Abstract
OBJECTIVE
To examine if delayed transfer to the intensive care unit (ICU) after physiologic deterioration is associated with increased morbidity and mortality.
DESIGN
Inception cohort.
SETTING
Community hospital in Ogden, Utah.
PATIENTS
Ninety-one consecutive inpatients with noncardiac diagnoses at the time of emergent transfer to the ICU. We determined the time when each patient first met any of 11 pre-specified physiologic criteria. We classified patients as “slow transfer” when patients met a physiologic criterion 4 or more hours before transfer to the ICU. Patients were followed until discharge.
INTERVENTIONS
None.
MEASUREMENTS
In-hospital mortality, functional status at hospital discharge, hospital resources.
MAIN RESULTS
At the time when the first physiologic criterion was met on the ward, slow- and rapid-transfer patients were similar in terms of age, gender, diagnosis, number of days in hospital prior to ICU transfer, prehospital functional status, and APACHE II scores. By the time slow-transfer patients were admitted to the ICU, they had significantly higher APACHE II scores (21.7 vs 16.2; P = .002) and were more likely to die in-hospital (41% vs 11%; relative risk [RR], 3.5; 95% confidence interval [95% CI], 1.4 to 9.5). Slow-transfer patients were less likely to have had their physician notified of deterioration within 2 hours of meeting physiologic criteria (59% vs 31%; P = .001) and less likely to have had a bedside physician evaluation within the first 3 hours after meeting criteria (23% vs 83%; P = .001).
CONCLUSIONS
Slow transfer to the ICU of physiologically defined high-risk hospitalized patients was associated with increased risk of death. Slow response to physiologic deterioration may explain these findings.
Keywords: intensive care unit, physiologic monitoring, mortality, length of stay, APACHE II score
Patients transferred from medical or surgical wards to the intensive care unit (ICU) provide a challenge to clinicians. A recent survey of 285 U.S. hospitals, including teaching and nonteaching institutions, observed an aggregate in-hospital mortality of 12% for ICU patients.1 In contrast, patients transferred from the ward to the ICU have a 20% to 65% in-hospital mortality rate.2–4 Patients transferred from the ward to the ICU are also much more costly to treat.3,5,6
For hospitalized patients, the timing of transfer to the ICU may be an important determinant of outcomes. The timing of several acute care interventions (thrombolytic agents, aspirin and β-blockers in myocardial infarction, thrombolytic agents in stroke, and emergency resuscitation after major trauma) has a substantial impact on mortality.7–9 Some authors have suggested that up to 50% of cardiopulmonary arrests on general medical and surgical wards could be prevented by earlier transfer to the ICU.10–12 However, these studies relied on expert opinion rather than explicit criteria to retrospectively judge which patients may have avoided respiratory or cardiac arrest. Other studies have proposed subjective criteria, such as the onset of acute dyspnea or failure to respond to treatment, to identify patient groups at risk for severe deterioration.13,14 Little is known about criteria that are both objective and simple that could be used prospectively to identify general ward patients at risk for catastrophic deterioration.15,16
We studied a consecutive series of noncardiac patients transferred to the intensive care unit at a community teaching hospital. We specified 11 physiologic or laboratory criteria indicative of physiologic instability. We determined the time, prior to transfer to the ICU, that each patient first met 1 or more of these criteria. We defined the timing of ICU transfer as the interval between first meeting a physiologic criterion on the hospital ward and transfer to the ICU. We followed patients' hospital course to determine if there was an association between the timing of ICU transfer and mortality, morbidity, and cost.
MATERIALS AND METHODS
The study was conducted over a 16-month period (April 1994 through July 1995) at McKay-Dee Medical Center, a 460-bed community hospital in Ogden, Utah. At the time of the study, the ICU was a 14-bed “open” medical-surgical unit where most physicians on the medical staff of the hospital had ICU admitting privileges. After transfer to the ICU, all patients were co-managed by a team of 3 full-time board certified intensivists. The study was approved by the Hospital Institutional Review Board, which waived the need for informed consent.
