Abstract
OBJECTIVES
To define racial similarities and differences in mobility among community-dwelling older adults and to identify predictors of mobility change.
DESIGN
Prospective, observational, cohort study.
PARTICIPANTS
Nine hundred and five community-dwelling older adults.
MEASURES
Baseline in-home assessments were conducted to assess life-space mobility, sociodemographic variables, disease status, geriatric syndromes, neuropsychological factors, and health behaviors. Disease reports were verified by review of medications, physician questionnaires, or hospital discharge summaries. Telephone interviews defined follow-up life-space mobility at 18 months of follow-up.
RESULTS
African Americans had lower baseline life-space (LS-C) than whites (mean 57.0 ± standard deviation [SD] 24.5 vs. 72.7 ± SD 22.6; P < .001). This disparity in mobility was accompanied by significant racial differences in socioeconomic and health status. After 18 months of follow-up, African Americans were less likely to show declines in LS-C than whites. Multivariate analyses showed racial differences in the relative importance and strength of the associations between predictors and LS-C change. Age and diabetes were significant predictors of LS-C decline for both African Americans and whites. Transportation difficulty, kidney disease, dementia, and Parkinson's disease were significant for African Americans, while low education, arthritis/gout, stroke, neuropathy, depression, and poor appetite were significant for whites.
CONCLUSIONS
There are significant disparities in baseline mobility between older African Americans and whites, but declines were more likely in whites. Improving transportation access and diabetes care may be important targets for enhancing mobility and reducing racial disparities in mobility.
Keywords: aging, mobility, function, African American, minority health, health disparities
Mobility is a complex function reflecting multiple domains of activity1 and may be the most important functional ability required to maintain social roles and pursuits among older adults.2 Mobility problems have been identified as the most common domain of disability in older men and women.3–5 Mobility restriction affects quality of life, independent living, and personal autonomy, and increases both formal and informal care needs.1,6,7
Studies have identified disparities between older African Americans and whites in functional ability and physical performance,8–15 but the underlying causes have not been established. Higher rates of disabling conditions, economic disadvantage, inadequate access to health care, and social factors potentially contribute to this disparity.
Seeman et al.16 found that older African Americans were more likely than whites to show both improvement and decline in measures of physical performance; however, the limited number of African Americans in most prospective studies has made it difficult to assess racial differences in predictors of function. Prospective studies are needed to identify modifiable risk factors for functional loss and to understand the causes for disparities in function for older African Americans and whites. Such data are also needed to guide the development of interventions to eliminate racial differences.
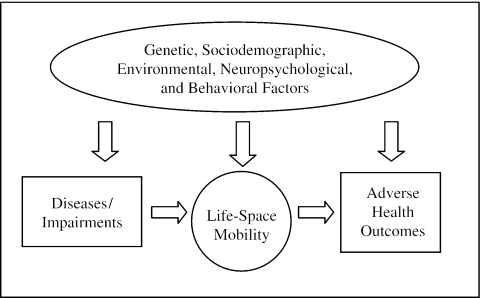
Life-space is a spatial measure of mobility describing the area through which a person moves over a specified time period.6,7,17–20 A life-space assessment incorporating where a person goes, the frequency of going there, and the need for assistance, can be used to define the full continuum and changes in mobility among community-dwelling older adults and provides a method to evaluate the impact of sociodemographic factors, diseases, geriatric syndromes, neuropsychological factors, and health behaviors on mobility change. We hypothesize that changes in life-space mobility precede adverse health outcomes such as nursing home placement and death (Fig. 1).18
FIGURE 1.
Conceptual model of the causes of adverse health outcomes among older adults.
Life-space reflects the person-environment relationship, permitting assessment of the effect of sociodemographic and community-level characteristics on mobility. Diseases, geriatric syndromes, and neuropsychological conditions occur in the context of sociodemographic factors and may lead to declines in physical performance and thereby reduce life-space. However, health behaviors may modify the impact of these factors on life-space. Life-space has been shown to correlate with measures of physical and mental health, including physical performance, activities of daily living (ADL), instrumental activities of daily living (IADL) depression, self-reported health, and the number of comorbid conditions.18
The University of Alabama at Birmingham (UAB) Study of Aging is a prospective, observational study of 1,000 community-dwelling older adults designed to understand subject-specific factors predisposing older adults to mobility loss and racial differences in mobility changes associated with aging. This article describes racial disparities in life-space mobility and health status observed at baseline (in-home assessments completed between 1999 and 2001) and predictors of life-space and life-space change for African Americans and whites over 18 months.
METHODS
Subject Recruitment
Subjects were participants in the UAB Study of Aging, a population-based, prospective, observational study of community-dwelling older adults. Subjects were a random sample of Medicare beneficiaries age 65 and older living in central Alabama, selected from a list of Medicare beneficiaries provided by the Centers for Medicare and Medicaid Services (CMS) and stratified by county, race, and gender. Two counties were classified as urban and three counties as rural.21 This study oversampled African Americans, males, and rural residents to provide a balanced sample in terms of race, gender, and urban-rural residence. The study protocol was approved by the UAB Institutional Review Board.
We mailed 3,100 persons a letter signed by the CMS administrator to explain the purpose of the study and to give them a toll-free number to call if they did not want to be contacted. Ten days after these letters were mailed, we began contacting potential subjects by telephone to arrange an in-home assessment. Up to 10 calls were made at different times and on different days over a 6-week period to schedule the in-home assessment.
