Abstract
The yeast Saccharomyces cerevisiae possesses two genes that encode phosphatidylinositol (PtdIns) 4-kinases, STT4 and PIK1. Both gene products phosphorylate PtdIns at the D-4 position of the inositol ring to generate PtdIns(4)P, which plays an essential role in yeast viability because deletion of either STT4 or PIK1 is lethal. Furthermore, although both enzymes have the same biochemical activity, increased expression of either kinase cannot compensate for the loss of the other, suggesting that these kinases regulate distinct intracellular functions, each of which is required for yeast cell growth. By the construction of temperature-conditional single and double mutants, we have found that Stt4p activity is required for the maintenance of vacuole morphology, cell wall integrity, and actin cytoskeleton organization. In contrast, Pik1p is essential for normal secretion, Golgi and vacuole membrane dynamics, and endocytosis. Strikingly, pik1ts cells exhibit a rapid defect in secretion of Golgi-modified secretory pathway cargos, Hsp150p and invertase, whereas stt4ts cells exhibit no detectable secretory defects. Both single mutants reduce PtdIns(4)P by ∼50%; however, stt4ts/pik1ts double mutant cells produce more than 10-fold less PtdIns(4)P as well as PtdIns(4,5)P2. The aberrant Golgi morphology found in pik1ts mutants is strikingly similar to that found in cells lacking the function of Arf1p, a small GTPase that is known to regulate multiple membrane trafficking events throughout the cell. Consistent with this observation, arf1 mutants exhibit reduced PtdIns(4)P levels. In contrast, diminished levels of PtdIns(4)P observed in stt4ts cells at restrictive temperature result in a dramatic change in vacuole size compared with pik1ts cells and persistent actin delocalization. Based on these results, we propose that Stt4p and Pik1p act as the major, if not the only, PtdIns 4-kinases in yeast and produce distinct pools of PtdIns(4)P and PtdIns(4,5)P2 that act on different intracellular membranes to recruit or activate as yet uncharacterized effector proteins.
INTRODUCTION
Phosphoinositides are essential phospholipids that serve as key regulators of numerous cellular processes. Although they make up only a small percentage of the total membrane lipid content, phosphoinositides have been found to play important roles in cell signaling, vesicle trafficking, cell proliferation, and cytoskeleton organization (reviewed by Fruman et al., 1998). Recently, a number of kinases from several organisms have been cloned that specifically use phosphoinositides as substrates. In the budding yeast Saccharomyces cerevisiae, five phosphoinositide kinases have been identified (reviewed by Odorizzi et al., 2000), each possessing well-conserved homologues in other organisms, including humans. Characterization of these enzymes is essential to unraveling the diverse and essential roles phosphoinositides play in eukaryotic cells.
Based on sequence analysis, at least two phosphatidylinositol (PtdIns) 4-kinases exist in the S. cerevisiae genome, namely STT4 and PIK1. A mutant allele of STT4 was initially identified in a genetic screen for mutations that confer hypersensitivity to the protein kinase inhibitor staurosporine (Yoshida et al., 1994a). This staurosporine sensitivity could be suppressed by overexpression of a yeast MAPK regulator, Pkc1p, providing a possible genetic link between the two enzymes. Additionally, MSS4 was identified as a multicopy suppressor of the stt4 temperature sensitivity (Yoshida et al., 1994b). Later, MSS4 was found to encode the major PtdIns(4)P 5-kinase in yeast, which is required for proper actin cytoskeleton organization (Desrivieres et al., 1998; Homma et al., 1998). More recently, Stt4p has been suggested to function in a transport step required for phosphatidylserine metabolism (Trotter et al., 1998). However, the precise cellular role of Stt4p in these pathways remains unclear.
Pik1p, the other PtdIns 4-kinase in yeast, shares 37% sequence identity with Stt4p. However, the majority of shared residues are restricted to the kinase domains of each protein, suggesting that the enzymes are not redundant. Initial characterization of Pik1p showed it to be enriched in the nucleus (Garcia-Bustos et al., 1994) and trans-Golgi compartments (Walch-Solimena and Novick, 1999). Although the nuclear function of Pik1p is unknown, this enzyme has been suggested to play a role in secretion from the late Golgi (Hama et al., 1999; Walch-Solimena and Novick, 1999).
In this study, we show that although Pik1p and Stt4p are essential to the production of the same second messenger, PtdIns(4)P, each of these proteins regulates distinct cellular processes. Inactivation of temperature-sensitive alleles of either PIK1 or STT4 results in markedly decreased production of PtdIns(4)P. One consequence of decreased PtdIns(4)P, the substrate of the PtdIns(4)P 5-kinase Mss4p, is the commensurate decrease in PtdIns(4,5)P2 levels in both of these temperature-conditional strains. Our results show that cells harboring a stt4 temperature-sensitive allele generate decreased levels of PtdIns(4)P and PtdIns(4,5)P2, and we find that Stt4 PtdIns 4-kinase activity is a critical regulator of vacuole morphology, actin cytoskeleton organization, and cell wall integrity, suggesting a pleiotropic role for Stt4p in the maintenance and organization of the cell. In contrast, we find that pik1ts cells exhibit severe protein trafficking defects and accumulate morphologically aberrant Golgi membranes, highlighted by the formation of membrane tubular ring structures. We postulate that the differential localization and/or timing of PtdIns(4)P production by Stt4p and Pik1p are critical to the cellular functions regulated by these lipid kinases.
MATERIALS AND METHODS
Strains and Media
Enzymes used for recombinant DNA techniques were purchased from commercial sources and used as recommended by the suppliers. Sources of growth media for yeast and bacterial strains have been described elsewhere (Gaynor et al., 1994). Transformation into yeast was performed by a standard lithium acetate method (Ito et al., 1983), and Escherichia coli transformations were done as described previously (Hanahan, 1983). Otherwise, standard yeast genetic methods were used throughout (Sherman et al., 1979; Maniatis et al., 1982). S. cerevisiae strains used in this study are listed in Table 1.
Table 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al. (1988) |
| SEY5188 | MATα sec18-1 ura3-52 leu2-3,112 suc2-Δ9 | Graham and Emr (1991) |
| AAY102 | SEY6210; stt4Δ::HIS3 carrying pRS415stt4-4 (LEU2 CEN6 stt4-4) | This study |
| AAY104 | SEY6210; pik1Δ::HIS3 carrying pRS314pik1-83 (TRP1 CEN6 pik1-83) | This study |
| AAY105 | SEY6210; stt4Δ::HIS3 pik1Δ::HIS3 carrying pRS415stt4-4 | This study |
| (LEU2 CEN6 stt4-4) and pRS314pik1-83 (TRP1 CEN6 pik1-83) | ||
| AAY107 | SEY6210; mss4Δ::HIS3MX6 carrying pYCplac111mss4-2 (LEU2 CEN6 mss4-2) | This study |
| 6210arf1Δ | SEY6210; arf1Δ::HIS3 | Gaynor et al. (1998) |
| AAY114 | SEY6210; stt4Δ::HIS3 arf1Δ::HIS3 carrying pRS415stt4-4 (LEU2 CEN6 stt4-4) | This study |
| AAY116 | SEY6210; pik1Δ::HIS3 arf1Δ::HIS3 carrying pRS314pik1-83 (TRP1 CEN6 pik1-83) | This study |
DNA Manipulations, Mutagenesis, and Plasmids
A plasmid that was used to generate a chromosomal deletion of the STT4 gene was constructed by inserting the HIS3 gene into Ycp50-STT4 (Trotter et al., 1998) that had been cleaved with StuI and NdeI. The resulting construct was then digested with EagI and SalI and transformed into 6210 a/α to eliminate the entire STT4 coding sequence on one chromosome. PCR was used to confirm the disruption with the use of two different sets of primers. A plasmid that was used to create a chromosomal deletion of PIK1 was made by inserting the HIS3 gene into pRS416-PIK1 that had been cleaved with SphI and SpeI. The resulting construct was then digested with SalI and SacI and transformed into SEY6210 a/α to eliminate the entire PIK1 coding sequence on one chromosome. Disruptions were confirmed by PCR with the use of two different sets of primers. Strain AAY106 (this study) was made by transforming a PCR-amplified mss4Δ::HIS3MX6 fragment from a genomic DNA preparation of SD102 (Desrivieres et al., 1998) into 6210 a/α. Disruptions were confirmed by PCR with the use of two different sets of primers. Strain AAY105 (this study) was made by mating strains AAY102 and AAY104, dissecting tetrads, and selecting spores with the appropriate markers. Disruptions were confirmed by PCR with the use of four sets of primers. Strain AAY114 (this study) was made by mating strains 6210 arf1Δ and AAY102, dissecting tetrads, and selecting spores with the appropriate markers. Disruptions were confirmed by PCR with the use of three sets of primers. Strain AAY116 (this study) was made by mating strains 6210 arf1Δ and AAY104, dissecting tetrads, and selecting spores with the appropriate markers. Disruptions were confirmed by PCR with the use of three sets of primers. STT4 temperature-sensitive alleles were made as described previously (Gaynor and Emr, 1997) except that the carboxyl terminus of STT4 was specifically targeted for mutagenesis. Plasmid pRS314-pik1-83ts (Hendricks et al., 1999) was kindly provided by J. Thorner (University of California, Berkeley, CA), and strain SD102 containing the mss4-2 temperature-sensitive allele (Desrivieres et al., 1998) was a generous gift of M. Hall (University of Basel, Basel, Switzerland). Plasmid pCYI-20 encoding invertase was described previously (Johnson et al., 1987).