Study Design and Patient Population
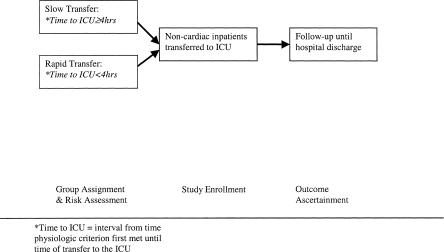
An overview of the study design is provided in Figure 1. We identified, at the time of ICU admission, all consecutive, noncardiac patients over the age of 12 years who were transferred to the ICU from the medical or surgical wards. We excluded patients admitted directly from the emergency department to the ICU, scheduled ICU admissions from the operating room, and patients admitted to the ICU for observation after invasive radiological procedures. We carried out retrospective chart review of the hospital course prior to transfer to the ICU to determine when each patient first met any 1 of 11 physiologic threshold criteria. We did not track the outcomes of patients who never met any physiologic criteria prior to ICU transfer. Patients were classified as slow transfers to the ICU if the time between first meeting a criterion and ICU admission was 4 hours or more. Patient risk status at the time the first criterion was met was assessed using data obtained from chart notes and laboratory reports. Also on the basis of chart review, we recorded the sequence of physician notification, physician bedside visits, and additional physiologic deterioration prior to transfer to the ICU. The study nurse (KM) prospectively gathered data to measure patients' risk status at the time of transfer to the ICU and recorded subsequent outcomes. The physicians referring patients to the ICU were unaware that this study was being conducted. The admission criteria used by the physician staff to evaluate patients for potential ICU admission were nonexplicit.
FIGURE 1.
Study enrollment and data collection process.
Measures
Table 1 describes the 11 physiologic threshold criteria used to define patients at risk for deterioration. These criteria were selected on the basis of a review of the measures included in APACHE II and SAPS17,18 and clinician judgment. Patients could meet a criterion through 2 mechanisms. First, patients could have values recorded in the chart or laboratory reports that met the specified threshold values. Second, the nurse could document in the chart the specified change in mental status, or intervention (e.g., providing mechanical airway support) that met physiologic threshold criterion.
Table 1.
Physiologic Threshold Criteria Measured on the Medical-Surgical Wards
| Criteria | Low | High |
|---|---|---|
| Respiratory | ||
| Respiratory rate | >35 breaths/min for >30 min | |
| Arterial blood gas | pH <7.25 | pC02 >60mm/Hg |
| Oxygen saturation | <95% of nonrebreather mask <30 min | |
| Peak flow | <250 L/min | |
| Airway protection | Absent cough, gag or need for artificial airway | |
| Cardiac | ||
| Systolic blood pressure | <85mm/Hg for >30 min | |
| Heart rate (sustained for >30 min) | <40 beats/min | >140 beats/min |
| Urine output | <200 cc/8 h in absence of chronic renal failure | |
| Other | ||
| Gastrointestinal bleeding | Hematocrit <22% | ≥4 units pRBCs transfused in <24 hours |
| Level of consciousness | Glasgow Coma Score <12 | Acute decrease in Glasgow Coma score ≥2 |
| Patient with abdominal pain and >60 years old and either: | WBC <3,000 | WBC >16,000 |
pCO2, partial pressure carbon dioxide; pRBC, packed red blood cells; WBC, white blood cell count.
Our classification of transferred patients as either “slow transfer” or “rapid transfer” was determined by examining the time recorded in the chart when the first criterion was met on the ward and the time of transfer to the ICU. Patients transferred to the ICU more than 4 hours after first meeting any of the physiologic threshold criteria were labeled as “slow transfer” while patients transferred in 4 hours or less were classified as “rapid transfer.” For example, a patient on the ward with a respiratory rate of 40 breaths/minute at 1 pm transferred to the ICU at 4 pm the same day would have the delay to ICU recorded as 3 hours and would be placed in the “rapid-transfer” group. Four hours was chosen as the threshold based upon prior studies indicating that delays over several hours in delivering essential therapies create increased risk for patients with acute myocardial infarction, stroke, and major trauma.7–9
Patients' characteristics at the time when patients first met a criterion were ascertained from chart review. Major diagnostic categories were defined by 2 of the study clinicians (KM, MY) on the basis of clinical data in the chart at the time the first criterion was met. Prehospital functional status was defined by the patient's or family's self-report of the patient's ability to perform activities of daily living using a modified functional independence measure.19 To obtain these reports, we relied on the nursing assessment on hospital admission and the physician history and physical. Patient prehospital functional status was classified as “independent” if patients were independent or mostly independent. Patients were classified as “dependent” if, prior to hospitalization, they were mostly or completely dependent.
