucleus was bleached at a set laser power of 15 mW for 40 iterations. Fluorescence in the bleached region and in the total nucleus was quantified at every time point using LSM software. In each experiment, two dishes (five cells per dish) were analyzed per treatment.
To correct for differences in expression level between individual cells, fluorescence data for the bleached region and the total nucleus were normalized to the level before bleaching. In addition, at all time points data were normalized to the fluorescence in the total nucleus in order to correct for the loss in fluorescence due to the bleach pulse and the imaging. Thus, in mathematical terms FRAP curves were created using the following equation: Ft = (T0 · Bt)/(Tt · B0), in which Ft is the normalized fluorescence at time point t, T0 and Tt are the fluorescence in the total nucleus at time points 0 and t, respectively, and B0 and Bt are the fluorescence in the bleached region at time points 0 and t. This way of presenting FRAP data was previously proposed by Phair and Misteli (43) and provides an accurate estimate of the immobile fraction. In each experiment (10 cells), these normalized data (Ft) were averaged and plotted. Using this FRAP curve, the t1/2 of maximal recovery was determined, which is defined as the time point after bleaching at which the normalized fluorescence has increased to half the amount of the maximal recovery. Every t1/2 shown is an average of at least two experiments. In addition, this curve was used to determine the fluorescence level at 40 s (in the experiments using MG132). Levels shown are an average of at least two experiments.
Chloramphenicol acetyltransferase (CAT) assays and Western blotting.
pEYFP-hGRα and the reporter plasmid pGRE2CAT (1) were transfected into COS-1 cells as described above. Twenty-four hours after transfection, dexamethasone was added (100 nM), and 24 h later cells were scraped off the culture dish into cold phosphate-buffered saline. Cells were pelleted, resuspended in a 0.25 M Tris-5 mM EDTA buffer (pH 8), lysed by tip sonication, and incubated at 65°C for 10 min. Volumes of extracts containing known amounts of protein were incubated overnight at 37°C with 14C-labeled chloramphenicol (Amersham) in the presence of 1 mM acetyl coenzyme A (Boehringer Mannheim, Indianapolis, Ind.). Acetylated chloramphenicol was extracted using mixed xylenes (Sigma) and quantitated by scintillation counting.
Aliquots of each protein extract were analyzed by Western blotting. Ten micrograms of protein was resolved by electrophoresis through 8% polyacrylamide gels (Novex) and electrophoretically transferred to nitrocellulose membranes (Novex). Membranes were blocked at room temperature for 30′ in Tris-buffered saline with Tween 20 (TBST; 10 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 0.1% Tween 20) containing 5% nonfat dry milk. Subsequently, the membrane was incubated overnight at 4°C with the indicated hGR antipeptide antibody 57 (10) at a dilution of 1:1,000 in TBST containing 5% nonfat dry milk. After three 15-min washes in TBST containing 5% nonfat dry milk, the membrane was incubated with a horseradish peroxidase-labeled goat anti-rabbit secondary antibody (Amersham Corp.) (1:10,000 dilution in TBST containing 5% nonfat dry milk) for 1.5 h at room temperature. Finally, after three 15-min washes in TBST, immunoreactivity was visualized by enhanced chemoluminescence (ECL; Amersham Corp.) according to the manufacturer's instructions.
Statistical analysis.
Statistical analysis was performed comparing individual groups by Student's t test. Dose-response curves were analyzed by an analysis of variance (ANOVA). Statistical significance was accepted at a P level of <0.05.
RESULTS
Characterization of YFP-hGRα.
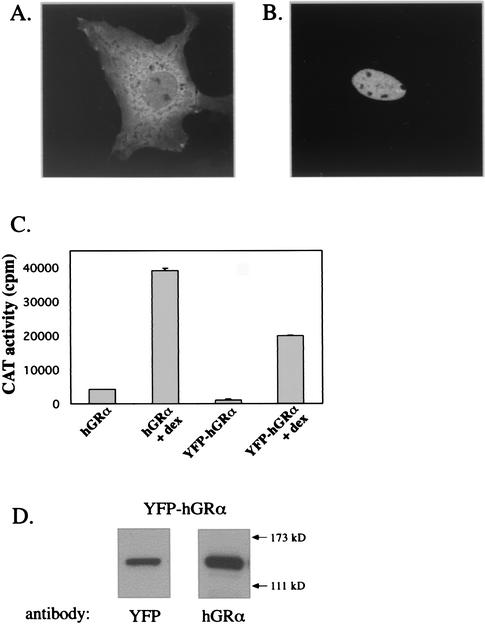
In all experiments described here, a fusion protein was studied in which YFP was tagged to the N terminus of hGRα. We compared the molecular properties of YFP-hGRα to those known for wild-type hGRα. Nuclear translocation of the YFP-hGRα fusion protein was studied by confocal microscopy in transfected COS-1 cells. In Fig. 1A a representative picture of the expression of YFP-hGRα is shown. YFP-hGRα is primarily located in the cytoplasm, although some staining can be observed in the nucleus. Figure 1B shows a representative image of YFP-hGRα-transfected cells 3 h after addition of 100 nM dexamethasone. Clearly, under these conditions YFP-hGRα has entirely translocated to the nucleus, as has been observed for the wild-type receptor (32). Subsequently, the transactivation properties on a GRE-driven promoter were analyzed. YFP-hGRα was transfected into COS-1 cells together with the reporter plasmid GRE2CAT, in which the CAT gene is driven by a promoter containing two GREs. Twenty-four hours after transfection, dexamethasone (100 nM) was added, and another 24 h later CAT activity was assayed. A representative experiment is shown in Fig. 1. Although the level of transactivation in the absence and presence of ligand of YFP-hGRα is lower than that of hGRα, the level of induction by the ligand is comparable (9.4-fold for hGRα, 16.6-fold for YFP-hGRα). Finally, protein extracts from YFP-hGRα-transfected cells were used for Western blotting. A representative blot is shown in Fig. 1D. Staining with either a YFP or an hGRα antibody resulted in a single band that migrated according to the predicted size of the fusion product (121 kDa). Thus, the yellow fluorescence in the transfected cells is solely due to the presence of the YFP-hGRα fusion protein.
FIG. 1.
Functional analysis of YFP-hGRα. (A) Expression of YFP-hGRα. COS-1 cells were transfected with pEYFP-hGRα, and 48 h later images were taken. Most of the fluorescence is located in the cytoplasm. (B) Nuclear translocation of YFP-hGRα. Forty-eight hours after transfection, dexamethasone (100 nM) was added. Three hours later, images were taken. Fluorescence could only be detected in the nucleus at this time point. (C) Transactivation induced by YFP-hGRα on a GRE-driven promoter, with pEYFP-hGRα and the reporter plasmid pGRE2CAT (comprising the CAT gene driven by a promoter that contains two GREs). Twenty-four hours after transfection, dexamethasone was added (100 nM), and 24 h later cells were harvested and CAT activity was analyzed by measuring the conversion of 14C-labeled chloramphenicol. Although less potent than hGRα, YFP-hGRα strongly induced transactivation upon dexamethasone addition. (D) Western blotting of lysates from YFP-hGRα-transfected COS-1 cells, using antibodies against YFP and hGRα. Both antibodies stain a band of ±120 kDa, which is the predicted size of YFP-hGRα.
FRAP analysis of YFP-hGRα.
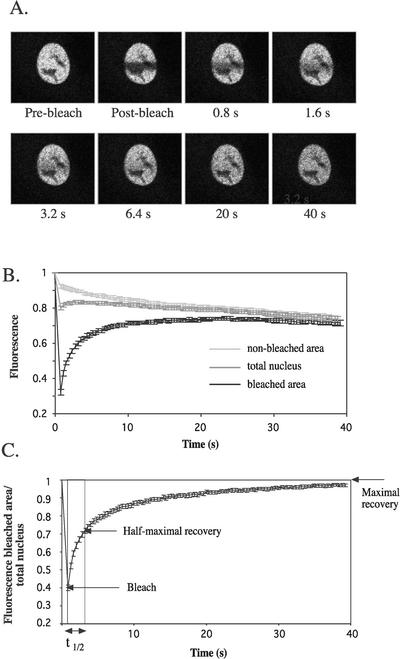
FRAP analysis was performed using the YFP-hGRα fusion protein. COS-1 cells were transfected with YFP-hGRα, and 48 h after transfection the analysis was undertaken. Figure 2 shows the analysis for cells treated with dexamethasone (100 nM) for 3 h. A rectangular region in the nucleus of fixed size was bleached by applying maximal laser power for approximately 800 ms, and images of the nucleus were taken every 400 ms. Figure 2A shows images taken right before bleaching (prebleach), immediately after bleaching (postbleach), and at several time points after bleaching.