Metabolic Labeling and Immunoprecipitation
Cell labeling and immunoprecipitations were performed as described previously (Gaynor et al., 1994) with noted variations. Log-phase cultures were concentrated to 4 OD600/ml and labeled with 1.5 μl/OD600 Tran 35S label (DuPont New England Nuclear, Boston, MA) for 10 min in YNB containing 100 μg/ml BSA and 20 μg/ml α2-macroglobulin. Cells were then chased with 5 mM methionine, 2 mM cysteine, and 0.2% yeast extract for the indicated times, and proteins were precipitated with 9% trichloroacetic acid (TCA). All temperature shifts, unless stated otherwise, were limited to 10 min at 37°C. Extracts were immunoprecipitated with antisera against carboxypeptidase Y (CPY), alkaline phosphatase (ALP), Hsp150p, invertase, or the hemagglutinin (HA) epitope that have been characterized previously (Cowles et al., 1997; Gaynor and Emr, 1997). Endoglycosidase H (DuPont New England Nuclear) treatment of radioactive invertase immunoprecipitates was performed as described (Gaynor et al., 1994). To assay internal and external fractions for the presence of invertase, cells harboring a plasmid expressing invertase (pCYI-20) were converted to spheroplasts after pulse chase by adding a 2× buffer containing 50 mM Tris (pH 7.5), 2 M sorbitol, 40 mM NaF, 40 mM NaN3, and 10 mM DTT and incubating on ice for 10 min. Zymolyase T100 (15 μg/OD600; Seikagaku Kogyo, Tokyo, Japan) was then added to the cell suspension and incubated for 30 min at 30°C. Cells were spun at 6000 rpm for 5 min, and the supernatant was removed. Both fractions were precipitated in the presence of 9% TCA. For secretion assay, cells were pulse labeled as described and spun at 6000 rpm to separate the cell fraction from the media fraction. Secreted proteins were TCA precipitated from the media, washed with acetone, and resuspended in sample buffer for resolution by SDS-PAGE. For Ste6p-HA analysis, cells were incubated at permissive temperature during the 10-min pulse and a 10-min chase. Cells were then shifted to restrictive temperature for up to an additional 70 min to determine the extent of Ste6p-HA degradation.
In Vivo Phosphoinositide Analysis
Analysis of phosphoinositide levels was done as described previously (Wurmser and Emr, 1998) with the following exceptions. Before labeling, cells were grown in YNB medium with the appropriate amino acids. Five OD600 of cells from a log-phase culture were harvested and washed with inositol-free synthetic medium (IFM). Cells were then resuspended in IFM and allowed to recover for 10 min at permissive temperature. Cells were next shifted to the appropriate temperature for 10 min, followed by the addition of 50 μCi of myo-[2-3H]inositol (Nycomed Amersham, Princeton, NJ). After 10 min, excess unlabeled myo-inositol was added to a final concentration of 300 μg/ml, and cells were quickly spun for 2 min at 8000 rpm. Cells were then resuspended in IFM containing 250 μg/ml unlabeled inositol and chased for 30 min at the appropriate temperature. Cells were lysed in 4.5% perchloric acid with glass beads to generate extracts. Further analysis of extracts was as described previously (Schu et al., 1993; Stack et al., 1995a). Fractions eluting from the HPLC column (catalogue number 4611-1505, Whatman, Clifton, NJ) were collected every 0.66 min.
Fluorescence and Electron Microscopy
Ultrastructural analysis was performed as described previously (Odorizzi et al., 1998) with the following exceptions. Fifty OD600 of log-phase cells were harvested from YPD medium and fixed in 3% glutaraldehyde, 0.1 M Na cacodylate (pH 7.4), 5 mM CaCl2, 5 mM MgCl2, and 2.5% sucrose for 1 h. Cells were washed and resuspended in buffer containing 100 mM Tris (pH 7.5), 25 mM DTT, 5 mM EDTA, and 1.2 M sorbitol for 10 min. Cells were then washed and resuspended in buffer (pH 5.9) containing 100 mM K2HPO4, 100 mM citrate, and 1 M sorbitol. Fifty microliters of β-glucuronidase and 400 μg/ml Zymolyase T100 were added, and cells were allowed to incubate for 40 min at 30°C. Cells were spun down at 4000 rpm, washed, and resuspended in ice-cold buffer containing 500 mM Na cacodylate (pH 6.8) and 25 mM CaCl2, followed by staining with osmium-thiocarbohydrazide. Cells were then further processed as described previously (Rieder et al., 1996). More than 100 cells were examined for each analysis.
Labeling with FM4-64 [N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenylhexatrienyl) pyridinium dibromide] (Molecular Probes, Eugene, OR) was done as described (Vida and Emr, 1995). Briefly, cells were grown to mid log phase in YPD, and OD600 of cells was harvested by centrifugation. Cells were then labeled with 16 nM FM4-64 in YPD prewarmed to the appropriate temperature for 15 min, followed by a chase in YPD without dye for 1 h. Cells were concentrated to 6 OD600/ml and visualized on slides coated with concanavalin A. For temperature-shift experiments, cells were pulse-chased with FM4-64 followed by a shift to 37°C for 2 h (samples were taken every 15 min). Observations are based on the examination of at least 100 cells.
For localization of actin, cells were grown to early log phase, shifted to the appropriate temperature for 2.5 h, fixed in 3.7% formaldehyde, and stained with rhodamine–phalloidin (Molecular Probes) as described previously (Benedetti et al., 1994). Observations are based on >200 cells viewed.
Cell Lysis Assay
To examine cell lysis, plates were incubated overnight at 37°C and then overlayed with a solution containing 50 mM glycine (pH 9.5), 1% agar, and 10 mM 5-bromo-4-chloro-3-indolyl-phosphate (Sigma Chemical, St. Louis, MO) for up to 60 min. Lysed cells turned blue after 15–30 min, whereas intact cells remained white for 90 min or longer, as described previously (Paravicini et al., 1992).
RESULTS
Isolation of Temperature-conditional stt4 Alleles
To address the primary function of Stt4p in vivo, we generated temperature-conditional alleles through PCR-mediated random mutagenesis of the kinase domain of Stt4p (Figure 1). A heterozygous diploid strain (STT4/stt4Δ::HIS3) was first created (see MATERIALS AND METHODS), and we found that STT4 is essential for growth. We then introduced mutant stt4 alleles into haploid yeast lacking a chromosomal copy of STT4 but expressing the wild-type STT4 gene from a URA3-marked plasmid. Strains expressing mutagenized, but not wild-type, STT4 were selected on 5-FOA–containing plates, making it possible to screen for temperature-conditional stt4 mutants. Among six candidate stt4ts mutants, one strain that grew robustly at 26°C but lost viability at temperatures > 35°C, stt4-4, was selected for further study. In contrast to previous results (Yoshida et al., 1994b), overexpression of MSS4 did not rescue the temperature sensitivity of any of the stt4ts strains made in this study.
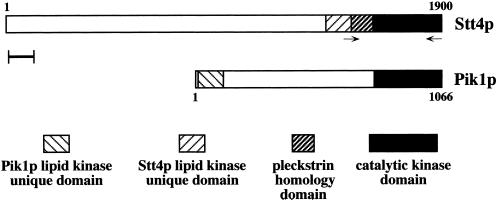
Figure 1.
Alignment of Stt4p and Pik1p. The STT4 gene encodes a high-molecular-weight protein possessing three conserved domains in its carboxyl terminus. Only the catalytic kinase domains of Stt4p and Pik1p share similarity, whereas the lipid kinase–unique domains of each protein diverge extensively. Additionally, Stt4p appears to contain a pleckstrin homology domain, whereas Pik1p does not. Neither protein contains obvious transmembrane domains. Oligonucleotides for PCR mutagenesis of STT4 are shown. Scale bar, 100 amino acids.
Both stt4ts and pik1ts Cells Produce Diminished Levels of PtdIns(4)P and PtdIns(4,5)P2 at Restrictive Temperature
Because previous work on PtdIns 4-kinases in S. cerevisiae has generated conflicting results (Yoshida et al., 1994a; Cutler et al., 1997; Trotter et al., 1998; Hama et al., 1999; Walch-Solimena and Novick, 1999), likely because of differences in strain background and the use of different mutant alleles, it has been difficult to compare independent results and determine precise roles for Stt4p and Pik1p. Therefore, we found it necessary to address the roles of yeast PtdIns 4-kinases with the use of a single strain background, SEY6210, and well-characterized alleles of STT4 and PIK1. We first determined whether the stt4 temperature-sensitive strains created by random mutagenesis lacked PtdIns 4-kinase activity at the nonpermissive temperature. To accurately assess changes in the rate of PtdIns(4)P synthesis, the newly synthesized pool of PtdIns(4)P was pulse labeled with myo-[2-3H]inositol at 26 and 38°C (York et al., 1999). Subsequent analysis of labeled lipids by HPLC enabled us to monitor the levels of glycero-phosphoinositols derived from PtdIns(3)P, PtdIns(4)P, PtdIns(3,5)P2, and PtdIns(4,5)P2. At the permissive temperature, phosphoinositide levels in stt4ts cells were nearly identical to those seen in wild-type cells, except for minor decreases in PtdIns(4)P and PtdIns(4,5)P2 (Figure 2, A and C). After a shift to 38°C, wild-type cells exhibited increases in PtdIns(4)P, PtdIns(3,5)P2, and PtdIns(4,5)P2 levels. However, in stt4ts cells, shifting to restrictive temperature resulted in a decrease in PtdIns(4)P and PtdIns(4,5)P2 levels by ∼60 and 40%, respectively, compared with wild-type cells (Figure 2, B and D). The decreased amount of PtdIns(4)P synthesized in stt4ts cells at the nonpermissive temperature indicates that this fraction of the total cellular pool of PtdIns(4)P is synthesized by the Stt4 PtdIns 4-kinase. Additionally, because a significant amount of PtdIns(4,5)P2 is synthesized in stt4ts cells after temperature shift, our results suggest that PtdIns(4,5)P2 is synthesized with the use of pools of PtdIns(4)P derived from both the Stt4 and Pik1 PtdIns 4-kinases.
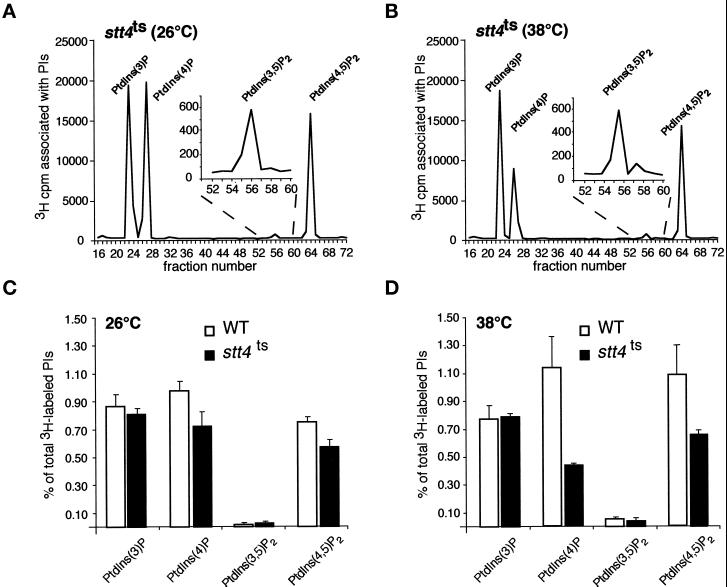
Figure 2.