We were unable to contact 451 (14.5%) potential subjects; 362 (11.7%) were not contacted because the recruitment goal of 1,000 subjects was reached before an appointment for an in-home assessment could be made; and we learned that 101 (3.2%) of persons listed on the Medicare beneficiary list were dead when we attempted to call them. Of 2,186 persons contacted to arrange in-home assessments, 1,143 (52.3%) refused and 43 (2.0%) were ineligible because the subject did not live in one of the five study counties, was under the age of 65, or was unable to communicate on the telephone to make arrangements for the in-home assessment. Thus, 1,000 subjects underwent a baseline in-home assessment.
In-home interviews lasting approximately 2 hours were conducted by trained interviewers after subjects signed informed consents. Telephone follow-up interviews to assess life-space were conducted at 6-month intervals; proxy responses were included. This analysis uses data available through the 18-month follow-up telephone interview.
Study Variables
Life-Space Mobility
The UAB Study of Aging Life-Space Assessment (LSA) measures mobility based on the distance through which a person reports moving during the 4 weeks preceding the assessment.7,18 Questions establish movement to specific life-space levels ranging from within one's dwelling to beyond one's town. Frequency of movement and use of assistance (from equipment or persons) are assessed. Life-space levels were assessed by asking: “During the past 4 weeks, have you 1) been to other rooms of your home besides the room where you sleep; 2) been to an area outside your home such as your porch, deck, or patio, hallway of an apartment building, or garage; 3) been to places in your neighborhood other than your own yard or apartment building; 4) been to places outside your neighborhood, but within your town; and 5) been to places outside your town?”
For each life-space level, persons were asked how many days within a week they attained that level and whether they needed help from another person or from assistive devices to move to that level. A life-space composite score (LS-C) was calculated based upon the life-space level, the degree of independence in achieving each level, and the frequency of attaining each level. Values were computed for each life-space level by multiplying the level (1 to 5), the degree of independence (2 if independent, i.e., no assistance from persons or equipment was reported; 1.5 if equipment was used; or 1 if personal assistance was reported), and the frequency of attainment (1 = less than once a week; 2 = one to three times a week; 3 = four to six times a week; and 4 = daily). The level-specific values were summed. LS-C scores ranged from 0 to 120 with higher levels representing greater mobility. (For a copy of the LSA, please contact the authors).
Change in life-space between the baseline interview and the 18-month follow-up interview was calculated by subtracting the baseline score from the follow-up score. A positive change score indicated an increase in life-space, while a negative change score indicated a decline. Although individuals may vary in how they define distances between specific life-space levels, these definitions are consistent between interviews for a specific participant.18 For purposes of comparison, the proportion of participants who experienced a change of 10 or more points in the life-space score was determined for both African American and white study participants.
Sociodemographic Variables
Age, gender, race, marital status, education, and income were self-reported. Total combined family income before taxes was reported in 9 categories (less than $5,000 through $50,000 or more). Responses indicating perceived income adequacy (“All things considered, would you say your income is not enough to make ends meet; gives you just enough to get by on; keeps you comfortable, but permits no luxuries; or allows you to do more or less what you want?”) were used to define an income category for persons with missing responses to reported income. These responses corresponded to income categories of $5,000 to $7,999, $8,000 to $11,999, $16,000 to $19,999, and $30,000 to $39,999, respectively. Education was categorized as 1 = completed 6th grade or less; 2 = completed 7th through 11th grade; 3 = completed high school or General Educational Development Test; 4 = any higher education. Transportation resources were assessed by asking, “Over the past 4 weeks, have you had any difficulty getting transportation to where you want to go?” and “Do you limit your activities because you don't have transportation?” Persons responding positively to either question were defined as having transportation difficulty. The 4-item AIMS2 social support subscale was used22; scores in the lowest quintile were defined as low social support.
Medical History
At baseline, participants were asked to report physician diagnoses from a listing of diseases and conditions, hypothesized to be associated with mobility limitation based on the literature23 and unpublished analyses of the Statewide Survey of Alabama's Elderly (1986–1987)24 (see Table 1). In addition, participants were asked to show the interviewer all prescription medications used at the time of the in-home assessment, and to indicate whether they were on any medications for the diseases they reported.
Table 1.