We used 2 measures of illness severity at the time of study transfer. We calculated APACHE II scores on the basis of laboratory and physiologic data available at the time the first criterion was met. APACHE II scores are based upon 12 weighted physiologic measures and a chronic health evaluation used to generate an individual patient score between 0 and 71. High scores mean greater severity of illness and increased predicted mortality. For example, an APACHE II score of 25 obtained after ICU admission is associated with an expected in-hospital mortality rate of 50%.20 To calculate the APACHE II score for ward patients prior to transfer to the ICU, we used data available at the time the patient first met a physiologic threshold and from the prior 24 hours. As is standard with APACHE II scoring, we assigned missing data a neutral weight.
As our second measure of illness severity prior to ICU transfer, we recorded the time prior to ICU transfer that a second physiologic threshold criterion was met. We hypothesized that if ward patients were similarly ill at the time they met the first criteria, a similar proportion would meet a second criterion within 2 hours of meeting the first.
We tracked 3 processes-of-care measures after a patient first met physiologic threshold criterion. First, we recorded the time, as documented by the nurse, that the patient's physician was first notified of the patient's condition. Second, we recorded the time of the first documented physician bedside evaluation after a patient first met a physiologic threshold criterion. Third, to compare the amount of physiologic deterioration that occurred on the ward prior to transfer to the ICU, we compared 2 severity-of-illness markers measured first on the ward and then again immediately after transfer to the ICU. We compared the APACHE II score generated at the time a physiologic criteria was first met on the ward with the APACHE II score generated later on transfer to the ICU. We also compared the proportion of patients meeting 2 or more physiologic threshold criteria at those same 2 points in time.
We prospectively ascertained our main outcomes by following the patients from the time of transfer to the ICU until hospital discharge. To measure the degree of deterioration that had occurred on the ward, we compared the illness severity on arrival at the ICU to the illness severity when a patient first met physiologic criteria. To estimate illness severity on arrival at the ICU, we recalculated an APACHE II score using only physiologic data available for the first 24 hours after transfer to the ICU. We used the difference in the 2 APACHE II scores as our measure of deterioration during the delay interval. Final outcomes included in-hospital mortality, functional status on hospital discharge, length of stay in-hospital, and costs. We tracked actual costs rather than charges by employing a costing system that reflected actual resources consumed by the patient during their hospital stay.21 We included only costs incurred after the patient met the first physiologic criterion. We recorded hospital length of stay as the time interval between first meeting a physiologic criteria and discharge from the hospital.
Statistical Analysis
We compared the baseline characteristics of slow-transfer and rapid-transfer patients at the time they first met a physiologic criterion. To calculate P values for categorical variables, we used the χ2 test or Fisher's exact with cell sizes of 5 data points or less. Because of the skewed distribution of costs and hospital length of stay, we tested for significance differences in these variables using the Wilcoxon rank sum.
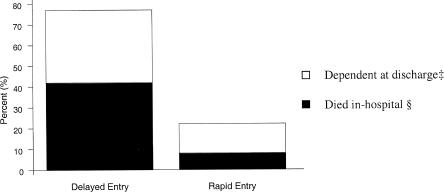
We studied the relationship between slow and rapid transfer and the outcomes of interest in both the crude and adjusted analysis. In our analysis, age and APACHE II score were modeled as both continuous and dichotomous endpoints. Because of the large number of baseline characteristics and the relatively small number of subjects, we built a series of regression models using all statistically significant and clinically important predictor variables in groups of three.22 Because odds ratios may exaggerate the true relative risk, the adjusted odds ratios were transformed into adjusted relative risks.23 The results in Figure 2 are based on a model adjusting for age, pre-ICU APACHE II score and number of days in-hospital pre-ICU transfer. All tests of statistical significance were 2-tailed. Analyses were conducted with STATA Statistics/ Data Analysis (Stata Corp., College Station, Tex).