Levels of PtdIns(4)P and PtdIns(4,5)P2 decrease in stt4ts cells at restrictive temperature. (A and C) stt4ts cells (AAY102) were labeled with myo-[2-3H]inositol for 10 min at 26°C, chased with excess unlabeled myo-inositol for 30 min, and glass bead lysed. Cellular lipids were extracted, deacylated, and separated by HPLC (see MATERIALS AND METHODS). Peaks corresponding to glycero-Ins(3)P, glycero-Ins(4)P, glycero-Ins(3,5)P2, and glycero-Ins(4,5)P2 are indicated. Quantitative comparisons of glycero-phosphoinositols generated by wild-type and stt4ts cells at 26°C are shown below the elution profile. (B and D) stt4ts cells were preincubated at 38°C for 10 min, labeled with myo-[2-3H]inositol for 10 min, and chased with excess unlabeled myo-inositol for 30 min. Quantitative comparisons of glycero-phosphoinositols generated by wild-type and stt4ts cells at 38°C are shown below the elution profile. These data represent means ± ranges of at least three independent experiments.
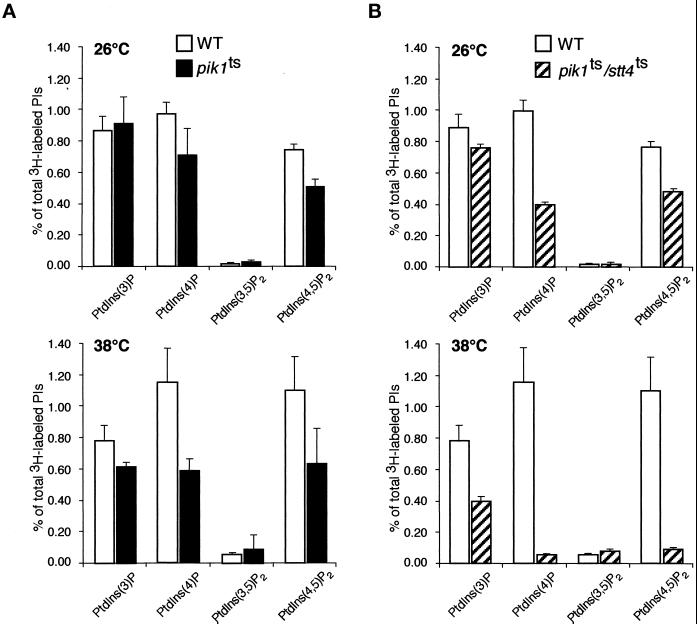
To determine if cells harboring a pik1ts allele show defects in PtdIns(4,5)P2 synthesis, we used plasmid shuffle techniques to introduce the temperature-sensitive pik1-83 allele, which contains mutations within the carboxyl-terminal catalytic kinase domain of Pik1p (Hendricks et al., 1999), into a pik1Δ strain made in the same strain background, SEY6210. We then labeled phosphoinositides in the pik1 temperature-sensitive strain and performed HPLC analysis. At 26°C, the levels of PtdIns(4)P and PtdIns(4,5)P2 in pik1ts cells were decreased significantly compared with the levels in wild-type cells (Figure 3A). The observed decreases in phosphoinositide levels indicate that this allele of PIK1 already exhibits reduced activity at 26°C. Upon shift to 38°C, PtdIns(4)P and PtdIns(4,5)P2 levels were decreased by 45 and 40%, respectively, compared with the levels in wild-type cells at this temperature (Figure 3A). This result suggests that, like Stt4p, the Pik1 kinase may also contribute PtdIns(4)P for the synthesis of PtdIns(4,5)P2.
Figure 3.
pik1ts cells show defects in both PtdIns(4)P and PtdIns(4,5)P2 synthesis. Wild-type (SEY6210), pik1ts (AAY104), and stt4ts/pik1ts double mutant (AAY105) cells were grown to mid log phase, shifted to the appropriate temperature for 10 min, labeled with myo-[2-3H]inositol for 10 min, and chased in the presence of excess unlabeled myo-inositol for 30 min. Cellular lipids were recovered, deacylated, and separated by HPLC as described in Figure 1. Levels of deacylated products corresponding to the indicated phosphoinositides are shown. (A) A comparison of phosphoinositide levels in wild-type and pik1ts cells at 26 and 38°C. (B) A comparison of phosphoinositide levels in wild-type and stt4ts/pik1ts double mutant cells at 26 and 38°C. These data represent means ± ranges of at least three independent experiments.
To confirm an essential role for Pik1p and Stt4p in the synthesis of PtdIns(4)P and, by virtue of Mss4p-mediated conversion of PtdIns(4)P to PtdIns(4,5)P2, the production of PtdIns(4,5)P2, we created a haploid strain harboring both the pik1ts and stt4ts alleles. At permissive temperature, this strain grew more slowly than either single mutant, and HPLC analysis of glycero-phosphoinositols isolated from the stt4ts/pik1ts double mutant cells at permissive temperature showed additive decreases in both PtdIns(4)P (50%) and PtdIns(4,5)P2 (40%) levels compared with the levels in wild-type cells (Figure 3B). Moreover, after a shift to the restrictive temperature, the levels of both PtdIns(4)P and PtdIns(4,5)P2 decreased >10-fold, close to background levels (Figure 3B). This provides strong evidence that Stt4p and Pik1p are likely the only PtdIns 4-kinases in yeast and that each contributes PtdIns(4)P as a substrate for the synthesis of PtdIns(4,5)P2. In support of these data, attempts to create double mutants harboring an mss4ts allele (Desrivieres et al., 1998), the only known PtdIns(4)P 5-kinase in yeast, in combination with either the pik1ts or stt4ts allele, resulted in synthetic lethality (our unpublished results).
Stt4p Is Required for Actin Cytoskeleton Organization and Cell Wall Integrity
Phosphoinositides have previously been implicated in the organization of the actin cytoskeleton (Janmey, 1994; Desrivieres et al., 1998; Homma et al., 1998). Because temperature shift of stt4ts and pik1ts cells resulted in diminished levels of PtdIns(4)P and PtdIns(4,5)P2, we investigated a possible role for Stt4p and Pik1p in controlling the actin organization. At both permissive and restrictive temperatures, stt4ts and pik1ts cells were fixed and stained with rhodamine–phalloidin (see MATERIALS AND METHODS). Because mss4 mutant cells have a defect in actin cytoskeleton organization, the temperature-sensitive mss4-2 allele (Desrivieres et al., 1998) was transferred into a mss4Δ strain made in the SEY6210 strain background to serve as a positive control in the assay (Figure 4) (Desrivieres et al., 1998; Homma et al., 1998). At the permissive temperature, stt4ts, mss4ts, and pik1ts cells displayed actin cables in the mother cell aligned toward concentrated cortical actin patches in the bud and septum, similar to wild-type cells (Figure 4). In contrast, after a shift to restrictive temperature, >90% of stt4ts and mss4ts cells displayed random cortical actin patches throughout both mother and daughter cells, indicating that cells lacking either Stt4 or Mss4 kinase activity fail to properly organize their actin cytoskeletons (Figure 4, B and C; Table 2). After an identical temperature shift, >90% of both pik1ts and wild-type cells exhibited normal patterns of actin filaments and cortical patches (Figure 4, A and D). Additionally, double mutants harboring temperature-sensitive alleles of both stt4 and pik1 displayed a disorganized actin cytoskeleton only at the restrictive temperature (Figure 4E). Together, these results demonstrate that Stt4, but not Pik1, PtdIns 4-kinase activity is required for proper actin organization.
Figure 4.
Cells lacking Stt4 kinase activity fail to reorganize their actin cytoskeleton after heat shock. Cells were grown to early log phase, shifted to the appropriate temperature for 2.5 h, and then fixed with 3.7% formaldehyde. Cells were labeled with rhodamine–phalloidin (see MATERIALS AND METHODS) and visualized. Pictures shown are representative of more than 200 cells observed (see Table 2). (A) Wild-type (SEY6210) cells at 26 and 37°C. (B) stt4ts (AAY102) cells at 26 and 37°C. (C) mss4ts (AAY107) cells at 26 and 37°C. (D) pik1ts (AAY104) cells at 26 and 37°C. (E) stt4ts/pik1ts double mutant (AAY105) cells harboring temperature-sensitive alleles of both PtdIns 4-kinases at 26 and 37°C.
Table 2.
Percentage of small budded cells with cortical actin patches restricted to the bud
| Yeast strain | 26°C | 37°C |
|---|---|---|
| Wild type | >95% | >95% |
| stt4ts | >90% | <10% |
| pik1ts | >90% | >90% |
| mss4ts | >80% | <5% |
| pik1ts/stt4ts | >85% | <5% |
Percentages are based on >200 cells observed for each strain at each condition. Wild-type (SEY6210), stt4ts (AAY102), pik1ts (AAY104), mss4ts (AAY107), and pik1ts/stt4ts double mutant (AAY105) cells at 26 and 37°C are described.