FIG. 2.
FRAP analysis of YFP-hGRα. (A) COS-1 cells were transfected with pEYFP-hGRα, and 48 h later dexamethasone (100 nM) was added. Between 3 and 6 h after hormone addition, a region in the nucleus was bleached, and single z-sections were taken at approximately every 400 ms. (B) Quantification of FRAP analysis. Fluorescence in the bleached area, nonbleached area, and the total nucleus was quantified and plotted against time. Bleaching occurred at t = 0, and took approximately 800 ms. (C) Fluorescence in bleached area corrected for the change in total fluorescence. Using this plot, t1/2 can be determined, which is the time in which the fluorescence has reached half of the maximal recovery. At least 10 cells were analyzed for each plot in the present study. All plots are representative of at least two individual experiments.
The fluorescence in the bleached area, the nonbleached area, and the total nucleus was quantified. Ten cells were analyzed, and the mean ± standard error of the mean was plotted against time (Fig. 2B). Between 0 and 800 ms, the cells were bleached and fluorescence decreased not only in the bleached area but also in the nonbleached area, which reflects the movement of bleached molecules out of the bleached area. Immediately after the bleach, the fluorescence in the bleached area increased as a result of the exchange of YFP-hGRα between bleached and nonbleached areas. As a result, the fluorescence in the nonbleached area decreased, and at 40 s fluorescence levels in the two areas were almost identical. Since the two curves converge it can be concluded that all molecules can be exchanged between the two areas, indicating that there is no immobile fraction of YFP-hGRα molecules under these conditions. For the total nucleus, immediately after the bleach pulse a small increase in fluorescence intensity was observed, after which it started to gradually decrease. The small increase resulted from nonbleached molecules entering from other focal planes, since the exchange is three-dimensional. The decrease can be explained by bleaching as a result of imaging. When the same experiment is performed without bleaching, a 13% decrease in fluorescence is observed in the total nucleus (data not shown). To control for the changes in total fluorescence, we normalized the fluorescence in the bleached area to the fluorescence in the total nucleus. These normalized data are shown in Fig. 2C and were used to determine the t1/2 of maximal recovery.
The mobility of YFP-hGRα is determined by ligand concentration and affinity.
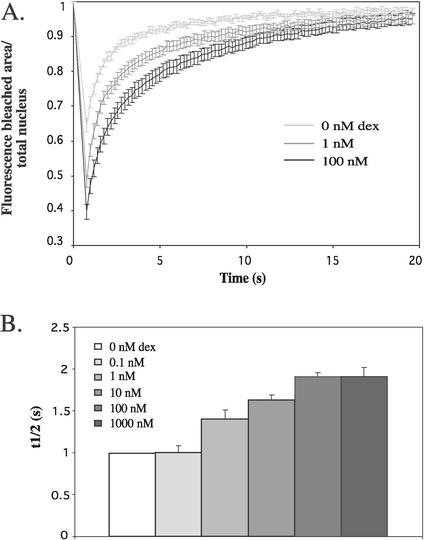
We first examined the effect of ligand on the mobility of YFP-hGRα. Different concentrations of dexamethasone were administered to YFP-hGRα-transfected cells, and FRAP analysis was performed as described above. All experiments were performed between 3 and 6 h after addition of hormone, because initial time course studies showed that the mobility of YFP-hGRα does not change between 1 and 12 h after addition of hormone (data not shown). In Fig. 3A, fluorescence curves are shown for 0, 1, and 100 nM dexamethasone. A higher concentration of dexamethasone results in a slower recovery after bleaching, indicating a decrease in receptor mobility induced by the ligand. This conclusion is confirmed by the measurement of t1/2 of maximal recovery, which is shown in Fig. 3B. Between 0 and 0.1 nM, t1/2 remains stable, and between 0.1 and 100 nM, t1/2 increases from 1.00 ± 0.08 s to 1.91 ± 0.04 s. At 100 nM, a plateau is reached. In addition, a higher concentration of dexamethasone results in a more efficient bleaching (Fig. 3A). These data reflect the movement of a smaller number of bleached molecules out of the bleached region during the bleaching period, since at a higher concentration of dexamethasone the fluorescence in the nonbleached area is higher than at a lower concentration (data not shown). All curves appear to approach a maximal recovery of approximately one, suggesting that there is no immobile fraction in the absence and presence of ligand.
FIG. 3.
Dexamethasone decreases the mobility of YFP-hGRα. A. Representative fluorescence curves from FRAP analysis of YFP-hGRα after addition of different concentrations of dexamethasone. Images were taken every 200 ms. Results indicate that increasing concentrations of dexamethasone resulted in decreasing YFP-hGRα mobility. (B) t1/2 from FRAP analysis of YFP-hGRα after addition of different concentrations of dexamethasone. Data are means ± standard errors of the means of at least two experiments. The diffusion time increased when the concentration of dexamethasone was increased between 1 and 100 nM. Maximal t1/2 was reached at 100 nM.
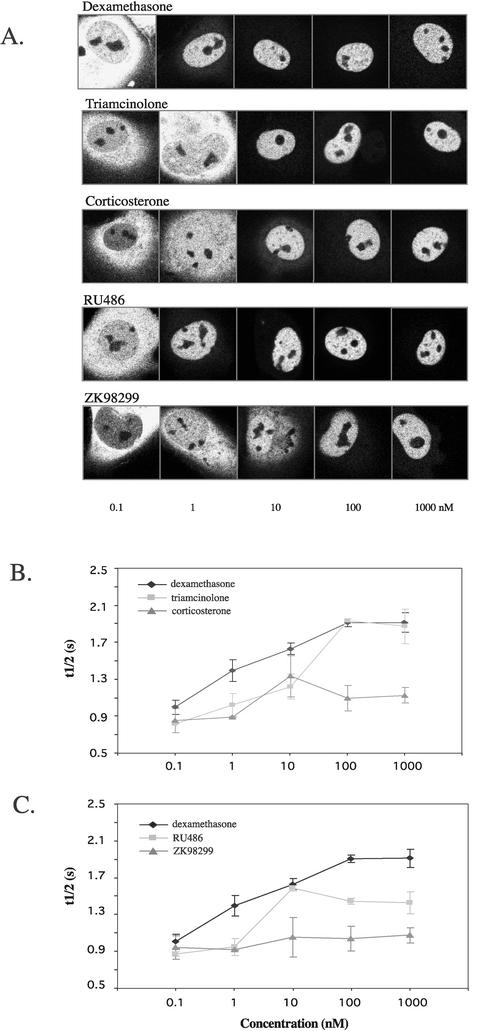
Subsequently, the effect of other ligands was studied in identically designed experiments. First, different concentrations of the agonists triamcinolone and corticosterone were administered and FRAP analysis was performed. Both these ligands, like dexamethasone, induce translocation of YFP-hGRα to the nucleus, although total translocation is reached at a higher concentration (Fig. 4A). However, the effect on mobility that these ligands induce differs. ANOVA of these data revealed an effect of the ligand between triamcinolone and corticosterone and between dexamethasone and corticosterone. The dose-response curves for their t1/2 both reached a plateau at 100 nM, but triamcinolone decreased YFP-hGRα mobility similar to dexamethasone (at 1,000 nM, t1/2 was 1.87 ± 0.18 s), whereas corticosterone induced a smaller decrease in mobility (at 1,000 nM, t1/2 =1.13 ± 0.08 s). ANOVA revealed an effect of the concentration for triamcinolone and dexamethasone, but not for corticosterone.
FIG. 4.
Natural and synthetic ligands decrease the mobility of YFP-hGRα. (A) Nuclear translocation induced by different concentrations of dexamethasone, triamcinolone, corticosterone, RU486, and ZK98299. All ligands were able to induce total translocation to the nucleus. (B) Dose-response curves of YFP-hGRα FRAP analysis for dexamethasone, triamcinolone, and corticosterone. t1/2 is plotted against concentration of the ligand. For all ligands, a plateau was reached at 100 nM. Dexamethasone and triamcinolone induced a lower mobility than corticosterone. (C) Dose-response curves of YFP-hGRα FRAP analysis for dexamethasone, RU486, and ZK28299. For all ligands, a plateau was reached at 100 nM. ZK 98299 decreased the mobility to the least extent; RU486 slowed the receptor down more, but less than dexamethasone.