FIGURE 2.
Adjusted outcomes (adjusted percent dead and adjusted percent dependent calculated from logistic model using means [see reference 22]): in-hospital mortality and percentage of patients dependent at time of discharge from the hospital (adjusted for pre-ICU APACHE II score, age, and number of days in hospital pre-ICU transfer). ‡Relative risk (RR) of functional dependence for delayed entry, 2.9; 95% confidence interval (95% CI), 1.01 to 5.4. §RR for death in-hospital for delayed entry, 4.9; 95% CI, 1.9 to 9.1.
RESULTS
Patient Characteristics
Table 2 summarizes the patient characteristics of slow- and rapid-transfer patients. We found no significant differences between slow- and rapid-transfer patients in terms of age, gender, the proportion functionally dependent prior to hospitalization, or the percentage of patients on the ward >72 hours prior to ICU transfer. At the time slow- and rapid-transfer patients first met a physiologic threshold criterion, both groups had similar pre-ICU APACHE II scores. The proportion of patients meeting a second physiologic threshold criterion less than 2 hours after meeting the first criterion was also similar in both groups.
Table 2.
Patient Characteristics on the Medical-Surgical Wards When a Physiologic Threshold Criterion Was First Met
| Slow Transfer (N = 56) | Rapid Transfer (N = 35) | P Value | |
|---|---|---|---|
| Demographics | |||
| Mean age, ±SE | 63 ± 4 | 61 ± 6 | .41 |
| Age >65 y, % | 54 | 49 | .65 |
| Female, % | 56 | 60 | .66 |
| >72 H in hospital pre-ICU transfer, % | 30 | 17 | .21 |
| Function and disease class | |||
| Functionally dependent prehospital, % | 30 | 23 | .44 |
| Pre-ICU diagnosis, % | .64 | ||
| Respiratory failure | 48 | 53 | |
| Sepsis | 36 | 26 | |
| Gastrointestinal bleeding | 16 | 21 | |
| Severity of illness on ward | |||
| Mean pre-ICU APACHE II score ±SE* | 16 ± 2 | 19 ± 2 | .09 |
| Pre-ICU APACHE II ≥20, %* | 37 | 32 | .8 |
| Second physiologic criterion met ≤2 h after first criterion, % | 21 | 29 | .5 |
Obtained from data at time physiologic criteria first met on the ward.
ICU, intensive care unit.
Process Markers
A physician was notified in less than 2 hours after the patient first met a physiologic criteria in 59% of slow-transfer patients compared with 91% of rapid-transfer patients (relative risk [RR], 0.6; 95% confidence interval [95% CI], 0.4 to 0.8). Only 23% of slow-transfer patients, compared with 86% of rapid-transfer patients, received a physician bedside evaluation less than 3 hours after meeting the first physiologic criterion (RR, 0.3; 95% CI, 0.2 to 0.5). As noted earlier, at the time slow- and rapid-transfer patients first met physiologic threshold criteria on the ward, they were equally ill by our 2 severity-of-illness markers, the APACHE II score and the proportion of patients meeting 2 or more physiologic threshold criteria. However, by the time slow-transfer patients were transferred to the ICU, they were far sicker than were rapid-transfer patients, as indicated by the same 2 severity-of-illness markers re-calculated immediately after transfer to the ICU. Fifty-nine percent of slow-transfer patients had APACHE II scores ≥20 on admission to the ICU compared to only 24% of rapid-transfer patients (RR, 1.5; 95% CI, 1.1 to 2.2). Slow-transfer patients were also more likely to meet a second physiologic criterion by the time they were transferred from the ward to the ICU (RR, 1.5; 95% CI, 1.04 to 2.1).