Baseline Characteristics of Participants by Race
| Factor | African American (N = 452) n (%) | White (N = 453) n (%) | P Value |
|---|---|---|---|
| Sociodemographic factors | |||
| Age group,* y | .050 | ||
| 65 to 74 | 219 (48) | 251 (55) | |
| 75 to 84 | 175 (39) | 162 (36) | |
| 85+ | 58 (13) | 40 (9) | |
| Female | 232 (51) | 233 (51) | .974 |
| Married | 178 (39) | 288 (64) | <.001 |
| Rural | 232 (51) | 232 (51) | .973 |
| Income† (<$8,000) | 169 (38) | 39 (9) | <.001 |
| Education† (<7th grade) | 159 (35) | 25 (6) | <.001 |
| Transportation difficulty | 106 (24) | 39 (9) | <.001 |
| Low social support* | 83 (18) | 70 (15) | .261 |
| Verified medical diagnoses | |||
| Hypertension | 357 (79) | 276 (61) | <.001 |
| Angina | 31 (7) | 45 (10) | .095 |
| Myocardial infarction | 29 (6) | 54 (12) | .004 |
| Heart failure | 63 (14) | 44 (10) | .049 |
| Cardiac arrhythmia | 42 (9) | 78 (17) | <.001 |
| Peripheral arterial disease | 44 (10) | 43 (10) | .902 |
| Stroke | 54 (12) | 43 (10) | .233 |
| COPD or asthma | 47 (10) | 72 (16) | .014 |
| Diabetes | 130 (29) | 88 (19) | .001 |
| Arthritis or gout | 230 (51) | 209 (46) | .153 |
| Osteoporosis | 18 (4) | 71 (16) | <.001 |
| Fractures | |||
| Lower extremity | 83 (18) | 115 (25) | .011 |
| Vertebral | 6 (1) | 28 (6) | <.001 |
| Degenerative discs | 33 (7) | 37 (8) | .625 |
| Spinal stenosis | 14 (3) | 23 (5) | .133 |
| Gastrointestinal disease | 99 (22) | 140 (31) | .002 |
| Gall bladder disease | 14 (3) | 27 (6) | .038 |
| Liver disease | 2 (.4) | 5 (1) | .256 |
| Kidney disease | 30 (7) | 17 (4) | .051 |
| Seizure disorder | 6 (1) | 8 (2) | .593 |
| Dementia | 18 (4) | 7 (2) | .025 |
| Parkinson's disease | 6 (1) | 11 (2) | .233 |
| Anemia | 64 (14) | 33 (7) | .001 |
| Eye disease | |||
| Untreated cataracts | 109 (24) | 121 (27) | .370 |
| Glaucoma | 59 (13) | 31 (7) | .002 |
| Other | 16 (4) | 26 (6) | .116 |
| Cancer, nonskin | 71 (16) | 81 (18) | .382 |
| Geriatric syndromes | |||
| History of falling | 120 (27) | 143 (32) | .096 |
| Incontinence† (any) | 162 (36) | 230 (51) | .008 |
| Constipation† (any) | 196 (43) | 180 (40) | .391 |
| Unexplained weight loss | 69 (15) | 65 (14) | .698 |
| Low body mass index (<20) | 25 (6) | 34 (8) | .254 |
| High body mass index (>30) | 189 (43) | 99 (22) | <.001 |
| Dizziness | 154 (34) | 147 (33) | .605 |
| Fainting | 11 (2) | 11 (2) | .996 |
| Symptoms of arthritis | 160 (35) | 172 (38) | .422 |
| Pain, frequent, severe | 89 (17) | 76 (20) | .257 |
| Hearing problems | 59 (13) | 65 (14) | .571 |
| Poor vision | 39 (9) | 30 (7) | .391 |
| Poor appetite | 84 (19) | 69 (15) | .179 |
| Neuropsychological factors | |||
| Depression* (Geriatric Depression Scale > 5) | 45 (10) | 36 (8) | .291 |
| Clock drawing (score < 10) | 214 (47) | 88 (19) | <.001 |
| High anxiety* | 96 (21) | 71 (16) | .031 |
| Fear of falling | 75 (39) | 140 (31) | .014 |
| Fear of crashing | 86 (19) | 49 (11) | .001 |
| Fear of personal attack | 122 (27) | 40 (9) | <.001 |
| Health behaviors | |||
| Irregular meals | 223 (49) | 108 (24) | <.001 |
| Smokers* (10+ pack years) | 146 (32) | 192 (42) | .002 |
| Alcohol use† (more than 6/week) | 18 (4) | 43 (10) | <.001 |
| Physical activity level,† kcal/week | <.001 | ||
| None | 111 (25) | 78 (17) | |
| 1 to 400 | 107 (24) | 91 (20) | |
| 401 to 1,000 | 112 (25) | 93 (21) | |
| 1,001 to 1,800 | 43 (10) | 79 (17) | |
| 1,801+ | 79 (18) | 112 (25) |
Used as a continuous variable in multivariate models.
Used as an ordinal variable in multivariate models.
COPD, chronic obstructive pulmonary disease.
Questionnaires were sent to the physician (or clinic) reported as the primary source of care to verify diagnoses. Discharge summaries of the most recent hospitalization were requested if subjects had been hospitalized in the previous 3 years. Diagnoses were considered verified if the participant reported the diagnosis and also reported currently using a medication to treat the disease (e.g., insulin for diabetes mellitus), or if the diagnosis was confirmed by the physician questionnaire or by a review of a hospital discharge summary. Only verified diagnoses were defined as present for purposes of analyses.
Geriatric Syndromes
The presence of geriatric syndromes was defined by participant self-reports because these conditions are frequently underreported to physicians. Persons were asked whether they had fallen in the past year. Problems with balance, dizziness, and fainting were assessed. Urinary incontinence was assessed by asking subjects whether they “leaked even a very small amount of urine” in the past year and “how much urine do you leak?” Severity of urinary incontinence ranged from 0 = none, 1 = drop or two, 2 = enough to dampen undergarments, and 3 = enough to wet outer clothing. Constipation was categorized as 0 = never, 1 = less than one time a month, 2 = one or more times a month, 3 = one or more times a week, and 4 = daily.
Pain was ascertained by asking participants, “How frequently over the past 4 weeks have you experienced pain?” Severity of pain was assessed using standardized descriptors.23,25 An ordinal variable for pain was created with no pain = 0; annoying or uncomfortable pain occurring three or fewer times per week = 1; dreadful or agonizing pain occurring three or fewer times per week = 2; annoying or uncomfortable pain occurring four or more times per week = 3; and dreadful or agonizing pain occurring four or more times per week = 4.