We next evaluated the effect of two glucocorticoid antagonists. We used RU486, a type I antagonist which is known to induce a receptor conformation that is able to bind DNA (8), and ZK98299, which induces a conformation that does not bind DNA (6). A different mobility of YFP-hGRα was observed when RU486 or ZK98299 was added (Fig. 4C). At 1,000 nM RU486, the t1/2 measured was 1.43 ± 0.12 s. In contrast, the type II antagonist ZK98299 did not significantly change the mobility of the receptor: at 1,000 nM, t1/2 was 1.07 ± 0.11 s. ANOVA of these data revealed that both antagonists have a different effect from dexamethasone and from each other. An effect of the concentration was only found for RU486.
Mobility correlates with affinity of ligand.
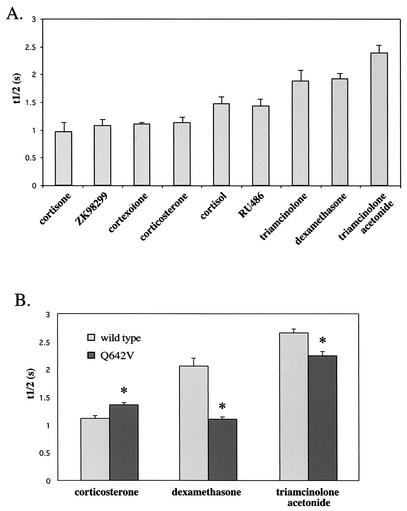
Since the mobility of YFP-hGRα appears to depend on the ligand to which it is bound, we examined the effect of four other ligands of varying affinity for hGRα. Triamcinolone acetonide, cortisol, cortexolone, and cortisone were administered at saturating levels (1,000 nM) and FRAP analysis was performed as described above. Again, large differences were observed in the decrease in mobility that the ligands induced. For triamcinolone acetonide, t1/2 was 2.38 ± 0.14 s, for cortisol it was 1.47 ± 0.12 s, for cortexolone it was 1.10 ± 0.03 s, and for cortisone it was 0.97 ± 0.16 s. In Fig. 5A the studied ligands and the t1/2 induced at 1,000 nM are presented in the order of their affinity for the hGR (based on references 19, 22, 23, 26, 34, 48, and 63). This graph reveals that low-affinity ligands like corticosterone, ZK98299, cortexolone, and cortisone (Kd > 10 nM) minimally alter the mobility of the receptor, whereas high-affinity ligands (triamcinolone acetonide and dexamethasone [Kd < 5 nM]) induce a profound decrease in receptor mobility. Ligands with an intermediate affinity (triamcinolone, RU486, and cortisol [5 nM < Kd < 10 nM]) reduce the receptor mobility to a degree that ranks it between the high- and low-affinity ligands.
FIG. 5.
Ligand affinity determines mobility of YFP-hGRα. (A) t1/2 for several ligands, presented in the order of their affinity, administered at a concentration of 1,000 nM. High-affinity ligands induce a larger decrease in receptor mobility than low-affinity ligands. (B) t1/2 for wild-type YFP-hGRα and mutant Q642V in the presence of 1,000 nM corticosterone, dexamethasone, and triamcinolone acetonide. Mutation Q642V slightly increased the affinity for corticosterone (50% inhibitory concentration (IC50) 2.3 times lower than wild type [34]), markedly decreased the affinity for dexamethasone (IC50 11-fold increased compared to wild type in a competition binding assay), and slightly decreases the affinity for triamcinolone acetonide (Kd 1.4 times higher than wild type in a binding assay). Consistent with the alterations in affinity, the mobility of the mutant receptor induced by these ligands was altered compared to the wild type. The mutant receptor shows a higher t1/2 in response to corticosterone than does the wild-type receptor, a lower t1/2 than the wild-type receptor after binding to dexamethasone receptor, and a slightly lower t1/2 in response to triamcinolone acetonide. *, significantly different from wild-type receptor value under similar conditions (P < 0.05).
To further determine if the ligand affinity is an important determinant for the mobility of the ligand-bound receptor, the GR mutant Q642V was utilized. The point mutation, in which at position 642 a glutamine is replaced by a valine, was previously described by Lind et al. (34). Glutamine 642 supposedly interacts with the 17α-OH group of glucocorticoid ligands (5), and therefore mutation of this residue markedly decreases the affinity for ligands with a 17α-OH group like dexamethasone, whereas it alters the affinity for ligands that lack this group only slightly (the mutant has decreased affinity for triamcinolone acetonide and increased affinity for corticosterone [34]). We made this mutation in YFP-hGRα and performed FRAP analysis after addition of 1,000 nM triamcinolone acetonide, dexamethasone, or corticosterone (Fig. 5B). Consistent with the alterations in affinity, the mobility of the mutant receptor induced by these ligands was altered compared to the wild type. The mutant receptor showed a slightly higher mobility in response to triamcinolone acetonide than with the wild-type receptor (t1/2, 2.24 ± 0.07 s versus 2.66 ± 0.07 s), a higher mobility than the wild-type receptor after binding to dexamethasone receptor (t1/2, 1.10 ± 0.04 s versus 2.06 ± 0.14 s), and a lower mobility than the wild type in response to corticosterone (t1/2, 1.36 ± 0.04 s versus 1.12 ± 0.05 s). Furthermore, the mutant Q642V showed a significantly higher mobility induced by dexamethasone than by corticosterone, reflecting its higher affinity for corticosterone than for dexamethasone.
Multiple domains of YFP-hGRα determine its mobility.
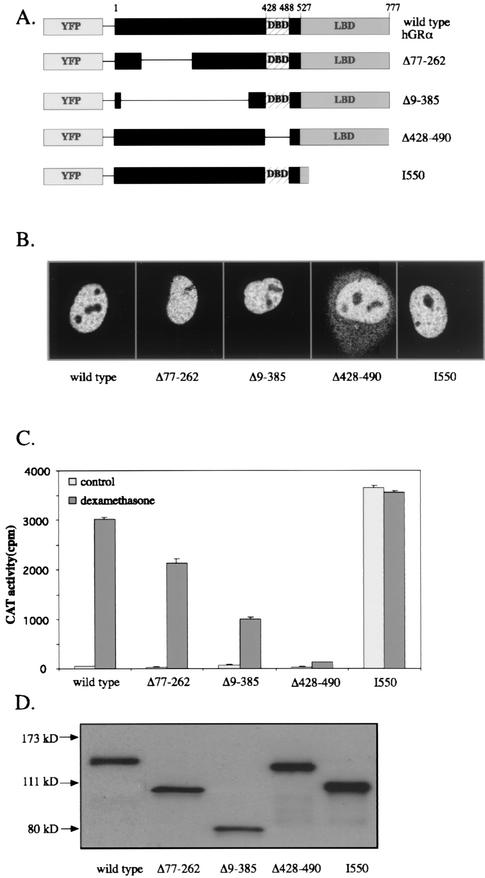
We next examined which domains in hGRα determine its mobility in the nucleus. Therefore, several YFP-hGRα deletion mutants were created (Fig. 6A). The mutants created were Δ77-262 and Δ9-385, in which a part of the N terminus was deleted, Δ428-490, in which the DNA-binding domain was deleted, and the truncation mutant I550 that lacks most of the ligand-binding domain. First, these mutants were functionally characterized. After addition of 100 nM dexamethasone, complete nuclear translocation of Δ77-262, Δ9-385, and I550 was observed (Fig. 6B; for I550 complete translocation was also observed without ligand [data not shown]). The mutant Δ428-490 also showed nuclear translocation, but a significant amount of fluorescence was still observed in the cytoplasm. These data are consistent with the nuclear translocation of these mutants without the YFP tag, as studied by immunohistochemistry (32).
FIG. 6.
Characterization of deletion mutants of YFP-hGRα. (A) Schematic diagram of the mutants used in the present study. (B) Nuclear translocation of the mutant receptors. All mutants translocate to the nucleus in the presence of 100 nM dexamethasone, although Δ428-490 did not do so completely. I550 shows the same distribution in the absence of ligand. (C) Transactivation properties of YFP-hGRα and deletion mutants on a GRE-driven promoter. CAT assays were performed as described in the legend for Fig. 2. The results indicate that Δ77-262 and Δ9-385 induce less transactivation than the wild type, Δ428-490 is inactive, and I550 induces strong transactivation in the presence and absence of ligand. (C) Western blot of lysates from COS-1 cells transfected with YFP-hGRα or a deletion mutant. Two hGRα antibodies were used in order to stain all deletion mutants. All mutants were expressed at approximately the level of the wild-type receptor, resulting in single bands which had the expected electrophoretic mobilities.