Mortality, Morbidity, and Costs
Table 3 compares mortality, morbidity, and costs for the 2 groups. Crude in-hospital mortality was 41% for slow-transfer patients compared to 11% for rapid-transfer patients (RR, 3.5; 95% CI, 1.4 to 9.5). Slow-transfer patients were also more likely to be dependent at the time of discharge, although this difference was not statistically significant (RR, 1.5; 95% CI, 0.96 to 2.4). Median hospital length of stay after ICU transfer was 14 days for slow-transfer patients and 9 days for rapid-transfer patients (P = .03). After study entry, median total hospital costs were $34,000 for slow-transfer patients and $21,000 for rapid-transfer patients (P = .01). As shown in Figure 2, the findings were not influenced by adjustment for potential confounders. In the multivariate analysis, slow transfer to the ICU was a significant predictor of death, discharge in a functionally dependent state, and higher costs. We repeated these multivariate analyses with different potential predictors included in the models in groups of 3. All subanalyses gave similar results.
Table 3.
Health Outcomes and Resources Consumed: Slow- Versus Rapid-ICU Transfer (Crude)
| Slow Transfer (N = 56) | Rapid Transfer (N = 35) | RR (95% CI) | P Value | |
|---|---|---|---|---|
| Health outcomes | ||||
| Death in-hospital | 41% | 11% | 3.5 (1.4 to 9.5) | .004 |
| Survivors functionally dependent at hospital discharge* | 11(33%) | 5(16%) | 1.5 (0.96 to 2.4) | .15 |
| Hospital Resources† | ||||
| Hospital length of stay–median (interquartile range) | 14 d (7 to 20) | 9 d (5 to 15) | .03 | |
| Median/hospital costs‡ (interquartile range) | 34 (18 to 58) | 21 (11 to 34) | .01 |
Mostly or totally dependent at time of hospital discharge (by modified functional independence measure score).
Hospital costs and hospital length of stay recorded from time each patient first met physiologic criteria until time of hospital discharge.
Costs in thousands of 1994 dollars.
RR, relative risk; CI, confidence interval.
DISCUSSION
Slow transfer to the ICU was strongly associated with increased mortality and costs. At the time patients first met a physiologic criterion, slow- and rapid-transfer groups were similar in terms of demographics, prior length of stay, illness class, and severity of illness. However, by the time they arrived in the ICU, slow-transfer patients were far sicker than were the rapid-transfer patients. Slow-transfer patients had much higher mortality and consumed significantly more hospital resources. Finally, the process of care appeared to differ for these 2 groups of patients. Slow-transfer patients were less likely to have a nurse notify their physician of their deteriorating condition and were less likely to experience an early bedside physician evaluation.
Two explanations for our findings deserve consideration. First, it is possible that slow-transfer patients differed in some way from rapid-transfer patients at the time they first met the physiologic criterion and that this difference, rather than the delay itself, led to their higher mortality. Because our effect size was large and we found no evidence of confounding by severity of illness or patient demographics, such an unmeasured risk factor would have to be both highly predictive of mortality and not correlated with the factors we were able to measure. This seems unlikely.
A more likely explanation is that the delay in responding to physiologic deterioration was itself responsible for the increased mortality. Our analysis of process-of-care markers indicates that physicians of slow-transfer patients were less likely to receive prompt notification of the patient's deterioration. Even if prompt physician notification occurred, slow-transfer patients were 61% less likely to experience a physician bedside evaluation within 3 hours of first meeting a physiologic criterion. We did not examine other potential causes of slow transfer such as ICU bed availability or physician's perception of prognosis. However, the differences in notification and examination were substantial and consistent with the theory that the delays were causal.