Poor visual acuity was defined using the Bailey-Lovie Chart.26 Acuity was tested in the best available light within a person's home. Poor vision was defined as vision 20/180 or worse wearing corrective lenses. Persons with missing visual acuity data were assigned a vision category based on questions from the Visual Function Questionnaire (VFQ 25).27 Subject reports of poor hearing or deafness even when using aids defined poor hearing.
Diet and Weight
Persons reporting fair or poor appetite were defined as having a poor appetite. Unexplained weight loss was defined as reported loss of 10 or more pounds in the year before the interview not explained by dieting, exercise, surgery, illness, or medication. Participants were weighed on a floor model scale if they were able to stand unassisted. Height was measured using a folding yard measure. Body mass index (BMI) was calculated from these measures and persons were classified as overweight if BMI was greater than 30 and underweight if less than 20. Mid-arm circumference and knee height were used to estimate BMI28 for persons unable to stand.
Neuropsychological Factors
A clock-drawing task, CLOX,29,30 was used to evaluate cognitive function as it is less related to education than other measures. The Geriatric Depression Scale (the 15-item short form, GDS)31 assessed symptoms of depression. The 5-item AIMS2 anxiety subscale was used to identify persons with high anxiety (the highest quintile).22 Self-efficacy was evaluated by questions about limiting activities due to fear of falling, car crashes, or being attacked.
Health Behaviors
Persons were asked about the number of meals eaten per day. Persons eating fewer than 2 meals a day or irregularly were classified as having irregular meals. Current alcohol use was classified as not drinking, drinking fewer than 6 drinks/week, or 6 or more/week. A history of pack years was calculated from the number of years and number of cigarettes a person had smoked. Leisure-time physical activity was defined in terms of kilocalories expended per week using the activity assessment instrument of the Cardiovascular Health Study.32 Persons were asked about activities they had done in the past year such as walking for exercise, doing moderately strenuous household chores, outside tasks such as mowing the lawn or gardening, exercise programs, and other recreational activities. Persons were asked about the average amount of time spent in these activities over the year and how often each activity had been performed in the past 2 weeks. Ordinal categories of physical activity level were defined with 0 = none, 1 = 1–400 kcal/week; 2 = 401–1,000 kcal/week; 3 = 1,001–1,800 kcal/week, and 4 = 1,801+ kcal/week.
Health Care Utilization Variables
Study subjects were asked whether they had a doctor that they usually saw for their general health care, when they were last seen for any medical care, and the number of times they saw any doctor in the previous year. We asked them to show all prescription medications used at the time of the baseline interview.
Statistical Procedures
Descriptive statistics and χ2 tests were used to compare persons interviewed at 18 months with those who had died and who were not contacted at the 18-month follow-up interview and to compare the characteristics of African Americans and whites. Spearman ρ correlations of life-space with measures of physical function, ADL, and IADL difficulty were calculated to validate the life-space measure for African Americans and whites separately. Subjects who reported typical declines in life-space were identified to describe reasons for observed declines in life-space. Bivariate partial correlations (controlling for baseline life-space) were used to identify potential predictors of 18-month LS-C scores.
A hierarchal approach was used to develop multivariate, linear regression models predicting 18-month LS-C for African Americans and whites separately to test the hypothesis that independent predictors of life-space mobility would differ. Because we hypothesized that life-space change would occur in the context of a relatively constant sociodemographic status (Fig. 1), the first step in model development involved entering all sociodemographic variables. These variables were retained in all subsequent steps whether they remained significant or not.
We hypothesized that life-space change would be predicted by variables from 4 categories: 1) verified diseases; 2) geriatric syndromes; 3) neuropsychological factors; and 4) health behaviors (Fig. 1). Variables from these categories significantly associated (P < .05) in bivariate partial correlations for either African Americans or whites were considered for inclusion in the multivariable, linear regression models. When two or more variables were obviously measuring the same construct or were strongly intercorrelated, only one of these was included in the model development.
All multivariate models controlled for all sociodemographic variables and for baseline composite life-space. At each subsequent step, variables were retained if they remained significant (P < .05) at that step in either the model for African Americans or for whites. Thus, the final models identify independent and significant predictors of 18-month life-space for African Americans and whites separately, while adjusting and controlling for all the same variables.
Variables with negative β coefficients were associated with a decline in 18-month life-space. The magnitudes of the standardized coefficients reflect the relative strength of the associations among the predictive variables. SPSS (Version Standard, 11.0.1, SPSS Inc., Chicago, Ill) was used for all analyses.
RESULTS
Baseline and 18-Month Interviews
Data from 18-month follow-up interviews were available for 905 persons. During this period 58 (5.8%) subjects died, and we were unable to ascertain the mobility status of 37 (3.9%) persons. Five of these 37 subjects (1 African American male, 1 African American female, 1 white male, and 2 white females) formally withdrew from the study. The 905 subjects for whom we had 18-month follow-up data were not significantly different by race but were more likely to be older, male, and have transportation difficulty than subjects who died, withdrew, or for whom 18-month follow-up data were missing. At the 18-month follow-up interview, 888 (98.2%) persons were living in the community and 17 were in a nursing home.