The transactivation properties of the mutants in the presence of 100 nM dexamethasone were studied in a CAT assay (Fig. 6C). I550 induced transactivation similar to the wild type, but the mutant showed this induction also in the absence of ligand. Mutants Δ77-262 and Δ9-385 induced transactivation, although less than the wild-type receptor, whereas Δ428-490 was virtually inactive. These data are also consistent with previously reported data on these mutants without the YFP tag (24, 25). Western blotting was performed on the cell extracts to validate the expression of the mutant receptors (Fig. 6D) and revealed comparable expression levels of protein with the appropriate size.
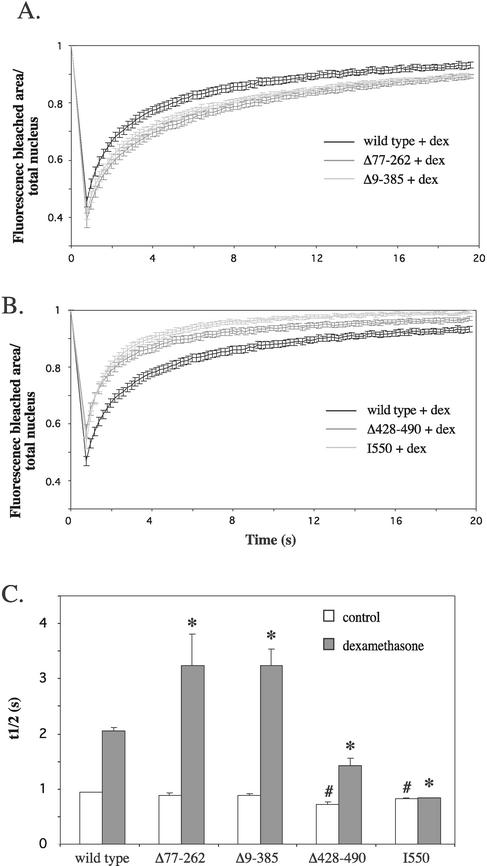
FRAP analysis was performed on these deletion mutants both in the absence of hormone and in the presence of 100 nM dexamethasone (Fig. 7). In the absence of hormone, all deletion mutants show a relatively high mobility, which is reflected in t1/2's that are all under 1 s (Fig. 7C). Mutants Δ428-490 and I550 had a slightly higher mobility than the wild-type receptor (t1/2, 0.72 ± 0.05 s for Δ428-490 and 0.83 ± 0.02 s for I550 versus 0.94 ± 0.01 for the wild type). No differences were observed between mutants Δ77-262, Δ9-385, and the wild-type receptor in the absence of hormone. In contrast, in the presence of hormone the two N-terminal deletion mutants showed a slower recovery than the wild type (Fig. 7A), which was reflected in their t1/2's (Fig. 7C), which were 3.24 ± 0.58 s for Δ77-262 s and 3.24 ± 0.58 s for Δ9-385 versus 2.05 ± 0.07 s for wild-type YFP-hGRα. This decrease in mobility is not maximal, since in a separate experiment we measured an even lower mobility of the Δ9-385 deletion mutant in response to triamcinolone acetonide (t1/2, 5.93 ± 0.51 s versus 3.73 ± 0.46 for dexamethasone). The mutants Δ428-490 and I550 showed a faster recovery rate than the wild type (Fig. 7B), and t1/2's were 1.42 ± 0.14 s and 0.84 ± 0.01 s, respectively (Fig. 7C). These data indicate that the absence of C-terminal domains in the GR increases the mobility of the receptor, whereas the absence of N-terminal domains makes the ligand-activated receptor less mobile in the presence of ligand.
FIG. 7.
Multiple domains determine the mobility of YFP-hGRα. (A) FRAP analysis of deletion mutants Δ77-262 and Δ9-385, in the presence of 100 nM dexamethasone. Representative fluorescence curves are presented for each mutant. The results indicate that Δ77-262 and Δ9-385 are more immobile in the presence of dexamethasone than the wild type receptor. (B) FRAP analysis of mutants Δ428-490 and I550, after addition of 100 nM dexamethasone. Mutants Δ428-490 and I550 displayed a higher mobility than the wild type receptor. (C) t1/2 for mutants Δ77-262, Δ9-385, Δ428-490, and I550 in the absence of hormone and after addition of 100 nM dexamethasone. Mutants Δ77-262 and Δ9-385 show a longer t1/2 than the wild-type receptor in the presence of dexamethasone, whereas Δ428-490 and I550 had a shorter t1/2 under these conditions. #, significantly different from control-treated wild type, *, significantly different from dexamethasone-treated wild type (P < 0.05).
Mobility of YFP-hGRα is cell type specific.
Since all experiments described above were performed in COS-1 cells (African green monkey kidney cells), we next examined if the mobilities induced by different ligands are similar in other cell types. In addition to COS-1 cells, we examined the human embryonic kidney cell line HEK293 and the human cervical carcinoma cell line HeLa.
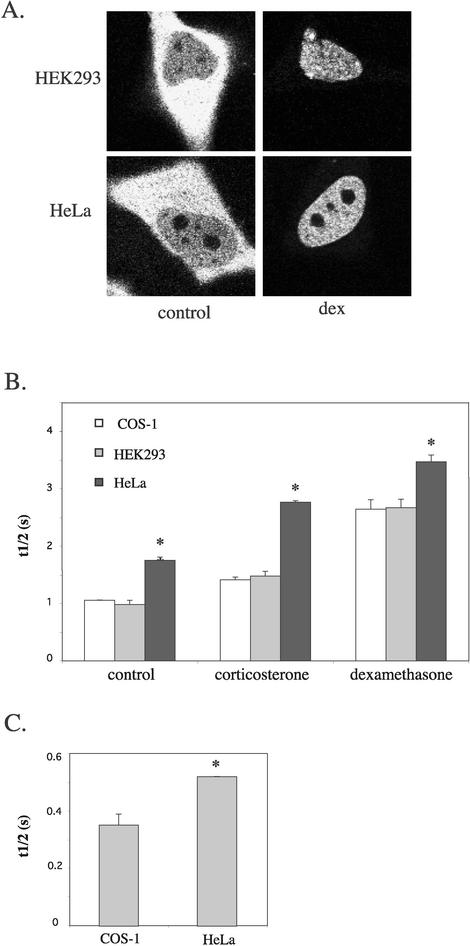
All three cell types were transfected with YFP-hGRα, and representative pictures of YFP-hGRα in HEK293 and HeLa cells are shown in Fig. 8A. FRAP was performed in the absence of hormone and after addition of corticosterone or dexamethasone (1 μM). The t1/2's are shown in Fig. 8B. In HEK293 cells the mobilities of unliganded, corticosterone- and dexamethasone-bound YFP-hGRα were similar to those in COS-1 cells. In contrast, the mobilities observed in HeLa cells differed. Unliganded (t1/2, 1.56 ± 0.05 s), corticosterone-bound (t1/2, 2.46 ± 0.02 s), and dexamethasone-bound (t1/2, 3.09 ± 0.10 s) YFP-hGRα moved slower in HeLa cells than in COS or HEK293 cells. In all three cell types, dexamethasone-bound YFP-hGRα displayed a lower mobility than corticosterone-bound YFP-hGRα, which in turn moved slower than the unliganded receptor. These data suggest that in all cell types ligands decrease receptor mobility to an extent depending on the affinity of the ligand. However, absolute mobilities differ among cell types. We next studied if the relatively low mobility of the GR in HeLa cells was a phenomenon specific for this protein. Therefore, we transfected YFP into COS-1 and HeLa cells and analyzed the mobility of YFP in the nucleus by FRAP. t1/2 for YFP in COS-1 and HeLa cells is shown in Fig. 8C. YFP appears to move more slowly in the nucleus of HeLa cells than in COS-1 nuclei (t1/2, 0.35 ± 0.04 and 0.52 ± 0.00 s, respectively), suggesting that proteins in general display a relatively low mobility in the nucleus of HeLa cells compared to other cell types.
FIG. 8.