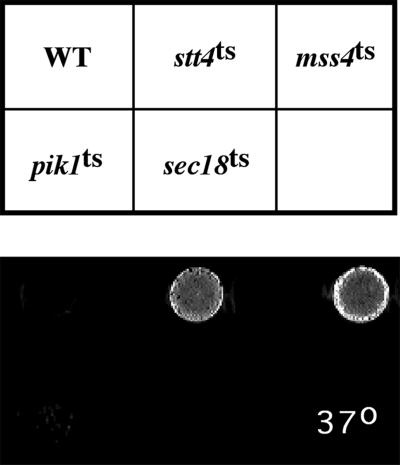
Mss4p PtdIns(4)P 5-kinase activity has also been suggested to play a role in yeast cell wall maintenance, because inactivation of Mss4p at high temperature led to the appearance of lysed “ghost cells” (Desrivieres et al., 1998). Therefore, we investigated whether stt4ts or pik1ts cells undergo a loss of cell wall integrity at restrictive temperature. After a 6- to 7-h shift to 37°C, we found that, like mss4ts cells, stt4ts cells undergo lysis as determined by both microscopic examination and overlay plate assay (Figure 5). However, this lysis defect in stt4ts cells could be suppressed by the presence of 1 M sorbitol, similar to the lysis suppression observed with cells lacking the protein kinase C Pkc1p, a MAPK regulator (Paravicini et al., 1992). This evidence suggests that Stt4p may function in the PKC1 pathway. Using the same overlay assay, we observed no lysis of a strain containing the pik1ts allele, again demonstrating distinct roles for the two PtdIns 4-kinases. Together, these data strongly indicate that, although both Pik1p and Stt4p contribute to the overall pool of PtdIns(4)P synthesized, only PtdIns(4)P synthesized by Stt4p is required for the maintenance of actin cytoskeleton organization and cell integrity.
Figure 5.
stt4ts cells exhibit a cell lysis defect. Equivalent numbers of wild-type (SEY6210), stt4ts (AAY102), mss4ts (AAY107), pik1ts (AAY104), and sec18ts (SEY5188) cells were incubated on YPD plates for 2 d at 26°C and then shifted for 15 h to 37°C. Plates were then overlayed with the 5-bromo-4-chloro-3-indolyl-phosphate solution described in MATERIALS AND METHODS, and pictures were taken after 30 min.
PtdIns 4-Kinase Activity of Pik1p but Not Stt4p Is Required for Secretion
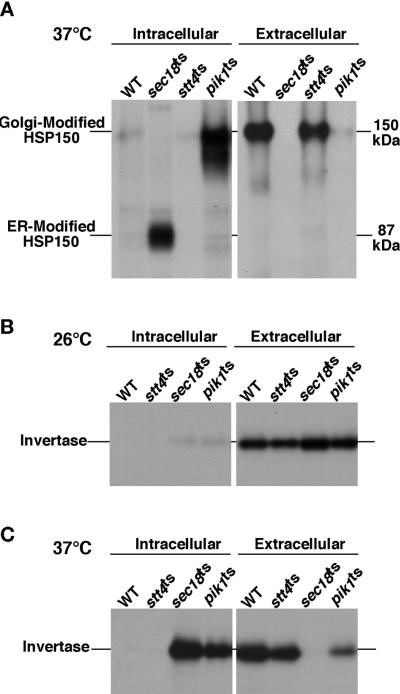
Because PtdIns(4)P generated by Pik1p did not contribute to the maintenance of the actin cytoskeleton or cell wall integrity, we investigated a role for Pik1p and Stt4p in protein trafficking. By performing a pulse-chase experiment, we directly tested whether pik1ts and stt4ts cells could normally secrete proteins at permissive and nonpermissive temperatures. Unlike stt4ts cells, pik1ts cells failed to efficiently secrete proteins into the medium at restrictive temperature. Most notably, Hsp150p (Russo et al., 1992), a high-molecular-weight glycoprotein that is normally secreted rapidly, was largely retained (>95%) within pik1ts cells at high temperature, predominantly in a Golgi-modified form (Figure 6A). As a negative control, sec18ts cells, which are blocked in protein transport from the endoplasmic reticulum (ER) to the Golgi at high temperatures, were included in all of our experiments (Novick et al., 1980; Graham and Emr, 1991).
Figure 6.
pik1ts but not stt4ts cells exhibit defects in protein secretion. Wild-type and mutant yeast cells were preincubated at 26 or 37°C for 10 min, metabolically labeled with a 35S-protein labeling mix during a 10-min pulse, and then chased in the presence of excess unlabeled methionine and cysteine for the times indicated. Each protein was immunoprecipitated with the appropriate antibody and resolved on SDS-PAGE. For Hsp150p secretion, labeled cells were spun down and Hsp150p was immunoprecipitated from both the media (external) fraction and the cellular (internal) fraction. Both the Golgi-modified and ER-modified forms of Hsp150p are shown. For invertase secretion, cellular transport was stopped with the addition of NaN3 and NaF after the pulse chase, and the cells were converted to spheroplasts. Internal and external fractions were separated by centrifugation and analyzed for the presence of invertase by immunoprecipitation. Before separation by SDS-PAGE, invertase was deglycosylated by endoglycosidase H treatment (see MATERIALS AND METHODS). (A) Analysis of Hsp150p secretion in wild-type (SEY6210), sec18ts (SEY5188), stt4ts (AAY102), and pik1ts (AAY104) cells. (B) Analysis of invertase secretion in wild-type, sec18ts, stt4ts, and pik1ts cells at 26°C. (C) Analysis of invertase secretion in wild-type, sec18ts, stt4ts, and pik1ts cells at 37°C. All data are representative of multiple experiments.
We also examined the trafficking of another well-characterized secreted protein, the enzyme invertase (Novick and Schekman, 1979). In wild-type cells, invertase is heavily glycosylated and secreted rapidly from cells, with a transport half-time of ∼1–2 min (Payne et al., 1987; Gaynor et al., 1998). By performing a pulse-chase experiment, we determined the relative intracellular and extracellular concentrations of the enzyme after a chase of 30 min. At permissive temperature, wild-type, stt4ts, pik1ts, and sec18ts cells exhibited normal secretion of invertase (Figure 6B). However, we observed that invertase secretion was >80% blocked in pik1ts cells at nonpermissive temperature (Figure 6C). Furthermore, when examining invertase glycosylation in the pik1ts cells at 37°C, two pools of the enzyme were observed. The intracellular pool (>80%) was highly glycosylated, indicating that the vast majority of invertase accumulated in the Golgi, but a smaller pool (<20%) was significantly underglycosylated, similar to the ER core glycosylated form of invertase (our unpublished results). In contrast to the pik1 phenotype, secretion and modification of invertase in cells lacking functional Stt4p were not affected. Together, these data demonstrate that Pik1p, but not Stt4p, activity is required for normal protein secretion.
pik1ts Cells Exhibit Kinetic Delays in Vacuole Protein Sorting
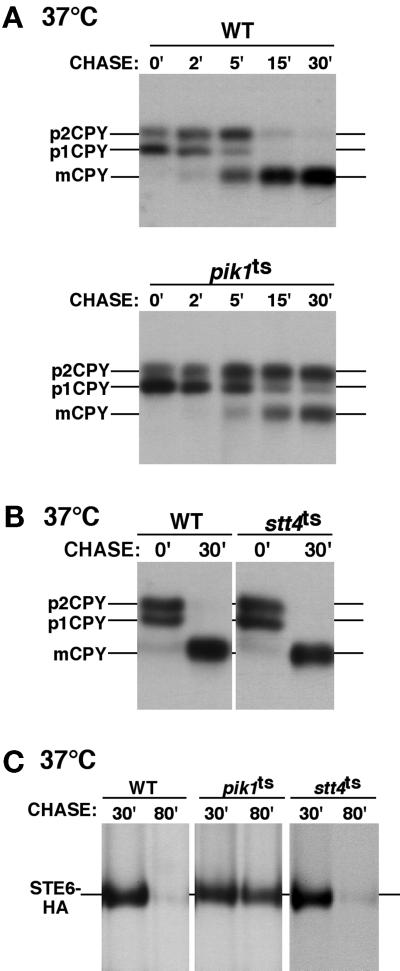
Because pik1ts cells display significant defects in secretory protein transport, we wanted to ascertain whether Pik1 or Stt4 kinase activity is required for vacuole protein sorting and transport. Using our pik1ts and stt4ts strains, we examined the transport/processing of multiple vacuolar hydrolases. CPY is one marker typically used for such an analysis. After a 10-min pulse with Tran 35S label, wild-type cells display two precursor forms of 35S-labeled CPY, representing an ER-modified form (p1) and a Golgi-modified form (p2). Upon delivery to the vacuole, proCPY is proteolytically processed, resulting in an active, lower-molecular-weight, mature form (m). At the permissive temperature, CPY transport appeared to be unaffected in both temperature-sensitive strains, but after a short 10-min preshift to 37°C and a standard pulse chase, a kinetic delay in the transport/processing of CPY was observed in the pik1ts cells (Figure 7A). The CPY transport block was not nearly as severe as that seen for the secreted proteins, Hsp150p and invertase, and extended chase points indicate that CPY maturation does occur (our unpublished results). Less than 50% of CPY accumulated as the prevacuolar, Golgi-modified p2 form, and <5% accumulated as the ER-modified p1 form. Unlike vacuolar protein sorting (vps) mutants, which missort and secrete vacuole proteins, we observed that pik1ts cells do not secrete accumulated p2 CPY. This distinguishes the pik1 mutant from the >40 S. cerevisiae vps mutants and further indicates a role for Pik1p in protein transport to the plasma membrane. In contrast to pik1ts cells, we observed no CPY transport/processing defects in stt4ts cells at restrictive temperature (Figure 7B). This result indicates that Stt4p is not required for protein transport to the vacuole. We also examined the transport of other vacuole resident proteins, ALP and carboxypeptidase S. Previous studies have demonstrated that CPY and ALP traffic to the vacuole with the use of two different pathways, namely the CPY pathway via the prevacuolar endosome and the ALP pathway, which bypasses the endosome (Cowles et al., 1997; Conibear and Stevens, 1998). Shifting pik1ts cells to restrictive temperature resulted in partial defects in the both maturation of ALP and carboxypeptidase S, suggesting a general defect in vacuole protein transport in pik1ts cells, but no transport/processing defects were observed in stt4ts cells. These data are consistent with a role for Pik1p, but not Stt4p, in protein trafficking from the Golgi.