Table 1 shows baseline characteristics of the 905 participants in this analysis by racial group. African American subjects were slightly older than whites (mean 75.8 vs. 74.6; P = .006). At baseline, 96.1% of subjects reported having a general care doctor. There were no racial differences in this variable or in the other measures of health care utilization.
Seventy-eight percent of subjects' doctors returned questionnaires verifying the presence or absence of medical diagnoses. Hospital discharge summaries were requested for 381 persons, and we received 75% of those requested. These hospital discharges provided disease verification information on 64 subjects for whom physician questionnaires were unavailable. Table 1 summarizes specific verified diseases by race.
Validating correlations of life-space and measures of physical function, ADL, and IADL difficulty were of similar magnitude and significance (P < .05) for both African Americans and whites. Baseline and 18-month life-space also were correlated (r = .69 for African Americans; r = .57 for whites; P < .001 for both).
Examples of Typical Life-Space Changes
Participants with baseline life-space scores between 54 and 57 were identified to illustrate 3-, 8-, and 10-point declines in life-space scores over the 18-month follow-up period. A 3-point decline in the life-space score was experienced by an 87-year-old African American woman who had begun to use a cane when she traveled within her neighborhood. An 8-point decline was noted for a 66-year-old African American male who previously used no equipment but at 18 months was using a cane at all levels of life-space. A 78-year-old white woman who traveled outside of town without equipment or help from another person at baseline, needed help from another person to travel within town and did not report ever leaving town at the 18-month follow-up interview. This resulted in a 10-point decline in her life-space score.
Racial Disparities in Life-Space and Life-Space Change over 18 Months
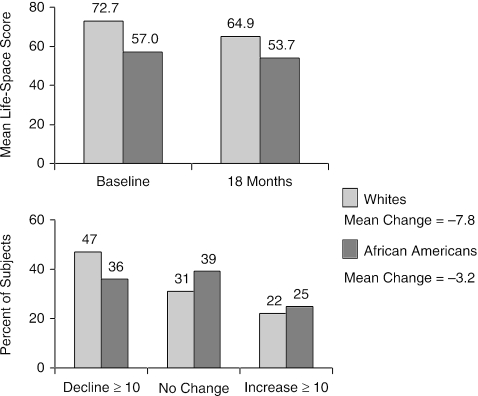
African-American subjects had significantly lower mean life-space levels both at baseline (57.0 ± standard deviation [SD] 24.5 vs. 72.7 ± SD 22.6) and after 18 months of follow-up (53.7 ± SD 24.5 vs. 64.9 ± SD 24.7) (Fig. 2). Mean life-space score decreased by 3.2 among African-American subjects and 7.8 among white subjects (Fig. 2). White subjects were more likely to show a decrease of 10 or more in life-space score than African Americans (P = .002; Fig. 2).
FIGURE 2.
Life-space change by race at 18 months.
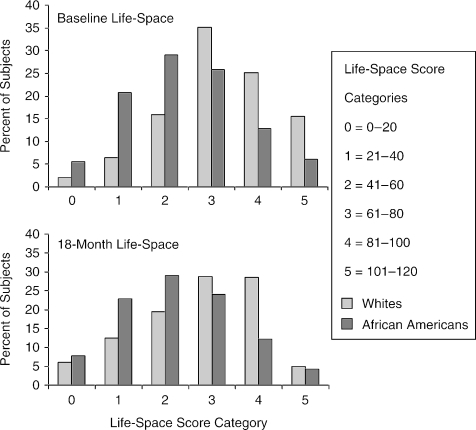
Figure 3 shows the distribution of life-space scores at baseline and at 18-month follow-up by race. Life-space scores showed a normal distribution for both African-American and white subjects at both time points. The figure demonstrates that life-space scores generally shifted downward (to the left) for both African American and whites after 18 months of follow-up.
FIGURE 3.
Distribution of life-space composite scores (LS-C) at baseline and 18 months by race.
Table 2 shows partial bivariate correlations (controlling for baseline life-space) of all sociodemographic variables with 18-month LS-C by race. Values are given for other statistically significant variables (verified diseases, geriatric syndromes, neuropsychological factors, and health behaviors) in bivariate partial correlations with 18-month life-space. Correlations adjusted for baseline life-space levels define race-specific, potential predictors of life-space change over 18-months of follow-up. Negative coefficients suggest that candidate variables predicted a decline in life-space. Similarities in direction and magnitude are noted for nearly all variables.
Table 2.
Partial Bivariate Correlation Coefficients for Potential Predictor Variables with 18-Month Life-Space
| Partial Bivariate Coefficients (Adjusted for Baseline Life-Space Composite Score) | ||
|---|---|---|
| Factor | African American N = 452 | White N = 453 |
| Sociodemographic variables | ||
| Age | −.134 | −.223‡ |
| Female | −.041 | −.056 |
| Married | .064 | .123† |
| Rural | −.105* | −.106* |
| Income <$8,000 | −.056 | −.154† |
| Education <7th grade | −.029 | −.164† |
| Transportation difficulty | −.183‡ | −.092 |
| Social support | −.092 | .039 |
| Verified medical diagnoses | ||
| Heart failure | −.043 | −.101* |
| Cardiac arrhythmia | −.041 | −.140† |
| Peripheral neuropathy | −.085 | −.140† |
| Stroke | −.011 | −.144† |
| Diabetes | −.118* | −.122* |
| Arthritis or gout | −.088 | −.136* |
| Liver disease | −.094* | −.068 |
| Kidney disease | −.112* | −.047 |
| Dementia | −.148† | −.062 |
| Parkinson's disease | −.150† | −.057 |
| Geriatric syndromes | ||
| History of falling | −.136* | −.133† |
| Urinary incontinence | −.097* | −.131* |
| Constipation | −.134† | −.145† |
| Unexplained weight loss | −.030 | −.146† |
| Pain | −.137† | −.121* |
| Hearing problems | −.044 | −.100* |
| Poor vision | −.119* | −.177‡ |
| Poor appetite | −.114 | −.170‡ |
| Neuropsychological factors | ||
| Depression | −.155† | −.209‡ |
| Clock drawing (score < 10) | −.128† | −.141† |
| Anxiety | −.140† | −.177‡ |
| Fear of falling | −.168‡ | −.150† |
| Fear of crashing | −.116* | −.025 |
| Health behaviors | ||
| Irregular meals | −.020 | −.094* |
| Physical activity, kcal/week | .063 | .158† |
P < .05.