The mobility of YFP-hGRα differs among cell types. (A) Representative pictures of HEK293 and HeLa cells transfected with YFP-hGRα, in the absence of hormone and after addition of 1 μM dexamethasone. (B) FRAP analysis. YFP-hGRα was transfected into COS-1, HEK293, and HeLa cells. FRAP was performed in the absence of hormone and after addition of 1,000 nM corticosterone or dexamethasone. t1/2's are presented for each combination of cell type and ligand. No difference was observed between COS-1 and HEK293 cells, but t1/2's measured in HeLa cells were higher than in the other cell types. In all cell types, dexamethasone induced a higher t1/2 than corticosterone, which in turn induced a t1/2 higher than the t1/2 observed in the absence of hormone. *, significantly different from that under similar conditions in COS-1 cells (P < 0.05). (C) The higher mobility in HeLa cells is not specific for the GR. YFP was transfected into COS-1 and HeLa cells and FRAP was performed. t1/2 is presented for YFP in each cell type. In HeLa cells, t1/2 for YFP was higher than in COS-1 cells, reflecting a lower mobility for YFP in this cell type. *, significantly different from that under similar conditions in COS-1 cells (P < 0.05).
The proteasome inhibitor MG132 immobilizes a fraction of the YFP-hGRα pool in the nucleus in the absence of ligand.
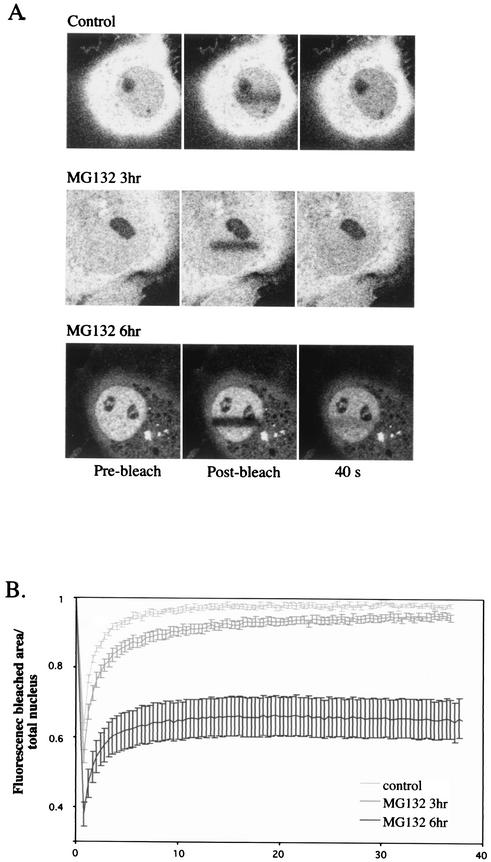
Stenoien et al. (54) have recently reported that the proteasome inhibitor MG132 dramatically decreases the mobility of ERα, and this observation has been confirmed for GR by DeRoo et al. (13), although neither report has provided quantitative information. In order to compare it to the effects we have described in the present study, we incorporated an experiment in which we subjected YFP-hGRα-transfected COS-1 cells to MG132 treatment. The transfected cells were treated with 10 μM MG132 for 3 or 6 h (no hormone was added). Six hours after MG132 addition the distribution of YFP-hGRα was predominantly nuclear (consistent with previous data [13]) (Fig. 9A). When FRAP analysis was performed at this time point, quantitation of the data revealed that MG132 creates an immobile fraction of YFP-hGRα molecules, as illustrated by the fact that the bleach line remains visible for up to 40 s (Fig. 9A). The fluorescence curve of this experiment (Fig. 9B) shows more efficient bleaching after 6 h of MG132 treatment, indicating a lower mobility. In addition, in the recovery phase the fluorescence does not asymptote towards 1.0 as in all other curves in our study presented thus far, but the level of fluorescence remains at around 0.6 after 20 s. This result indicates that there is no full exchange of fluorescent molecules between the bleached region and the rest of the cell; hence, a fraction of molecules is immobile during this time period. Thus, rather than decreasing the mobility of all YFP-hGRα molecules in the nucleus, MG132 treatment completely immobilizes a subpopulation of molecules. The relatively long period of time before this effect appears suggests that the effect is due to the accumulation of one or more proteins affecting hGRα's mobility, rather than the inhibition of the proteasome per se, since inhibition of the proteasome is observed as soon as 15 min after addition of MG132 (41).
FIG. 9.
MG132 immobilizes a fraction of YFP-hGRα molecules. COS-1 cells were transfected with YFP-hGRα, treated with the proteasome inhibitor MG132, and subjected to FRAP analysis. (A) Representative pictures showing intracellular distribution of YFP-hGRα and FRAP under control conditions and 3 and 6 h after addition of 10 μM MG132. MG132 treatment resulted in nuclear translocation of YFP-hGRα and fluorescence recovery in the bleached region is not complete within the time period studied. (B) Quantitation of FRAP analysis. At 6 h after MG132 addition, the FRAP curve does not asymptote to 1, but becomes a horizontal line around 0.6, indicating that a subpopulation of molecules has been immobilized.
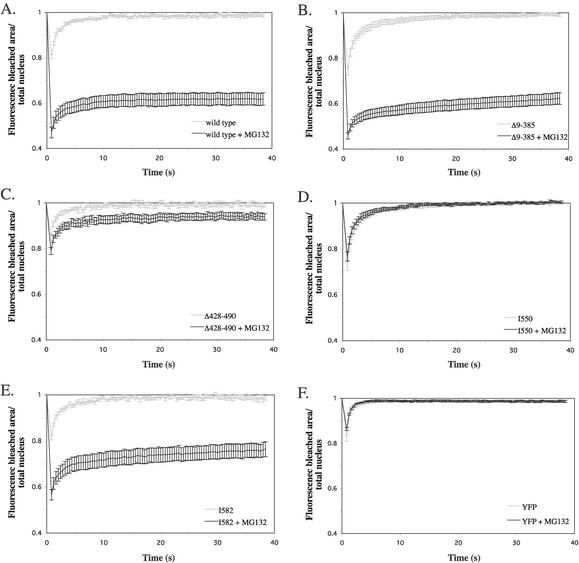
To understand the basis for the MG132 effect on hGRα, we examined which regions in the receptor are required for the immobilization by MG132. We used the previously mentioned YFP-tagged hGRα mutants Δ9-385, Δ428-490, and I550 and an additional mutant I582, which is truncated after amino acid 582. The wild-type and mutant receptors were transfected into COS-1 cells, and 6 h after MG132 addition (10 μM) FRAP analysis was performed (Fig. 10A to E). At this time point, mutant Δ9-385 (Fig. 10B) showed a fluorescence recovery which was similar to that of the wild-type receptor (Fig. 10A). In contrast, mutant Δ428-490 displayed an almost complete recovery of the fluorescence after MG132 treatment (Fig. 10C), indicating that the DNA-binding domain is required for the immobilization induced by MG132. The truncation mutant I550 also showed complete recovery of the fluorescence in the presence of MG132 (Fig. 10D). Interestingly, I582 displayed a very low level of recovery under these conditions (Fig. 10E), although the recovery was slightly higher than that displayed by the wild-type receptor. Together, these data indicate that the regions between amino acids 428 to 490 and 550 to 582 play a major role in this effect. In order to confirm the specificity of the MG132 effect, we measured the effect of MG132 treatment on the mobility of YFP by performing FRAP analysis on YFP-transfected cells. No effect of MG132 treatment on YFP mobility was detected, as shown by the identical FRAP curves that were obtained in the absence and presence of MG132 (Fig. 10F).
FIG. 10.
Multiple domains determine the immobilization of unliganded YFP-hGRα by MG132. FRAP analysis was performed in the absence and presence of MG132 (10 μM, 6 h) of the YFP-tagged wild-type receptor (A), deletion mutants Δ9-385 (B), Δ428-490 (C), truncation mutants I550 (D) and I582 (E), and YFP alone (F). Representative fluorescence curves of at least two experiments are presented for each mutant. MG132 treatment did not alter the mobility of I550 and hardly changed the fluorescence curve of Δ428-490. In contrast, a large immobilizing effect of MG132 was observed when administered to Δ9-385 and I582, similar to that observed for the wild type. MG132 did not affect the mobility of YFP.
Ligand binding induces resistance to immobilization by MG132 in an affinity-dependent manner.