Figure 7.
pik1ts cells exhibit defects in vacuole protein sorting and endocytosis. (A and B) Yeast cells were treated as described in Figure 5. The migration positions of precursor and mature forms of CPY are indicated on the left side of each panel. (A) A kinetic experiment showing the maturation of CPY in wild-type (SEY6210) and pik1ts (AAY104) cells at 37°C. (B) The maturation of CPY in wild-type and stt4ts (AAY102) cells at 37°C. (C) For Ste6p-HA endocytosis, cells were metabolically labeled for 10 min and chased for 10 min at 26°C. Cells were then shifted to 37°C, and samples were taken 10 and 50 min after the shift. A time course comparing the degradation of Ste6p-HA in wild-type, stt4ts, and pik1ts cells at 37°C is shown. Times shown indicate total time of label and chase. All data are representative of multiple experiments.
pik1ts Cells but Not stt4ts Cells Exhibit Defects in Endocytosis
In addition to intracellular and secretory protein trafficking defects, we examined pik1ts cells for possible endocytic dysfunction. We determined whether endocytosis of Ste6p, a plasma membrane transporter that is efficiently endocytosed and targeted to the vacuole for degradation, is altered in pik1ts and stt4ts cells. Wild-type, stt4ts, and pik1ts cells were transformed with a Ste6p-HA construct and subjected to pulse-chase labeling. To allow for efficient transit of Ste6p to the plasma membrane in pik1ts and stt4ts cells at restrictive temperature, cells were Tran 35S labeled for 10 min and chased for 10 min at permissive temperature before being shifted to 37°C. Our studies with multiple secreted proteins indicate that this period of time at permissive temperature (20 min) is sufficient for protein transport to the cell surface in pik1ts cells, thereby allowing Ste6p to efficiently reach the plasma membrane before cells are shifted to nonpermissive temperature. Degradation of the Ste6p-HA protein was analyzed by immunoprecipitation with antibody to the HA epitope at various chase times. At both 26 and 37°C, Ste6p-HA was efficiently degraded in wild-type and stt4ts cells (Figure 7C). Also, Ste6p-HA degradation was normal in pik1ts cells at 26°C (our unpublished results). However, at nonpermissive temperature, pik1ts cells fail to degrade the fusion protein, clearly indicating a delay in Ste6p transit from the plasma membrane to the vacuole. Using the dye FM4-64, we were further able to identify an endocytic defect in pik1ts cells. At 37°C, FM4-64 fails to exclusively label vacuoles in pik1ts cells but instead accumulates in several dots throughout the cytoplasm (our unpublished results). These data show that Pik1, but not Stt4, kinase activity is required for normal endocytosis.
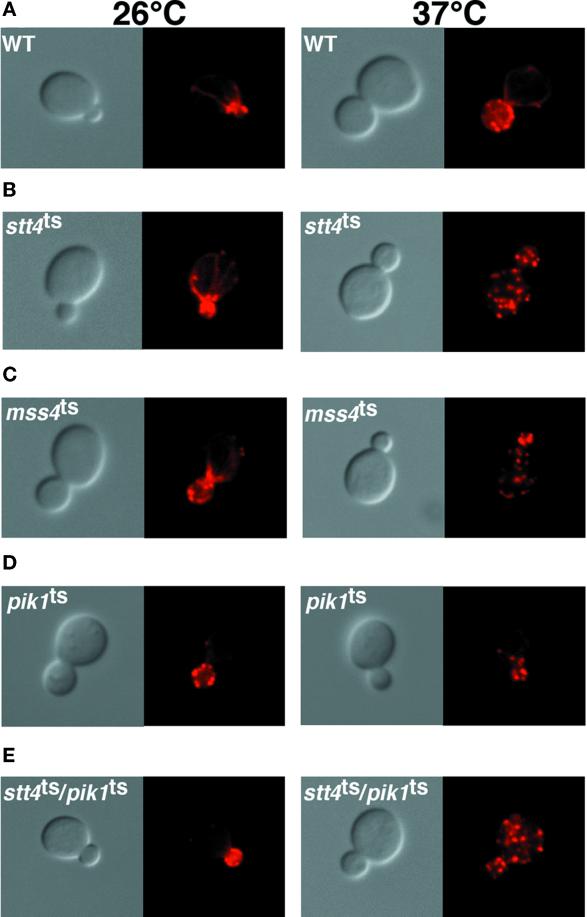
stt4ts Cells Exhibit an Abnormal Vacuole Morphology, whereas pik1ts Cells Accumulate Aberrant Golgi-like Structures
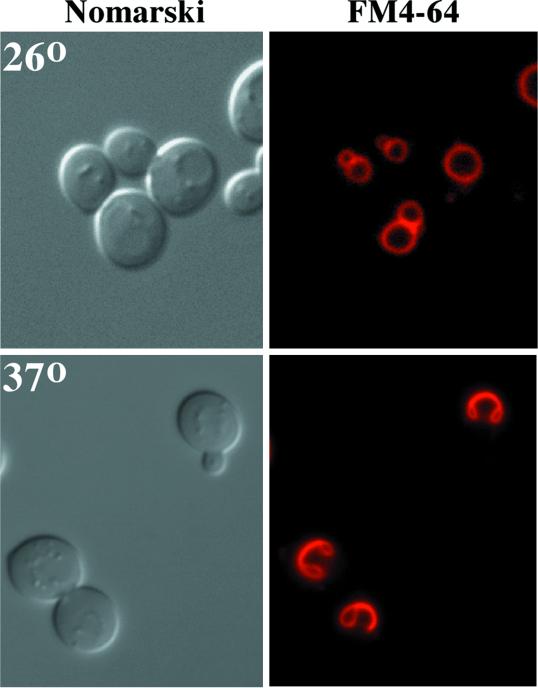
Phosphoinositides have previously been implicated in intracellular trafficking and maintenance of organelle morphology (Stack et al., 1995b; Gary et al., 1998; Odorizzi et al., 2000). To determine if Stt4 or Pik1 kinase activity plays a role in intracellular organelle morphology, stt4ts and pik1ts cells were examined by labeling the mutant cells with the vital dye FM4-64 (described in MATERIALS AND METHODS). At permissive temperature, stt4ts cells show normal uptake of the dye, staining morphologically normal vacuoles (Figure 8). However, shifting stt4ts cells to 37°C for 45 min profoundly altered the vacuole appearance, whereas endocytosis of the dye was unaffected. Within 1 h at the nonpermissive temperature, vacuoles began to fuse together and “collapse.” Strikingly, within 1–2 h, >80% of stt4ts cells contained collapsed vacuoles (Figure 8). This observation indicates that Stt4p kinase activity is required for maintaining normal vacuole morphology. In contrast to stt4ts cells, the vacuoles in pik1ts cells appeared to be distorted and fragmented at both permissive and nonpermissive temperatures (our unpublished results). These effects on the vacuole were clearly distinguishable from the phenotype observed in stt4ts cells, suggesting that normal vacuole morphology depends on both Pik1 and Stt4 kinase activities in distinct manners (see below).
Figure 8.
Stt4 kinase activity is required for normal vacuole morphology. stt4ts cells (AAY102) were grown to early log phase at 26°C, and vacuoles were labeled with the vital dye FM4-64 for 15 min (see MATERIALS AND METHODS). After labeling, cells were chased for 45 min and shifted to the indicated temperature for 1 h. Samples were taken every 15 min to examine vacuole morphology. On the left, cells were observed by Nomarski optics. On the right, identical fields are shown under fluorescent illumination (rhodamine channel). stt4ts cells at 26°C are shown on the top, and stt4ts cells after a 1-h shift to 37°C are shown on the bottom. These cells are representative of >80% of cells observed.
The altered organelle morphology in stt4ts and pik1ts cells was further characterized at the ultrastructural level. Electron microscopy revealed that at permissive temperature, stt4ts cells display features typical of wild-type cells, including multiple round, electron-dense vacuoles (Figure 9A). However, incubation at restrictive temperature before fixation resulted in the appearance of electron-dense, collapsed vacuoles in >65% of stt4ts cells, whereas wild-type cells treated in a similar manner remained unaffected (Figure 9B). Another striking feature of stt4ts cells viewed at restrictive temperature was the presence of 80- to 100-nm vesicles throughout the cytoplasm. In only a few cases did these vesicles appear polarized. In contrast, similar vesicles were observed in wild-type cells, but unlike in stt4ts cells, localization of these vesicles was restricted to the growing bud.
Figure 9.
Ultrastructure of stt4ts cells. stt4ts cells (AAY102) were grown to early log phase, shifted to the appropriate temperature for 3 h, and fixed with 3% glutaraldehyde (see MATERIALS AND METHODS). (A) stt4ts cells at 26°C. (B) stt4ts cells at 38°C. These cells are representative of >65% of cells observed. v, vacuole; n, nucleus. Bars, 1 μm.
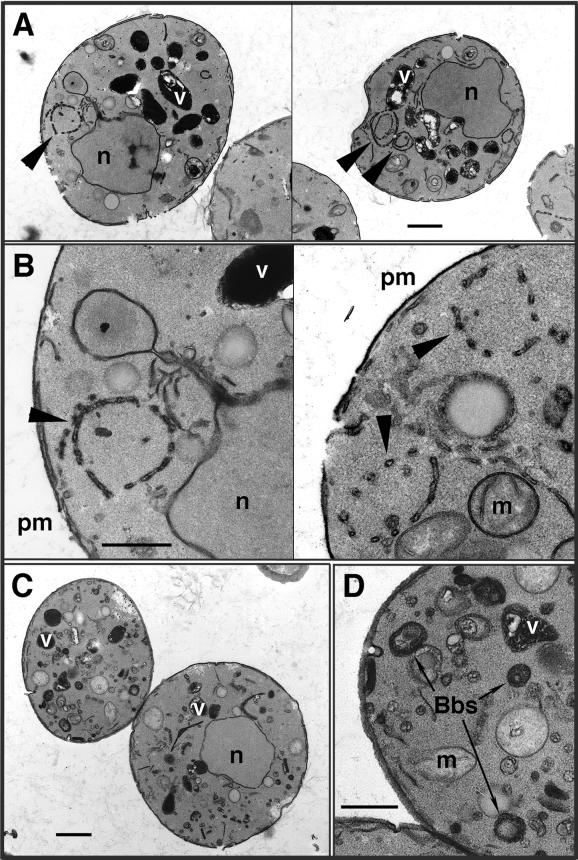
In contrast to both wild-type and stt4ts cells, >85% of pik1ts cells, when grown at permissive temperature, displayed an accumulation of atypical membrane ring structures that were ∼500 nm in diameter (Figure 10, A and B). This abnormal morphology is consistent with the diminished levels of PtdIns(4)P detected in pik1ts cells at permissive temperature, even though protein transport from the Golgi remains unaffected. The structures appear to be interconnected membrane tubules and vesicles that form discontinuous rings in most thin sections. However, grazing sections that appeared to be near the surface of these rings suggest that they are composed of a reticular network of membrane tubules organized as a cylinder or sphere. The area contained within the rings appears to be similar in electron density to the surrounding cytoplasm, indicating continuity with the cytoplasm. Consistent with distinct roles for the two PtdIns 4-kinases, <30% of stt4ts cells displayed similar structures, and they were observed only at restrictive temperature.