P < .01.
P < .001.
Table 3 shows the final models developed to predict 18-month life-space for African-American and white subjects. Despite bivariate similarities, there are different patterns of significant predictors for 18-month life-space.
Table 3.
Final Multivariate Regression Models
| African American | White | |||||
|---|---|---|---|---|---|---|
| Unstandardized Coefficients | Standardized Coefficients | Unstandardized Coefficients | Standardized Coefficients | |||
| Factor | B | Std. Error | Beta | B | Std. Error | Beta |
| Baseline life-space | .543 | .046 | .544‡ | .319 | .052 | .292‡ |
| Age | −.379 | .127 | −.108† | −.685 | .160 | −.172‡ |
| Low education | .127 | .910 | .005 | −3.388 | 1.149 | −.127† |
| Transportation difficulty | −7.042 | 2.090 | −.122† | −5.137 | 3.537 | −.059 |
| Arthritis or gout | −.815 | 1.682 | −.017 | −4.024 | 1.845 | −.081* |
| Diabetes | −4.584 | 1.917 | −.085* | −5.934 | 2.332 | −.095* |
| Stroke | −.535 | 2.629 | −.007 | −7.182 | 3.146 | −.085* |
| Neuropathy | −2.331 | 3.267 | −.025 | −11.415 | 3.876 | −.109† |
| Kidney | −7.865 | 3.304 | −.081* | 3.399 | 4.811 | .026 |
| Dementia | −9.225 | 4.590 | −.070* | −7.409 | 7.181 | −.037 |
| Parkinson's disease | −15.690 | 7.473 | −.074* | −6.948 | 5.761 | −.044 |
| Depression | −.670 | .424 | −.062 | −1.138 | .441 | −.106† |
| Poor appetite | −3.403 | 2.175 | −.054 | −5.661 | 2.724 | −.082* |
| Physical activity level, kcal/week | .736 | .643 | .042 | 1.540 | .683 | .089* |
P < .05;
P < .01;
P < .001.
Controlling for gender, income, marital status, urban/rural residence, and social support, all of which were not significant.
Model R2 = 0.55, P < .001 for African Americans; R2 = .48, P ≤ .001 for whites.
DISCUSSION
This is the first study to document racial differences in life-space mobility among community-dwelling older African Americans and whites (Fig. 2). This disparity in mobility was accompanied by evidence of older African Americans being disadvantaged in terms of income, education, and transportation availability (Table 1). African Americans were also less likely to be married and to have leisure time physical activity, and more likely to have verified diagnoses of diabetes and dementia. Despite this, African Americans were less likely to report declines in life-space than whites and average life-space declines were smaller for African Americans than whites after 18 months of follow-up (Fig. 2).
While African Americans and whites both showed declines in average life-space, approximately one quarter of all subjects experienced increases in life-space during follow-up (Fig. 2). Significant predictors of 18-month life-space declines included older baseline age and diabetes for both African Americans and whites (Table 3). However, the significance and relative importance of sociodemographic variables, specific diseases, geriatric syndromes, neuropsychological factors, and health behaviors as predictors of life-space changes generally differed by racial group.
In addition to age and diabetes, significant independent predictors of mobility loss for African-American participants included transportation difficulty, kidney disease, dementia, and Parkinson's disease. Significant predictors of declines in life-space for white, but not African-American, participants included low educational attainment, arthritis/gout, stroke, neuropathy, the number of depressive symptoms, and poor appetite. In addition, higher levels of reported leisure time physical activity were associated with significant increases in 18-month life-space levels for whites but not African Americans. Even though they did not reach statistical significance, the associations of nearly all predictor variables with life-space in the final multivariate models (Table 3) were in the same direction for both African Americans and whites.
The greater magnitude in life-space change observed in whites than in African Americans may reflect a “floor” effect in life-space scores for African Americans. Alternatively, this difference may be explained by a survival bias, with surviving African Americans having less vulnerability to functional decline than whites. Other explanations for racial differences in life-space mobility may include age-by-racial differences in susceptibilities to certain disabling diseases33; cohort effects34; cultural differences in response to disease throughout the life cycle35,36; and a lack of acculturation that may adversely affect health and functioning.37 The association of race with a lower level of baseline life-space mobility may be explained by differences in socioeconomic status.38
Although the 3-point average decline in the life-space score noted for African-American participants was somewhat smaller than the average 7.8-point decline observed for white participants, the data suggest that such changes in the LS-C reflect clinically meaningful transitions in mobility for community-dwelling older adults. For example, a 3-point decline in the LS-C identified a participant who started to use a cane to travel within her neighborhood over the 18-month period of follow-up. A 10-point decline in the LS-C was associated with beginning to have assistance from another person to go to town and totally stopping travel outside of town.