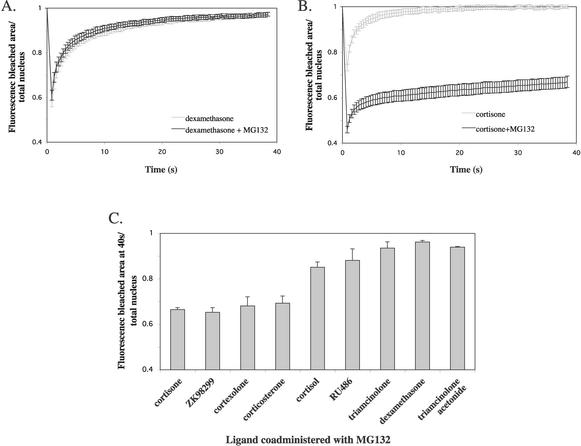
Finally, we evaluated the effect of proteasome inhibition on a liganded receptor by treating YFP-hGRα-transfected cells with MG132 (10 μM) and dexamethasone (1 μM) simultaneously and performing FRAP analysis 6 h after administration. Surprisingly, the fluorescence curve from the MG132- and dexamethasone-treated cells overlapped with the curve derived from cells treated with dexamethasone alone (Fig. 11A). Apparently, the receptor ligated with dexamethasone is resistant to the immobilizing effect of MG132. Subsequently, we performed a similar experiment coadministering MG132 and the low-affinity ligand cortisone. Under these conditions, the receptor is not resistant to the effect of MG132, as shown by the fluorescence curve (Fig. 11B), which shows a low level of recovery that is similar to that with the unliganded receptor. To study which ligands induce resistance to hGRα immobilization by MG132, we performed FRAP experiments in the presence of MG132 for seven more ligands (ZK98299, cortexolone, corticosterone, cortisol, RU486, triamcinolone, and triamcinolone acetonide). For each ligand, the fluorescence level in the bleached region at 40 s after bleaching was determined, and results are presented in Fig. 11C. In the presence of high-affinity ligands like triamcinolone acetonide, dexamethasone, and triamcinolone the fluorescence level at 40 s approached 1.0 (0.94 ± 0.00, 0.96 ± 0.01, and 0.94 ± 0.03, respectively), indicating almost complete recovery at this time point, which means that no subpopulation of receptors has been immobilized. In contrast, in the presence of low-affinity ligands like cortisone, ZK98299, cortexolone, and corticosterone, the fluorescence at 40 s was significantly lower (0.66 ± 0.08, 0.65 ± 0.02, 0.68 ± 0.04, and 0.69 ± 0.03, respectively), indicating very low recovery of the fluorescence and a large pool of receptors immobilized by MG132 action. In the presence of cortisol and RU486, intermediate levels of fluorescence were observed at 40 s (0.85 ± 0.05 and 0.88 ± 0.05, respectively). Apparently, the affinity of the ligand determines the extent to which it is able to induce resistance to the immobilizing effect of MG132.
FIG. 11.
Ligand binding induces resistance to YFP-hGRα immobilization by MG132 in an affinity-dependent way. FRAP analysis was performed 6 h after coadministration of MG132 (10 μM) and different ligands (1 μM). (A and B) Representative fluorescence curves for dexamethasone (A) and cortisone (B) in the absence and presence of MG132. Dexamethasone induced resistance to MG132, whereas in the presence of cortisone a subpopulation of receptors was still immobilized by MG132. (C) Fluorescence levels at 40 s after bleaching in the presence of MG132 and a ligand. Ligands are presented in the order of their affinity. In the presence of a high-affinity ligand, the fluorescence level at 40 s approached 1.0, whereas in the presence of a low-affinity ligand the level was significantly lower. This indicates that high-affinity ligand binding prevents the immobilization of a subpopulation of receptors by MG132, and low-affinity binding does not.
DISCUSSION
In the present study, we have evaluated the mobility of hGRα in the nucleus. Our results show that ligand-induced activation of hGRα decreases receptor mobility, an observation which is consistent with recent studies on the mobility of ERα (54). Our results further show that the extent of this decrease is ligand dependent and that the affinity of the ligand is an important determinant of receptor mobility. High-affinity ligands like dexamethasone and triamcinolone acetonide appeared to decrease the mobility to a larger extent than low-affinity ligands like cortexolone and corticosterone. The transcriptional properties of a ligand do not correlate with receptor mobility, since the mobilities induced by the antagonists RU486 and ZK98299 could be predicted based on their affinity. Virtually all hGRα molecules that are present in the nucleus move relatively fast through this organelle, except after addition of the proteasome inhibitor MG132, which immobilizes a fraction of hGRα molecules in the nucleus. However, under all other conditions studied, no evidence for an immobile fraction was found.
What could be the mechanism of this affinity-based difference between ligands? Obviously, at low concentrations of the ligand, the difference in mobility can be explained by the number of ligand-bound receptors. However, at a concentration of 1 μM even low-affinity ligands like cortexolone and corticosterone saturate hGRα binding. Therefore, a difference in receptor mobility at this concentration of ligand cannot simply be explained by a difference in receptor binding. We hypothesize that upon ligand binding the receptor undergoes a conformational change, of which the degree correlates with the affinity of the ligand. This conformational change enables the receptor to transiently bind nuclear structures or domains that are immobile or moving slowly, which is reflected in a decrease in the average mobility of the receptors in the nucleus.
This hypothesis raises the question of what structures ligand-activated hGRα is binding in the nucleus. DNA binding is not likely to affect the average mobility of hGRα in the nucleus, since most likely the vast majority of GRs in the nucleus are not associated with chromatin. This assumption is based on the limited number of GREs in the genome compared to the number of receptors in the nucleus. In addition, it has been shown that the majority of nuclear GR clusters are not the sites where transcription initiation occurs (21, 59). The lack of effect of DNA binding on decreasing receptor mobility was confirmed by our results with the deletion mutants Δ428-490 and I550. Mutant Δ428-490 lacks the complete DNA-binding domain and is therefore unable to bind DNA, but its mobility decreases significantly upon ligand binding. In contrast, mutant I550 is able to bind DNA but moves with a higher mobility than Δ428-490.
It was suggested by Stenoien et al. (53) that the mobility of ERα depends on binding to the nuclear matrix, and the mobility changes of GR observed in the present study may be explained in a similar way. The nuclear matrix is originally defined as the nonchromatin structural components of the nucleus. In most experimental reports, it is referred to as the structure that is left after extraction of most of the chromatin and soluble and loosely bound proteins. Although a nuclear matrix is still a controversial concept (42), the idea is accepted by many researchers (39). Several laboratories have studied steroid receptors in nuclear matrix preparations. ER, AR and GR are present in these preparations only after ligand binding (2, 52, 60). The presence of GR and AR in these preparations appears to depend on the ligand-binding domain and DNA-binding domain of the receptor (60). Tang et al. (56) have suggested that the τ2 region of the rat GR contains a nuclear matrix targeting signal, which can interact with the RNA-binding hnRNP U, a protein which is one of the main constituents of nuclear matrix preparations (17, 35) and which has been shown to be associated with GR in intact cells (16). Overexpression of hnRNP U inhibits GR-induced transactivation, and this effect appears to depend on the physical interaction between these proteins (15, 56). It will be interesting to determine if the affinity of the ligand determines the extent of nuclear matrix binding, which is presently unknown. However, early studies by Cidlowski and Munck (11) showed that dexamethasone- or triamcinolone acetonide-bound GR is more resistant to high salt extraction from the nucleus than is corticosterone-bound GR, indicating stronger binding to nuclear structures induced by the high-affinity ligands.
The mobilities observed for the deletion mutants are consistent with a model in which nuclear matrix binding decreases the mobility of the ligand-bound receptor. In our study, the mobility of ligand-bound hGRα was decreased when the ligand-binding domain or the DNA-binding domain was deleted. Van Steensel et al. (60) have shown absence of these mutants in nuclear matrix preparations. In addition, deletion of amino acids 77 to 262 showed a lower mobility of GR after dexamethasone binding compared to the wild type. Since this region contains all eight putative phosphorylation sites, this effect may be due to decreased phosphorylation of the mutant receptor. GRs in ATP-depleted cells are dephosphorylated (30) and bind strongly to the nuclear matrix (55).
In addition, we have demonstrated that the mobility of hGRα is cell type dependent, although the difference between unliganded, corticosterone-bound, and dexamethasone-bound receptor was similar among all cell types studied. Under all conditions studied, hGRα had a lower mobility in HeLa cells than in COS-1 or HEK293 cells, and this effect is most likely a general phenomenon for nuclear proteins in this cell type, since YFP as well displayed a lower mobility in HeLa cells compared to COS-1 cells. What cell characteristic determines this difference in mobility of nuclear proteins remains to be elucidated.