Figure 10.
pik1ts cells accumulate morphologically abnormal Golgi and vacuole compartments. pik1ts cells (AAY104) were incubated at either 26 or 37°C for 90 min, fixed in 3% glutaraldehyde, and processed for electron microscopy (see MATERIALS AND METHODS). (A) pik1ts cells at 26°C. Bar, 1.0 μm. (B) Enlarged view of membrane ring structures found in pik1ts cells at 26°C. Bar, 0.4 μm. (C) pik1ts cells at 37°C. Bar, 1.0 μm. (D) Enlarged view of Berkeley bodies observed in pik1ts cells at 37°C. Bar, 0.4 μm. All cells shown are representative of >85% of cells observed. Arrowheads indicate atypical ring structures. Vacuoles (v), mitochondria (m), Berkeley bodies (Bbs), the plasma membrane (pm), and nuclei (n) are indicated.
Upon shift of pik1ts cells to nonpermissive temperature for 90 min, the shape of the vacuoles appeared even more distorted, and the membrane ring structures observed at low temperature were almost completely absent. Additionally, a dramatic accumulation of new membrane structures was seen (Figure 10, C and D). Specifically, we observed the appearance of numerous Berkeley bodies, previously observed in secretory mutants defective in Golgi transport (Novick et al., 1980), further supporting a role for Pik1p in Golgi trafficking and dynamics. In contrast to the pik1ts cells, stt4ts cells at 37°C did not accumulate Berkeley bodies, but the mutant stt4ts cells did increase in size (∼60%) relative to both wild-type and pik1ts cells treated in the same manner (Figure 9). This result indicates that Stt4p may have a role in regulating cell size or polarized growth, possibly through the actin cytoskeleton or cell wall remodeling.
Evidence for a Functional Interaction between Pik1p and Arf1p
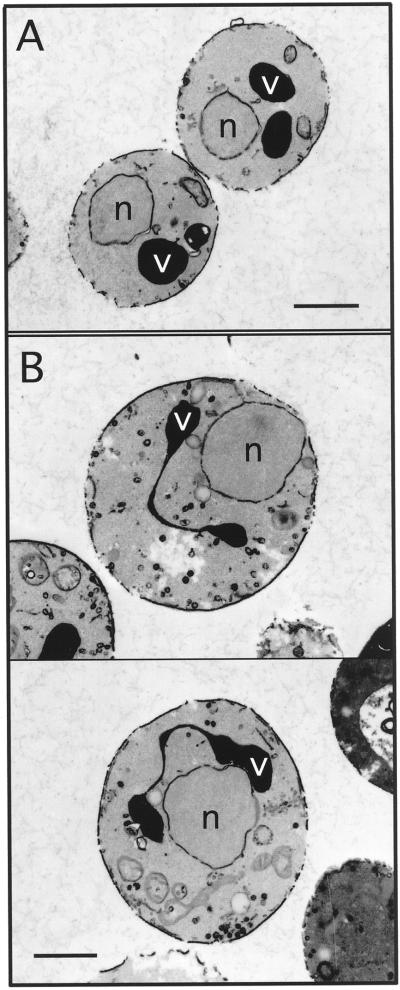
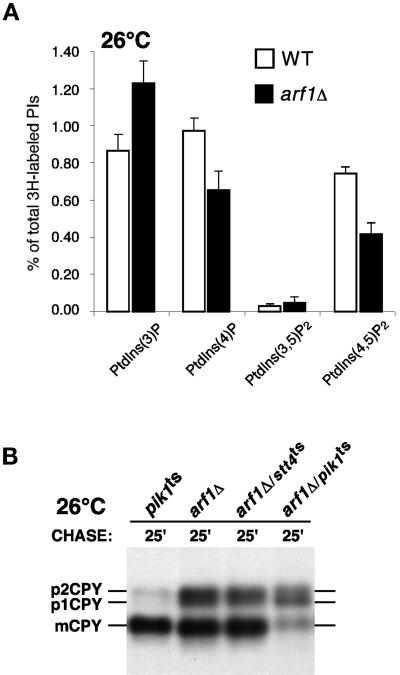
The morphology displayed by pik1ts cells at permissive temperature is similar to that of yeast cells that lack the gene encoding ARF1, a small GTP-binding protein required for the maintenance of Golgi membrane composition and dynamics (Gaynor et al., 1998). In arf1 mutant cells, early and medial Golgi glycosyltransferases localize to ring structures. Consistently in pik1ts cells, Sec7p, a Golgi component of the yeast secretory pathway (Franzusoff et al., 1991), also localizes to ring structures, likely corresponding to the tubular networks observed as membrane rings by electron microscopy (our unpublished results). Interestingly, we have found that, like pik1ts cells, cells lacking ARF1 exhibit significant decreases in PtdIns(4)P and PtdIns(4,5)P2 levels (Figure 11A), suggesting that PtdIns(4)P synthesis is partially dependent on Arf1p activity. Supporting a specific connection between ARF1 and PIK1, we found that arf1/pik1 double mutants are viable but exhibit synthetic protein trafficking defects at permissive temperature, whereas arf1/stt4 double mutant cells do not (Figure 11B). We propose that Pik1p, together with Arf1p, plays a key role in maintaining Golgi transport activity as well as the organization and dynamics of the Golgi.
Figure 11.
Cells lacking Arf1p produce diminished levels of PtdIns(4)P and PtdIns(4,5)P2. (A) arf1 mutant cells were treated as described in Figure 2A. Quantitative comparisons of glycerophosphoinositols generated by wild-type (SEY6210) and arf1Δ cells at 26°C are shown. These data represent means ± ranges of at least three independent experiments. (B) pik1ts (AAY104), arf1Δ, stt4/arf1 double mutant (AAY114), and pik1/arf1 double mutant (AAY116) cells were metabolically labeled with a 35S-protein labeling mix during a 10-min pulse at 26°C and then chased in the presence of excess unlabeled methionine and cysteine for the times indicated. Each protein was immunoprecipitated with an antibody specific to CPY and resolved on SDS-PAGE.
DISCUSSION
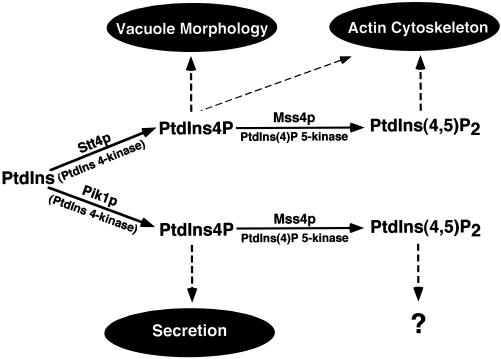
In this study, we have characterized two yeast PtdIns 4-kinases, identifying distinct roles for each in membrane trafficking and cell morphology. Our data demonstrate that Stt4p functions in maintaining normal vacuole morphology and actin polarization. In contrast, Pik1p activity is crucial for the maintenance of Golgi structure and for protein secretion. Additionally, pik1ts cells display a defect in vacuole morphology that is distinct from the defect observed in stt4ts cells. Our results indicate that both the Pik1 and Stt4 kinases contribute to the synthesis of distinct pools of PtdIns(4)P and PtdIns(4,5)P2. The unique roles for the two pools of PtdIns(4)P provide an understanding of why each gene is essential. We propose a model (Figure 12) in which the Stt4 and Pik1 PtdIns 4-kinases function in distinct subcellular locations and synthesize two essential and independent pools of PtdIns(4)P and PtdIns(4,5)P2 that activate effector proteins, which in turn direct membrane trafficking, cytoskeleton organization, and organelle homeostasis.
Figure 12.
The pathways for biosynthesis of PtdIns(4)P and PtdIns(4,5)P2 in yeast, indicating cellular role(s) for each phosphoinositide produced. The Stt4p- or Pik1p-dependent phosphorylation of PtdIns leads to the production of distinct pools of PtdIns(4)P. Arrows indicate the proposed roles for each phosphorylated lipid product. Stt4p-dependent PtdIns(4)P is required for maintenance of vacuole morphology and the actin cytoskeleton, whereas Pik1p-dependent PtdIns(4)P is essential for secretory vesicle formation and Golgi/endosome maintenance. Both Stt4 and Pik1 kinase activities appear to be required for generating the cellular pool of PtdIns(4,5)P2. Although we propose that Stt4p-dependent generation of PtdIns(4,5)P2 is required for actin polarization, a role for Pik1p-dependent PtdIns(4,5)P2 synthesis is not yet apparent.
Stt4p Is an Essential Component of the PKC1 Pathway and Is Required for Maintaining Vacuole Morphology
The STT4 gene encodes a hydrophilic protein of ∼216 kDa (Figure 1). Sequence analysis indicates that it is likely a soluble protein, but Stt4p has been shown to tightly associate with a particulate fraction in yeast that is enriched for membranes (Cutler et al., 1997). Our attempts to more specifically define the localization of Stt4p have not succeeded, because of insufficient expression levels and instability of the protein in cell extracts. Stt4p has been implicated in an ER-to-Golgi/vacuole aminophospholipid transport step (Trotter et al., 1998), suggesting that Stt4p may be localized to the ER. However, a recent two-hybrid screen identified a protein–protein interaction between Stt4p and Std1p (Uetz et al., 2000), a protein that has been localized to the cell periphery and nucleus (Schmidt et al., 1999). From our analysis of stt4ts cells, we have identified multiple roles for this PtdIns 4-kinase, perhaps suggesting that Stt4p is localized to multiple subcellular compartments likely distinct from Pik1p.