Previous studies have suggested that older African Americans have a higher prevalence of health problems than whites.9 This study found that hypertension, diabetes, and glaucoma were more common among older African Americans than whites, but racial differences in patterns of comorbidity were complex. Some disabling diseases were more commonly reported in whites, such as myocardial infarction and osteoporosis (Table 1). Such differentials in disease status may reflect true differences in disease prevalence, or could be due to access to diagnostic services. The older African Americans in this study may have suffered the long-term consequences of limited access to educational and health services in the southern United States.9,39 However, African Americans and whites reported similar degrees of health care utilization.
Our prospective data are consistent with the hypothesis that modifiable risk factors may lead to life-space declines. Diagnosing and preventing complications of diabetes may be important for both African-American and white older adults. Increasing physical activity levels may be particularly important for maintaining or enhancing life-space over time. Interventions aimed at improving transportation access may be most important when attempting to enhance the mobility of older African Americans. Interventions may need to be targeted to educationally disadvantaged white older adults. Identification and treatment of depression may be particularly important to maintaining life-space mobility for older whites.
In addition to identification of potentially modifiable risk factors for mobility loss, our findings may help primary care providers to target older adults vulnerable to life-space declines who may benefit from a comprehensive geriatric assessment and the development of a treatment plan to address risk factors for functional decline. This study suggests that African-American older adults with a diagnosis of diabetes, kidney disease, Parkinson's disease, or dementia are one such high-risk group. Older white patients with low educational attainment, a diagnosis of arthritis, gout, stroke, neuropathy, or depression may benefit from such a comprehensive evaluation and treatment plan.
Other investigators suggest that racial differentials in function between African American and whites under the age of 75 may decline or even “crossover” with increasing age.40 Longer-term follow-up is planned to see whether the slower and smaller declines in life-space observed among African Americans after 18-months of follow-up persist over time.
This study has a number of strengths including a racially balanced, population-based sample of community-dwelling older adults, its prospective follow-up, and use of a life-space assessment that detects both increases and decreases in mobility over a relatively short (18-month) period of follow-up. However, the smaller magnitude of life-space change observed among African Americans lowered the power to detect predictors of life-space decline in this group. Although life-space mobility reflects individual, environmental, and community-level characteristics (Fig. 1), identification of predictive factors was limited to those for which we had data.
Despite these limitations, several predictors were detected among African-American older adults, including transportation difficulty. It is possible that some of the predictors of 18-month life-space in white participants may become significant predictors in African Americans with longer follow-up. Some of the factors that are not significant predictors for life-space decline may be very important for any given older adult and for long-term maintenance of life-space for both racial groups.
Out findings confirm disparities in mobility and health status between older African Americans and whites at baseline, and support the hypothesis that there are racial differences in subject-specific predictors of life-space among community-dwelling older adults. Identification of such risk factors may permit the development and testing of interventions to enhance mobility and reduce racial disparities.
Acknowledgments
The authors acknowledge Eric Bodner for statistical support and data management of the UAB Study of Aging. This research was funded by the National Institute on Aging (grant NIA AG15062).
REFERENCES
- 1.Ettinger WH., Jr . Immobility. In: Hazzard WR, Bierman E, Blass JP, Ettinger W Jr, Halter JB, editors. Principles of Geriatric Medicine and Gerontology. New York, NY: McGraw-Hill, Inc; 1994. pp. 1307–11. [Google Scholar]
- 2.Verbrugge LM, Gruber-Baldini AL, Fozard JL. Age differences and age changes in activities: Baltimore Longitudinal Study of Aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:S30–S41. doi: 10.1093/geronb/51b.1.s30. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger WH, Jr, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the Cardiovascular Health Study. CHS Collaborative Research Group. J Am Geriatr Soc. 1994;42:1035–44. doi: 10.1111/j.1532-5415.1994.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The women's health and aging study: health and social characteristics of older women with disability. In: Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME, editors. The Women's Health and Aging Study. Vol. 95–4009. Bethesda, Md: National Institute on Aging; 1995. [Google Scholar]
- 5.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–57. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 6.Owsley C, Allman R, Gossman M, Kell S, Sims R, Baker P. Mobility Impairment and Its Consequences in the Elderly: A Theoretical Perspective. Amityville, NY: Baywood Publishing; 2000. pp. 305–10. [Google Scholar]
- 7.Parker M, Baker PS, Allman RM. A life-space approach to functional assessment of mobility in the elderly. J Gerontol Soc Work. 2001;35:35–55. [Google Scholar]