Our data do not allow us to specify the type of medical interventions that may account for the substantial differences in outcomes between slow- and rapid-transfer patients. One possibility is that slow access to ICU technology, such as pulmonary artery catheters, intra-aortic balloon pumps, and vasopressor agents may strongly influence outcomes.4 However, the benefit of some technologies routinely used in the ICU has been recently questioned.24,25 In our study, the delays to physician notification and delays to physician bedside evaluation suggest that, during the first several hours after meeting a physiologic criterion, slow-transfer patients received medical treatment different from that received by the rapid-transfer group. Slow transfer from the ward to the ICU may be an indicator of inadequate basic care, such as timely physician evaluation, receipt of sufficient intravenous fluids, prompt diagnostic testing, antibiotics, and respiratory support. Our analysis suggests that delays in transfer to the ICU may be a surrogate marker for the failure to undertake essential changes in therapy stimulated by nurse-to-physician communication and physician bedside evaluation. Other studies also indicate that clinical response to severe physiologic alterations is variable and too often inadequate.12–14,26 These studies found that 66% to 84% of patients who suffered a cardiopulmonary arrest on a medical or surgical ward had documented evidence of significant clinical deterioration for at least 6 to 24 hours prior to the arrest. Survival rate for this group of patients was under 10%. Rivers et al. determined that the outcome of patients with severe sepsis and septic shock was largely determined by therapies given during the first 6 hours after presentation.27 Our study and the investigations cited above suggest that more attention must be given to the evaluation and management of patients on the ward who experience physiologic deterioration. This has implications for the nurse-to-patient ratio used in the care of ward patients, physician availability, and systems of communication between physicians and nurses.
Three additional limitations of our study must be acknowledged. First, our small sample size constrained our ability to simultaneously adjust for multiple variables. Our basic model adjusted only for age, severity of illness, and number of days in-hospital prior to study entry. To address this concern, we repeated the analyses with all other potential predictor variables introduced in blocks of 3. Our results were unchanged.
Second, our adjustment for severity of illness of patients on the ward may not reflect true differences between slow and rapid transfers. We used the APACHE II system to measure illness severity pre-ICU and again after admission to the ICU. The APACHE II and III are well validated for use among patients admitted to the ICU. Less is known about the use of the APACHE II and III in the pre-ICU setting. However, the APACHE II and III systems have been used to adjust for severity of illness in the pre-ICU setting in a number of well-known clinical investigations.26,27,28 We chose APACHE II because of its relative simplicity.
Finally, the predictive performance of the physiologic criteria must be carefully considered: how well do our physiologic threshold criteria predict the need for eventual transfer to the ICU? Eighty-five percent of patients (91 of 107 subjects) who were urgently transferred to the ICU from the surgical or medical wards during the study period met a physiologic criterion prior to transfer. To further evaluate the measure's performance, we took advantage of the subsequent implementation of computerized nurse charting at the study hospital to carry out a 2-month prospective analysis of all noncardiac patients admitted to the surgical or medical wards. During this period, our physiologic threshold criteria had a sensitivity of 88% and specificity of 13% for predicting ICU transfer. The positive predictive value of our physiologic threshold criteria was 7.5%. Thus, at the study institution, our threshold criteria were highly sensitive but only modestly predictive of transfer from the ward to the ICU. Whether the measures will perform similarly at other institutions is uncertain.
CONCLUSIONS
Slow transfer of ward patients to the ICU after patients met explicit physiologic threshold criteria was associated with increased mortality, morbidity, and costs. Lack of physician notification and delays in physician bedside evaluation appear to have contributed to the delays in transfer. Our findings should be generalized with caution. However, this investigation suggests that timely evaluation and treatment of hospitalized patients showing evidence of physiologic instability may reduce the high mortality rate currently seen in hospitalized patients transferred to the ICU.
Acknowledgments
We especially thank Harold Sox, MD and H. Gilbert Welch, MD, MPH for their helpful insights in reviewing multiple drafts of the manuscript. We also thank Lisa M. Schwartz, MD and Steven Woloshin, MD for their thoughtful critique of our manuscript.