Previous studies have implicated STT4 in the yeast PKC1 protein kinase pathway (Yoshida et al., 1994a,b). Pkc1p is the regulator of a MAPK cascade, activated by heat shock and other external stimuli, to control the actin cytoskeleton and transcription of genes encoding cell wall biosynthetic enzymes (Davenport et al., 1995; Kamada et al., 1995; Helliwell et al., 1998; Zhao et al., 1998). After heat shock, wild-type cells undergo transient Pkc1p-dependent depolarization of both the actin cytoskeleton and the Fks1 protein, an integral plasma membrane protein that synthesizes a major component of the cell wall, β-1,3-glucan (Delley and Hall, 1999). This process is thought to aid in repairing general cell wall damage induced by heat shock. After 90 min, cells are able to repolarize their actin cytoskeletons, but cells lacking components of the Pkc1p kinase cascade are unable to do so (Delley and Hall, 1999). This result suggests that integrity of the PKC1 pathway is required for actin reorganization after heat shock. Our findings indicate that the Stt4 PtdIns 4-kinase may be involved in the PKC1 pathway, because stt4ts cells are unable to repolarize their actin cytoskeletons at restrictive temperature. This phenotype is not caused by cell death, because stt4ts cells remain viable at high temperature for several hours. In contrast to stt4ts cells, we find that loss of Pik1p kinase activity does not result in persistent actin disorganization, a finding that does not agree with previous work (Walch-Solimena and Novick, 1999). However, the pik1 allele used in that study was highly defective in actin organization at both low and high temperatures and was not directly tested for changes in phosphoinositide levels. These findings, therefore, may be an indirect consequence of the steady-state defect in Pik1p function in this mutant strain.
The involvement of phosphoinositides in actin polarization is well documented (reviewed by Janmey, 1994). We and others have shown that Mss4p, the major yeast PtdIns(4)P 5-kinase, is also required for actin organization (Figure 4) (Desrivieres et al., 1998; Homma et al., 1998). In a simple model for the role of STT4 and MSS4 in regulating the actin cytoskeleton, the Stt4 kinase generates PtdIns(4)P that is then converted by the Mss4 kinase to PtdIns(4,5)P2. The PtdIns(4,5)P2 then could recruit and/or activate actin-binding proteins such as profilin or cofilin, both of which are known to bind PtdIns(4,5)P2 (reviewed by Martin, 1998). Alternatively, Stt4p and/or Mss4p may signal activation of a RHO-type GTPase by recruitment/activation of Rom2p, a guanine nucleotide exchange factor that contains a phosphoinositide-binding pleckstrin homology domain (Ozaki et al., 1996). Previous studies have suggested that pleckstrin homology domains can bind phosphoinositides to regulate protein function (reviewed by Bottomley et al., 1998); thus, Rom2p may act as a downstream effector of Stt4p and/or Mss4p.
As described above, cells undergoing heat shock respond by activating the PKC1 pathway to induce expression of cell wall biosynthesis genes required to prevent cell lysis. We have observed that cells lacking Stt4p function are almost twice as large as wild-type cells, and stt4ts cells undergo cell lysis in the absence of osmotic support at restrictive temperature (Figure 9). The increase in cell size may be due to delocalized secretion caused by severe actin disorganization, leading to isotropic cell growth. Consistent with this idea, we observed 80- to 100-nm vesicles in stt4ts cells at restrictive temperature that resemble secretory vesicles observed in cells lacking Slt2p, the MAPK downstream of Pkc1p (Mazzoni et al., 1993). In virtually all cases, the vesicles in stt4ts cells were not polarized toward the site of bud formation, suggesting that secretion to the bud was defective. In addition, mutants lacking Stt4p and/or Mss4p activity undergo cell lysis similar to cells lacking PKC1. These results further support a model in which STT4 and MSS4 play important roles in the PKC1 pathway.
Unlike mss4ts cells, upon shift to restrictive temperature stt4ts cells undergo a dramatic change in vacuole morphology. The involvement of phosphoinositide kinase activity in regulating vacuole size has been reported previously (Gary et al., 1998). Cells lacking the PtdIns(3)P 5-kinase activity of Fab1p display single, abnormally large, swollen vacuoles, but the data presented in this study represent the first demonstration of PtdIns 4-kinase involvement in the maintenance of vacuole morphology. We have observed that after only a 45-min shift to restrictive temperature, vacuoles in stt4ts cells appear to collapse. Unexpectedly, pik1ts cells also undergo a dramatic, but distinct, change in vacuole morphology in which vacuoles appear to be fragmented at both permissive and nonpermissive temperatures. This suggests a general role for phosphoinositides in regulating organelle homeostasis, perhaps by controlling membrane channels or transporters required for osmotic control. These data also suggest a potential role for phosphoinositides in the regulation of vacuole fusion and/or fission. Consistent with this idea, PtdIns(4)P and PtdIns(4,5)P2 have recently been shown to be important for vacuole fusion (Mayer et al., 2000).
Pik1 PtdIns 4-Kinase Activity Is Required for Protein Secretion and Endocytosis
Our results demonstrate that the Pik1 kinase regulates cellular activities distinct from those of the Stt4 kinase, most likely as a result of different subcellular localization patterns for these enzymes. Pik1p is a hydrophilic 125-kDa protein that has been shown to associate tightly with a small Ca2+-binding protein, Frq1p (Flanagan and Thorner, 1992; Hendricks et al., 1999). In vitro, Frq1p can stimulate Pik1p activity and may target Pik1p to a particular membrane. A large pool of the Pik1 kinase appears to be localized within the nucleus and is suggested to have a role in the nuclear phosphoinositide cycle (Garcia-Bustos et al., 1994). Another pool of Pik1p is localized in the cytoplasm and enriched on Golgi membranes (Walch-Solimena and Novick, 1999). Recent studies have implicated Pik1p enzyme activity in secretory function at the trans-Golgi (Hama et al., 1999; Walch-Solimena and Novick, 1999). In mammalian cells, PtdIns 4-kinase activity has been associated with stimulated secretion of granules from chromaffin cells (Wiedemann et al., 1996). Here we show the first direct biochemical evidence that multiple secreted proteins are aberrantly retained within cells lacking Pik1 kinase activity. Our data are most consistent with an essential role for the Pik1 kinase in the maintenance of Golgi morphology and transport activity, possibly by controlling the fission of secretory vesicles from the late Golgi. First, pik1 mutants accumulate Golgi-modified proteins. Second, vacuole protein transport from the Golgi is perturbed. Third, pik1ts cells show an accumulation of Golgi-related structures. Fourth, arf1Δ cells, which fail to maintain Golgi structure and function, display specific decreases in PtdIns(4)P and PtdIns(4,5)P2, similar to pik1ts cells. Finally, arf1/pik1 double mutants exhibit synthetic protein trafficking defects.
The enzyme invertase and the secreted protein Hsp150p are normally rapidly secreted from cells after synthesis. However, in pik1ts cells, the secretion of both proteins is blocked. Each instead accumulates intracellularly in its Golgi-modified form at restrictive temperature (Figure 6, A and B). This suggests that the Golgi glycosylation machinery is only modestly affected by eliminating Pik1 kinase activity, whereas secretion from the Golgi is blocked. Additionally, transport from the Golgi to the vacuole appears to be kinetically delayed, indicated by an accumulation of p2 CPY in the pik1ts mutant at 37°C (Figure 7A). Studies with another allele of pik1 have also indicated a weaker defect in CPY maturation (Walch-Solimena and Novick, 1999). However, this may be a secondary consequence of the potent block in secretion out of the Golgi complex resulting from Pik1 kinase inactivation. In another strain background (YES102), the same allele of pik1 that was used in this study failed to produce a significant defect in CPY maturation (Hama et al., 1999). However, with only slightly longer preshifts (15 min instead of 10 min), we have observed a kinetic defect in CPY maturation in YES102 (our unpublished results), suggesting that differences in strain background can affect the kinetic delay in vacuole protein sorting in pik1 mutant cells. Together, these data implicate Pik1p kinase activity in the control of general Golgi structure and transport function. Based on these observations, we propose that the pool of PtdIns(4)P generated by the Pik1 kinase is required for transport between the Golgi and the cell surface (formation of secretory vesicles). Transport between the Golgi and the endosome/vacuole primarily requires PtdIns(3)P generated by the Vps34 PtdIns 4-kinase (Wurmser et al., 1999). However, an additional role of Pik1p-generated PtdIns(4)P is indicated by the defect in endocytic trafficking of Ste6p and FM4-64.
In agreement with a role for Pik1p in Golgi structure and function, a recent study has suggested that PtdIns4Kβ, the mammalian homologue of Pik1p, is required for structural integrity of the Golgi complex (Godi et al., 1999). Recruited by the small GTPase ARF to the Golgi, the PtdIns 4-kinase activity of PtdIns4Kβ contributes to the regulation of Golgi structure and function. Also, a PtdIns4Kβ point mutant that lacks kinase activity exerts a dominant-negative effect on Golgi organization in COS cells (Godi et al., 1999). We have observed a similar disorganization of the Golgi complex in pik1ts cells at the ultrastructural level. Specifically, we detected the presence of interconnected membrane tubules that appear as ring structures in thin sections (Figure 10). At permissive temperature, pik1ts cells produce a diminished amount of PtdIns(4)P, which may give rise to the tubular networks. When shifted to restrictive temperature, PtdIns(4)P produced in pik1ts cells further decreases compared with that in wild-type cells, and the rings disappear. Instead, numerous Berkeley bodies accumulate, and secretory traffic stops. In a previous report, ring structures similar to those observed in pik1ts cells were shown to accumulate in an arf1 mutant and to correspond to abnormal Golgi and/or endosomal compartments (Gaynor et al., 1998). This suggests that Arf1p- and Pik1p-dependent pathways may overlap in a conserved manner in yeast and mammals. Supporting a connection between ARF1 and PIK1, we observed synthetic growth and transport defects in arf1/pik1ts double mutants at permissive temperature, whereas no synthetic phenotypes were observed in arf1/stt4ts double mutants. Finally, cells lacking Arf1p function have decreased cellular levels of PtdIns(4)P and PtdIns(4,5)P2, similar to that observed in pik1ts cells at permissive temperature (Figure 11A). As in higher organisms, we speculate that Arf1p may recruit/activate Pik1p to the Golgi in yeast, in which both proteins are required to maintain the structure and function of the organelle.