- 8.Clark DO, Maddox GL. Racial and social correlates of age-related changes in functioning. J Gerontol. 1992;47:S222–S232. doi: 10.1093/geronj/47.5.s222. [DOI] [PubMed] [Google Scholar]
- 9.Cockerham WC. Medical Sociology. Upper Saddle River, NJ: Prentice Hall; 2001. [Google Scholar]
- 10.Ferraro KF. Double jeopardy to health for black older adults? J Gerontol. 1987;42:528–33. doi: 10.1093/geronj/42.5.528. [DOI] [PubMed] [Google Scholar]
- 11.Gibson RC. The age-by-race gap in health and mortality in the older population: a social science research agenda. Gerontologist. 1994;34:454–62. doi: 10.1093/geront/34.4.454. [DOI] [PubMed] [Google Scholar]
- 12.Gibson RC. Age-by-race differences in the health and functioning of elderly persons. J Aging Health. 1991;3:335–51. doi: 10.1177/089826439100300302. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Kaplan GA. Predictors of healthy aging: prospective evidence from the Alameda County study. Am J Public Health. 1989;79:703–8. doi: 10.2105/ajph.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macken CL. A profile of functionally impaired elderly persons living in the community. Health Care Financ Rev. 1986;7:33–49. [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RJ, Wolinsky FD. Gender, race, and health: the structure of health status among older adults. Gerontologist. 1994;34:24–35. doi: 10.1093/geront/34.1.24. [DOI] [PubMed] [Google Scholar]
- 16.Seeman TE, Charpentier PA, Berkman LF, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol. 1994;49:M97–M108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- 17.Tinetti ME, Ginter SF. The nursing home life-space diameter. A measure of extent and frequency of mobility among nursing home residents. J Am Geriatr Soc. 1990;38:1311–5. doi: 10.1111/j.1532-5415.1990.tb03453.x. [DOI] [PubMed] [Google Scholar]
- 18.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–4. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 19.Stalvey B, Owsley C, Sloane ME. The Life-Space Questionnaire: a measure of the extent of mobility of older adults. J Appl Gerontol. 1999;18:460–78. [Google Scholar]
- 20.May D, Nayak US, Isaacs B. The life-space diary: a measure of mobility in old people at home. Int Rehabil Med. 1985;7:182–6. doi: 10.3109/03790798509165993. [DOI] [PubMed] [Google Scholar]
- 21.Alabama Rural Health Association. Health Status of Rural Alabamians. Montgomery, AL: 1998. [Google Scholar]
- 22.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 23.Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–69. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 24.Alabama Commission on Aging and University of Alabama at Birmingham Center for Aging. Statewide Survey of Alabama's Elderly. Birmingham, AL: 1986–1987. [Google Scholar]
- 25.Acute Pain Management Guideline Panel. Acute Pain Management: Operative or Medical Procedures and Trauma. Vol. AHCPR Pub. No. 92-0032. Rockville, Md: Agency for Health Care Policy and Research, Public Health Service, U.S. Department of Health and Human Services; 1992 Clinical Practice Guideline; 1992. [Google Scholar]
- 26.Ferris FL, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103:181–2. doi: 10.1016/s0161-6420(96)30742-2. [DOI] [PubMed] [Google Scholar]
- 27.National Eye Institute. 25 Item Visual Functioning Questionnaire. Santa Monica, CA: RAND; 1997. [Google Scholar]
- 28.Chumlea WC, Roche AF, Mukherjee D. Nutritional assessment of the elderly through anthropometrics. Columbus, Ohio: Ross Laboratories; 1987. [Google Scholar]
- 29.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–94. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royall DR, Mulroy AR, Chiodo LK, Polk MJ. J Gerontol B Psychol Sci Soc Sci. Vol. 54. 1999. Clock drawing is sensitive to executive control: a comparison of six methods; pp. P328–P333. [DOI] [PubMed] [Google Scholar]
- 31.Sheikh JL, Yesavage JA. Geriatric Depression Scales (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:164–74. [Google Scholar]
- 32.Taylor HL, Jacobs DR, Schuker B, et al. Questionnaire for assessment of leisure time physical activities. J Chron Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 33.Manton KG, Soldo BJ. Dynamics of health changes in the oldest old: new perspectives and evidence. Milbank Mem Fund Q Health Soc. 1985;63:206–85. [PubMed] [Google Scholar]
- 34.Gibson RC, Jackson JS. The black oldest old. In: Suzman R, Wuillis D, Manton KG, editors. The Oldest Old. New York: Oxford University Press; 1992. [Google Scholar]
- 35.Anderson RM, Mullner RM, Cornelius LJ. Black-white differences in health status: methods or substance? Milbank Q. 1987;65(suppl 1):72–99. [PubMed] [Google Scholar]
- 36.Ford AB, Haug MR, Jones PK, Roy AW, Folmar SJ. Race-related differences among elderly urban residents: a cohort study, 1975–1984. J Gerontol. 1990;45:S163–S171. doi: 10.1093/geronj/45.4.s163. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell R. Functional disability among the Puerto Rican elderly. Journal Aging Health. 1989;1:244–64. [Google Scholar]
- 38.Mendes de Leon CF, Fillenbaum GG, Williams CS, Brock DB, Beckett LA, Berkman LF. Functional disability among elderly blacks and whites in two diverse areas: the New Haven and North Carolina EPESE. Established Populations for the Epidemiologic Studies of the Elderly. Am J Public Health. 1995;85:994–8. doi: 10.2105/ajph.85.7.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferraro KF, Farmer MM. Double jeopardy, aging as leveler, or persistent health inequality? A longitudinal analysis of white and black Americans. J Gerontol B Psychol Sci Soc Sci. 1996;51:S319–S328. doi: 10.1093/geronb/51b.6.s319. [DOI] [PubMed] [Google Scholar]
- 40.Gibson RC, Jackson JS. The health, physical functioning, and informal supports of the black elderly. Milbank Q. 1987;65(suppl 2):421–53. [PubMed] [Google Scholar]