Finally, we have demonstrated that the proteasomal inhibitor MG132 decreases the mobility of hGRα, confirming a previous study with GR (13) and an earlier report on ERα (54). In contrast to these studies, the quantitative nature of our analysis has allowed us to show that MG132 immobilizes only a fraction of receptors in the nucleus, rather than decreasing the mobility of the entire GR pool. Experiments using receptor deletion mutants revealed that the DNA-binding domain and the N-terminal part of the ligand-binding domain (until amino acid 582) are largely responsible for the receptor immobilization by MG132. In addition, we have shown for the first time that ligand binding makes the receptor resistant to the immobilizing effect of MG132. This ligand effect appears to be dependent on its affinity for the receptor. Our data suggest that upon inhibition of the proteasome by MG132, a nuclear protein accumulates which traps hGRα in the nucleus by binding to specific regions in the DNA-binding domain and the ligand-binding domain. Upon ligand binding, the receptor undergoes an affinity-dependent conformational change that alters or hides these regions, thereby inhibiting the effect of MG132 on receptor mobility.
Acknowledgments
We thank Jeff Reece and Gary Bird for their assistance with the FRAP experiments, Masahiko Negishi for providing the plasmid pEYFP-hGRα, and Gary Bird and Tatsuya Sueyoshi for critical reading of the manuscript.
REFERENCES
- 1.Allgood, V. E., F. E. Powell-Oliver, and J. A. Cidlowski. 1990. Vitamin B6 influences glucocorticoid receptor-dependent gene expression. J. Biol. Chem. 265:12424-12433. [PubMed] [Google Scholar]
- 2.Barrack, E. R. 1987. Steroid hormone receptor localization in the nuclear matrix: interaction with acceptor sites. J. Steroid Biochem. 27:115-121. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, C. T., P. Maruvada, G. L. Hager, and P. M. Yen. 2001. Nuclear cytoplasmic shuttling by thyroid hormone receptors. Multiple protein interactions are required for nuclear retention. J. Biol. Chem. 276:11237-11245. [DOI] [PubMed] [Google Scholar]
- 4.Beato, M., and A. Sanchez-Pacheco. 1996. Interaction of steroid hormone receptors with the transcription initiation complex. Endocr. Rev. 17:587-609. [DOI] [PubMed] [Google Scholar]
- 5.Bledsoe, R. K., V. G. Montana, T. B. Stanley, C. J. Delves, C. J. Apolito, D. D. McKee, T. G. Consler, D. J. Parks, E. L. Stewart, T. M. Willson, M. H. Lambert, J. T. Moore, K. H. Pearce, and H. E. Xu. 2002. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93-105. [DOI] [PubMed] [Google Scholar]
- 6.Bocquel, M. T., J. Ji, T. Ylikomi, B. Benhamou, A. Vergezac, P. Chambon, and H. Gronemeyer. 1993. Type II antagonists impair the DNA binding of steroid hormone receptors without affecting dimerization. J. Steroid Biochem. Mol. Biol. 45:205-215. [DOI] [PubMed] [Google Scholar]
- 7.Burnstein, K. L., C. M. Jewell, M. Sar, and J. A. Cidlowski. 1994. Intragenic sequences of the human glucocorticoid receptor complementary DNA mediate hormone-inducible receptor messenger RNA down-regulation through multiple mechanisms. Mol. Endocrinol. 8:1764-1773. [DOI] [PubMed] [Google Scholar]
- 8.Cadepond, F., A. Ulmann, and E. E. Baulieu. 1997. RU486 (mifepristone): mechanisms of action and clinical uses. Annu. Rev. Med. 48:129-156. [DOI] [PubMed] [Google Scholar]
- 9.Caldenhoven, E., J. Liden, S. Wissink, A. Van de Stolpe, J. Raaijmakers, L. Koenderman, S. Okret, J. A. Gustafsson, and P. T. Van der Saag. 1995. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Mol. Endocrinol. 9:401-412. [DOI] [PubMed] [Google Scholar]
- 10.Cidlowski, J. A., D. L. Bellingham, F. E. Powell-Oliver, D. B. Lubahn, and M. Sar. 1990. Novel antipeptide antibodies to the human glucocorticoid receptor: recognition of multiple receptor forms in vitro and distinct localization of cytoplasmic and nuclear receptors. Mol. Endocrinol. 4:1427-1437. [DOI] [PubMed] [Google Scholar]
- 11.Cidlowski, J. A., and A. Munck. 1980. Multiple forms of nuclear binding of glucocorticoid-receptor complexes in rat thymocytes. J. Steroid Biochem. 13:105-112. [DOI] [PubMed] [Google Scholar]
- 12.Collingwood, T. N., F. D. Urnov, and A. P. Wolffe. 1999. Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol. 23:255-275. [DOI] [PubMed] [Google Scholar]
- 13.Deroo, B. J., C. Rentsch, S. Sampath, J. Young, D. B. DeFranco, and T. K. Archer. 2002. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol. Cell. Biol. 22:4113-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drouin, J., M. A. Trifiro, R. K. Plante, M. Nemer, P. Eriksson, and O. Wrange. 1989. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol. Cell. Biol. 9:5305-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggert, H., M. Schulz, F. O. Fackelmayer, R. Renkawitz, and M. Eggert. 2001. Effects of the heterogeneous nuclear ribonucleoprotein U (hnRNP U/SAF-A) on glucocorticoid-dependent transcription in vivo. J. Steroid Biochem. Mol. Biol. 78:59-65. [DOI] [PubMed] [Google Scholar]
- 16.Eggert, M., J. Michel, S. Schneider, H. Bornfleth, A. Baniahmad, F. O. Fackelmayer, S. Schmidt, and R. Renkawitz. 1997. The glucocorticoid receptor is associated with the RNA-binding nuclear matrix protein hnRNP U. J. Biol. Chem. 272:28471-28478. [DOI] [PubMed] [Google Scholar]
- 17.Fackelmayer, F. O., and A. Richter. 1994. Purification of two isoforms of hnRNP-U and characterization of their nucleic acid binding activity. Biochemistry 33:10416-10422. [DOI] [PubMed] [Google Scholar]
- 18.Fejes-Toth, G., D. Pearce, and A. Naray-Fejes-Toth. 1998. Subcellular localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc. Natl. Acad. Sci. USA 95:2973-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannopoulos, G., and D. Keichline. 1981. Species-related differences in steroid-binding specificity of glucocorticoid receptors in lung. Endocrinology 108:1414-1419. [DOI] [PubMed] [Google Scholar]
- 20.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 21.Grande, M. A., I. van der Kraan, L. de Jong, and R. van Driel. 1997. Nuclear distribution of transcription factors in relation to sites of transcription and RNA polymerase II. J. Cell Sci. 110:1781-1791. [DOI] [PubMed] [Google Scholar]
- 22.Heikinheimo, O., K. Kontula, H. Croxatto, I. Spitz, T. Luukkainen, and P. Lahteenmaki. 1987. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. J. Steroid Biochem. 26:279-284. [DOI] [PubMed] [Google Scholar]
- 23.Hellal-Levy, C., B. Couette, J. Fagart, A. Souque, C. Gomez-Sanchez, and M. Rafestin-Oblin. 1999. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 464:9-13. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg, S. M., and R. M. Evans. 1988. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell 55:899-906. [DOI] [PubMed] [Google Scholar]
- 25.Hollenberg, S. M., V. Giguere, P. Segui, and R. M. Evans. 1987. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell 49:39-46. [DOI] [PubMed] [Google Scholar]
- 26.Hoschutzky, H., and O. Pongs. 1985. Characterization of glucocorticoid receptor in HeLa-S3 cells. Biochemistry 24:7348-7356. [DOI] [PubMed] [Google Scholar]
- 27.Houtsmuller, A. B., S. Rademakers, A. L. Nigg, D. Hoogstraten, J. H. Hoeijmakers, and W. Vermeulen. 1999. Action of DNA repair endonuclease ERCC1/XPF in living cells. Science 284:958-961. [DOI] [PubMed] [Google Scholar]