As noted above, ARF1 has also been implicated in endosome function (Gaynor et al., 1998). We have presented evidence that Pik1 kinase activity is required for normal endocytic transport of Ste6p from the plasma membrane to the vacuole (Figure 7C). Additionally, we and others have observed endocytic defects in pik1ts cells by staining with the vital vacuolar dye FM4-64 (Walch-Solimena and Novick, 1999; our unpublished results). However, the data provided here represent the first evidence that Pik1p is required for transport of a plasma membrane protein to the vacuole. These results suggest that Pik1p activity is essential not only for normal Golgi membrane dynamics but also for normal endocytic trafficking. It is not yet clear whether this is due to a direct role of Pik1p at the endosome or plasma membrane, because at present Pik1p has been detected only in the nucleus and Golgi.
The Cellular Pool of PtdIns(4,5)P2 Depends on Both Stt4 and Pik1 Kinase Activities
Our results indicate that STT4 and PIK1 are likely the only PtdIns 4-kinases in S. cerevisiae. HPLC analysis of phosphoinositide levels in a stt4ts/pik1ts double mutant strain demonstrates that loss of both Stt4 and Pik1 kinase activity results in a >95% decrease in PtdIns(4)P and PtdIns(4,5)P2 levels compared with that in wild-type cells after a short shift to nonpermissive temperature. The small amount of PtdIns(4)P and PtdIns(4,5)P2 produced in this strain is likely due to residual activity generated by the temperature-sensitive PtdIns 4-kinases. However, we cannot definitely rule out the possibility that another PtdIns 4-kinase may exist, although we do not favor such a hypothesis. We also observed a decrease in the levels of PtdIns(3)P in stt4ts/pik1ts double mutant cells, but this is likely an indirect consequence of the growth defect observed in these cells. In addition, our data indicate that both Stt4p and Pik1p contribute to the synthesis of PtdIns(4,5)P2. In either stt4ts or pik1ts single mutant cells at nonpermissive temperature, PtdIns(4,5)P2 continues to be produced. The role of PtdIns(4,5)P2 in the regulation of membrane trafficking or organelle morphology is not yet clear. Our initial analysis of the mss4ts allele suggests that PtdIns(4)P 5-kinase activity is not required for normal secretion or vacuole morphology; however, the onset of the kinase defects in this temperature-sensitive mutant is slow (Desrivieres et al., 1998). New mss4 alleles as well as additional genetic and biochemical analyses of both Pik1p and Stt4p should help resolve the precise roles of PtdIns(4)P and PtdIns(4,5)P2 in protein trafficking as well as in membrane dynamics and cytoskeletal organization.
ACKNOWLEDGMENTS
We are indebted to Dr. Jeremy Thorner for many helpful discussions and for generously providing the pik1-83 allele. We also thank Dr. Michael Hall for the mss4-2 strain and Dr. Dennis Voelker for the Ycp50-STT4 plasmid. We thank Chris Hofeditz for assisting with the electron microscopic analysis (Immunoelectron Microscopy Core B of Program Project grant CA58689 headed by M. Farquhar), and Erin Gaynor and Daryll DeWald for helpful technical advice. We also thank Chris Stefan, Greg Odorizzi, Markus Babst, and Beth Weaver for useful discussions, and Andrew Wurmser, Tamara Darsow, and Dr. Daryll DeWald for critical reading of the manuscript. This work was supported by grant CA58689 from the National Institutes of Health (to S.D.E.). S.D.E. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- Benedetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley MJ, Salim K, Panayotou G. Phospholipid-binding protein domains. Biochim Biophys Acta. 1998;1436:165–183. doi: 10.1016/s0005-2760(98)00141-6. [DOI] [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta. 1998;1404:211–230. doi: 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Synder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler NS, Heitman J, Cardenas ME. STT4 is the essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J Biol Chem. 1997;272:27671–27677. doi: 10.1074/jbc.272.44.27671. [DOI] [PubMed] [Google Scholar]
- Davenport KR, Sohaskey M, Kamada Y, Levin DE, Gustin MC. A second osmosensing signal transduction pathway in yeast: hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J Biol Chem. 1995;270:30157–30161. doi: 10.1074/jbc.270.50.30157. [DOI] [PubMed] [Google Scholar]
- Delley P, Hall MN. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J Cell Biol. 1999;147:163–174. doi: 10.1083/jcb.147.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivieres S, Cooke FT, Parker PJ, Hall MN. Mss4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- Flanagan CA, Thorner J. Purification and characterization of a soluble phosphatidylinositol 4-kinase from the yeast Saccharomyces cerevisiae. J Biol Chem. 1992;267:24117–24125. [PubMed] [Google Scholar]
- Franzusoff A, Redding K, Crosby J, Fuller RS, Schekman R. Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J Cell Biol. 1991;112:27–37. doi: 10.1083/jcb.112.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos JF, Marini F, Stevenson I, Frei C, Hall MN. Pik1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J. 1994;13:2352–2361. doi: 10.1002/j.1460-2075.1994.tb06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary JG, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Chen C, Emr SD, Graham TR. ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol Biol Cell. 1998;9:653–670. doi: 10.1091/mbc.9.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, te Heesen S, Graham TR, Aebi M, Emr SD. Signal-mediated retrieval of a membrane protein from the Golgi to the E.R. in yeast. J Cell Biol. 1994;112:27–37. doi: 10.1083/jcb.127.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi A, Pertile P, Meyers R, Marra P, DiTullio G, Iurisci C, Luini A, Corda D, DeMatteis M. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuole protein sorting events defined in a yeast Sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, De-Wald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helliwell SB, Schmidt A, Ohya Y, Hall MN. The Rho1 effector, Pkc1, but not Bni1, mediates signaling from Tor2 to the actin cytoskeleton. Curr Biol. 1998;8:1211–1214. doi: 10.1016/s0960-9822(07)00511-8. [DOI] [PubMed] [Google Scholar]
- Hendricks KB, Wang B, Schnieders E, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- Homma K, Terui S, Minemura M, Qadota H, Anraku Y, Kanaho Y, Ohya Y. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J Biol Chem. 1998;273:15779–15786. doi: 10.1074/jbc.273.25.15779. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cation. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Johnson LM, Bankaitis VA, Emr SD. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987;48:875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE. The protein kinase-C activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995;9:1559–1571. doi: 10.1101/gad.9.13.1559. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- Martin TFJ. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- Mayer A, Scheglmann D, Dove S, Glatz A, Wickner W, Haas A. Phosphatidylinositol 4,5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol Biol Cell. 2000;11:807–817. doi: 10.1091/mbc.11.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C, Zarzov P, Rambourg A, Mann C. The SLT2 (MPK1) MAP kinase is involved in polarized cell growth in Saccharomyces cerevisiae. J Cell Biol. 1993;123:1821–1833. doi: 10.1083/jcb.123.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Phosphoinositide signaling and regulation of membrane trafficking in yeast. Trends Biochem Sci. 2000;25:229–235. doi: 10.1016/s0968-0004(00)01543-7. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Tanaka K, Imamura H, Hihara T, Kameyama T, Nonaka H, Hirano H, Matsuura Y, Takai Y. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:2196–2207. [PMC free article] [PubMed] [Google Scholar]
- Paravicini G, Cooper M, Friedli L, Smith DJ, Carpenter J, Klig LS, Payton MA. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol Cell Biol. 1992;12:4896–4905. doi: 10.1128/mcb.12.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GS, Hasson TB, Hasson MS, Schekman R. Genetic and biochemical characterization of clathrin-deficient Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:3888–3898. doi: 10.1128/mcb.7.11.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Banta LM, Kohrer K, McCaffery JM, Emr SD. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuole protein sorting mutant. Mol Biol Cell. 1996;7:527–538. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defects in the delivery and sorting of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P, Kalkkinen N, Sareneva H, Paakkola J, Makarow M. A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci USA. 1992;89:3671–3675. doi: 10.1073/pnas.89.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MC, McCartney RR, Zhang X, Tillman TS, Solimeo H, Wolfl S, Almonte C, Watkins SC. Std1 and Mth1 proteins interact with the glucose sensors to control glucose-regulated gene expression in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4561–4571. doi: 10.1128/mcb.19.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Lawrence LW. Methods in Yeast Genetics: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1979. [Google Scholar]
- Stack JH, DeWald DB, Takegawa K, Emr SD. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J Cell Biol. 1995a;129:321–334. doi: 10.1083/jcb.129.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995b;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Trotter PJ, Wu W, Pedretti J, Yates R, Voelker DR. A genetic screen for aminophospholipid transport mutants identifies the phosphatidylinositol 4-kinase, Stt4p, as an essential component in phosphatidylserine metabolism. J Biol Chem. 1998;273:13189–13196. doi: 10.1074/jbc.273.21.13189. [DOI] [PubMed] [Google Scholar]
- Uetz P, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- Wurmser AE, Emr SD. Phosphoinositide signaling and turnover: PtdIns(3)P, a regulator of membrane traffic, is transported to the vacuole and degraded by a process that requires lumenal vacuolar hydrolase activities. EMBO J. 1998;17:4930–4942. doi: 10.1093/emboj/17.17.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmser AE, Gary JD, Emr SD. Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J Biol Chem. 1999;274:9129–9132. doi: 10.1074/jbc.274.14.9129. [DOI] [PubMed] [Google Scholar]
- York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohya Y, Goebl M, Nakano A, Anraku Y. A novel gene, Stt4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem. 1994a;269:1166–1171. [PubMed] [Google Scholar]
- Yoshida S, Ohya Y, Nakano A, Anraku Y. Genetic interactions among genes involved in the STT4-PKC1 pathway of Saccharomyces cerevisiae. Mol Gen Genet. 1994b;242:631–640. doi: 10.1007/BF00283416. [DOI] [PubMed] [Google Scholar]
- Zhao C, Jung US, Garrett-Engele P, Roe T, Cyert MS, Levin DE. Temperature-induced expression of yeast FKS2 is under dual control of protein kinase C and calcineurin. Mol Cell Biol. 1998;18:1013–1022. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]