- 28.Htun, H., J. Barsony, I. Renyi, D. L. Gould, and G. L. Hager. 1996. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc. Natl. Acad. Sci. USA 93:4845-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Htun, H., L. T. Holth, D. Walker, J. R. Davie, and G. L. Hager. 1999. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Mol. Biol. Cell. 10:471-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu, L. M., J. Bodwell, J. M. Hu, E. Orti, and A. Munck. 1994. Glucocorticoid receptors in ATP-depleted cells. Dephosphorylation, loss of hormone binding, HSP90 dissociation, and ATP-dependent cycling. J. Biol. Chem. 269:6571-6577. [PubMed] [Google Scholar]
- 31.Jenkins, B. D., C. B. Pullen, and B. D. Darimont. 2001. Novel glucocorticoid receptor coactivator effector mechanisms. Trends Endocrinol. Metab. 12:122-126. [DOI] [PubMed] [Google Scholar]
- 32.Jewell, C. M., J. C. Webster, K. L. Burnstein, M. Sar, J. E. Bodwell, and J. A. Cidlowski. 1995. Immunocytochemical analysis of hormone mediated nuclear translocation of wild type and mutant glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 55:135-146. [DOI] [PubMed] [Google Scholar]
- 33.Jonat, C., H. J. Rahmsdorf, K. K. Park, A. C. Cato, S. Gebel, H. Ponta, and P. Herrlich. 1990. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62:1189-1204. [DOI] [PubMed] [Google Scholar]
- 34.Lind, U., P. Greenidge, M. Gillner, K. F. Koehler, A. Wright, and J. Carlstedt-Duke. 2000. Functional probing of the human glucocorticoid receptor steroid-interacting surface by site-directed mutagenesis. Gln-642 plays an important role in steroid recognition and binding. J. Biol. Chem. 275:19041-19049. [DOI] [PubMed] [Google Scholar]
- 35.Mattern, K. A., B. M. Humbel, A. O. Muijsers, L. de Jong, and R. van Driel. 1996. hnRNP proteins and B23 are the major proteins of the internal nuclear matrix of HeLa S3 cells. J. Cell Biochem. 62:275-289. [DOI] [PubMed] [Google Scholar]
- 36.McKenna, N. J., R. B. Lanz, and B. W. O'Malley. 1999. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20:321-344. [DOI] [PubMed] [Google Scholar]
- 37.McNally, J. G., W. G. Muller, D. Walker, R. Wolford, and G. L. Hager. 2000. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287:1262-1265. [DOI] [PubMed] [Google Scholar]
- 38.Nakai, Y., T. Usui, T. Tsukada, H. Takahashi, J. Fukata, M. Fukushima, K. Senoo, and H. Imura. 1991. Molecular mechanisms of glucocorticoid inhibition of human proopiomelanocortin gene transcription. J. Steroid Biochem. Mol. Biol. 40:301-306. [DOI] [PubMed] [Google Scholar]
- 39.Nickerson, J. 2001. Experimental observations of a nuclear matrix. J. Cell Sci. 114:463-474. [DOI] [PubMed] [Google Scholar]
- 40.Oakley, R. H., M. Sar, and J. A. Cidlowski. 1996. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 271:9550-9559. [DOI] [PubMed] [Google Scholar]
- 41.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 42.Pederson, T. 2000. Half a century of “the nuclear matrix.” Mol. Biol. Cell. 11:799-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phair, R. D., and T. Misteli. 2000. High mobility of proteins in the mammalian cell nucleus. Nature 404:604-609. [DOI] [PubMed] [Google Scholar]
- 44.Picard, D., and K. R. Yamamoto. 1987. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 6:3333-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pratt, W. B., and K. D. Dittmar. 1998. Studies with purified chaperones advance the understanding of the mechanism of glucocorticoid receptor-hsp90 heterocomplex assembly. Trends Endocrinol. Metab. 9:244-252. [DOI] [PubMed] [Google Scholar]
- 46.Racz, A., and J. Barsony. 1999. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J. Biol. Chem. 274:19352-19360. [DOI] [PubMed] [Google Scholar]
- 47.Ray, A., and K. E. Prefontaine. 1994. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 91:752-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupprecht, R., J. M. Reul, B. van Steensel, D. Spengler, M. Soder, B. Berning, F. Holsboer, and K. Damm. 1993. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur. J. Pharmacol. 247:145-154. [DOI] [PubMed] [Google Scholar]
- 49.Saitoh, M., R. Takayanagi, K. Goto, A. Fukamizu, A. Tomura, T. Yanase, and H. Nawata. 2002. The presence of both the amino- and carboxyl-terminal domains in the AR is essential for the completion of a transcriptionally active form with coactivators and intranuclear compartmentalization common to the steroid hormone receptors: a three-dimensional imaging study. Mol. Endocrinol. 16:694-706. [DOI] [PubMed] [Google Scholar]
- 50.Scheinman, R. I., A. Gualberto, C. M. Jewell, J. A. Cidlowski, and A. S. Baldwin, Jr. 1995. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol. Cell. Biol. 15:943-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schule, R., P. Rangarajan, S. Kliewer, L. J. Ransone, J. Bolado, N. Yang, I. M. Verma, and R. M. Evans. 1990. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell 62:1217-1226. [DOI] [PubMed] [Google Scholar]
- 52.Stenoien, D. L., M. G. Mancini, K. Patel, E. A. Allegretto, C. L. Smith, and M. A. Mancini. 2000. Subnuclear trafficking of estrogen receptor-alpha and steroid receptor coactivator-1. Mol. Endocrinol. 14:518-534. [DOI] [PubMed] [Google Scholar]
- 53.Stenoien, D. L., A. C. Nye, M. G. Mancini, K. Patel, M. Dutertre, B. W. O'Malley, C. L. Smith, A. S. Belmont, and M. A. Mancini. 2001. Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor alpha-coactivator complexes in living cells. Mol. Cell. Biol. 21:4404-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenoien, D. L., K. Patel, M. G. Mancini, M. Dutertre, C. L. Smith, B. W. O'Malley, and M. A. Mancini. 2001. FRAP reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat. Cell Biol. 3:15-23. [DOI] [PubMed] [Google Scholar]
- 55.Tang, Y., and D. B. DeFranco. 1996. ATP-dependent release of glucocorticoid receptors from the nuclear matrix. Mol. Cell. Biol. 16:1989-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang, Y., R. H. Getzenberg, B. N. Vietmeier, M. R. Stallcup, M. Eggert, R. Renkawitz, and D. B. DeFranco. 1998. The DNA-binding and tau2 transactivation domains of the rat glucocorticoid receptor constitute a nuclear matrix-targeting signal. Mol. Endocrinol. 12:1420-1431. [DOI] [PubMed] [Google Scholar]
- 57.Tomura, A., K. Goto, H. Morinaga, M. Nomura, T. Okabe, T. Yanase, R. Takayanagi, and H. Nawata. 2001. The subnuclear three-dimensional image analysis of androgen receptor fused to green fluorescence protein. J. Biol. Chem. 276:28395-28401. [DOI] [PubMed] [Google Scholar]
- 58.Tyagi, R. K., Y. Lavrovsky, S. C. Ahn, C. S. Song, B. Chatterjee, and A. K. Roy. 2000. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol. Endocrinol. 14:1162-1174. [DOI] [PubMed] [Google Scholar]
- 59.van Steensel, B., M. Brink, K. van der Meulen, E. P. van Binnendijk, D. G. Wansink, L. de Jong, E. R. de Kloet, and R. van Driel. 1995. Localization of the glucocorticoid receptor in discrete clusters in the cell nucleus J. Cell Sci. 108:3003-3011. [DOI] [PubMed] [Google Scholar]
- 60.van Steensel, B., G. Jenster, K. Damm, A. O. Brinkmann, and R. van Driel. 1995. Domains of the human androgen receptor and glucocorticoid receptor involved in binding to the nuclear matrix. J. Cell Biochem. 57:465-478. [DOI] [PubMed] [Google Scholar]
- 61.Yang-Yen, H. F., J. C. Chambard, Y. L. Sun, T. Smeal, T. J. Schmidt, J. Drouin, and M. Karin. 1990. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62:1205-1215. [DOI] [PubMed] [Google Scholar]
- 62.Zelko, I., T. Sueyoshi, T. Kawamoto, R. Moore, and M. Negishi. 2001. The peptide near the C terminus regulates receptor CAR nuclear translocation induced by xenochemicals in mouse liver. Mol. Cell. Biol. 21:2838-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, S., and M. Danielsen. 1995. 8-Br-cAMP does not convert antagonists of the glucocorticoid receptor into agonists. Recent Prog. Hormone Res. 50:429-435. [DOI] [PubMed] [Google Scholar]