Funding for this project was provided in part by the McKay-Dee Foundation of Ogden, Utah
REFERENCES
- 1.Zimmerman JE, Wagner DP, Draper EA, Wright L, Alzola C, Knaus WA. Evaluation of acute physiology and chronic health evaluation III predictions of hospital mortality in an independent database. Crit Care Med. 1998;26:1317–26. doi: 10.1097/00003246-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg JS, Perl TM, Wiblin T, Costigan MD, Dawson J, Nettelman MD, Wenzel RP. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1988;26:1020–4. doi: 10.1097/00003246-199806000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport J, Teres D, Lemeshow S, Harris D. Timing of intensive care unit admission in relation to ICU outcome. Crit Care Med. 1990;18:1231–6. doi: 10.1097/00003246-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Berensen RA. Office of Technology Assessment Case Study 28. Washington DC: U.S. Government Printing Office; 1984. Intensive Care Units (ICU's) Clinical Outcomes, Costs and Decision Making. [Google Scholar]
- 5.Oye RK, Bellamy PF. Patterns of resource consumption in medical intensive care. Chest. 1991;99:685–8. doi: 10.1378/chest.99.3.685. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder SA, Showstack JA, Schwartz J. Survival of adult high-cost patients. Report of a follow-up study from nine acute-care hospitals. JAMA. 1981;245:1446–9. [PubMed] [Google Scholar]
- 7.Simmons J, Willens HJ, Kessler KM. Acute myocardial infarction. Chest. 1996;107:1732–43. doi: 10.1378/chest.107.6.1732. [DOI] [PubMed] [Google Scholar]
- 8.Cales RH, Trunkey DD. Preventable trauma deaths: a review of trauma care systems development. JAMA. 1995;254:1059–63. doi: 10.1001/jama.254.8.1059. [DOI] [PubMed] [Google Scholar]
- 9.Phillips P. New pharmaceutical approaches to stroke prevention, treatment. JAMA. 1999;281:2075–6. [PubMed] [Google Scholar]
- 10.Hersey CH, Fisher L. Why outcome of cardiopulmonary resuscitation in general medical wards is poor. Lancet. 1982;1:32–4. doi: 10.1016/s0140-6736(82)92567-3. [DOI] [PubMed] [Google Scholar]
- 11.Franklin C, Mathew J. Developing strategies to prevent in-hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event. Crit Care Med. 1987;22:244–47. [PubMed] [Google Scholar]
- 12.Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98:1388–92. doi: 10.1378/chest.98.6.1388. [DOI] [PubMed] [Google Scholar]
- 13.Sax L, Charlson M. Medical patients at high risk for catastrophic deterioration. Crit Care Med. 1987;15:510–5. doi: 10.1097/00003246-198705000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Dubois RW, Brook RH. Preventable deaths: who, how often, and why? Ann Intern Med. 1988;109:582–9. doi: 10.7326/0003-4819-109-7-582. [DOI] [PubMed] [Google Scholar]
- 15.Buist MD, Jarmolowski E, Burton PR, Bernartd SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999;171:22–5. doi: 10.5694/j.1326-5377.1999.tb123492.x. [DOI] [PubMed] [Google Scholar]
- 16.Bone RC, McElwee NE, Eubanks DH, Gluck EH. Analysis of indications for intensive care unit admission. Chest. 1993;104:1806–11. doi: 10.1378/chest.104.6.1806. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;10:818–29. [PubMed] [Google Scholar]
- 18.LeGall JR, Loirat P, Alperovitch A. Simplified acute physiology score for intensive care patients. Lancet. 1983;2:741–6. doi: 10.1016/s0140-6736(83)92278-x. [DOI] [PubMed] [Google Scholar]
- 19.Nyein K, Mc Michael L, Turner-Stokes L. Can a Barthel score be derived from the FIM? Clin Rehabil. 1999;13:56–63. doi: 10.1191/026921599701532135. [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. An evaluation of outcomes from intensive care in major medical centers. Ann Intern Med. 1986;104:410–8. doi: 10.7326/0003-4819-104-3-410. [DOI] [PubMed] [Google Scholar]
- 21.Talbot B. Intermountain Health Care Procedural Costing Summary. Salt Lake City, Utah: Intermountain Health Care; 1995. [Google Scholar]
- 22.Concato J, Feinstein AR, Holford T. The risk of determining risk with multivariate models. Ann Intern Med. 1993;118:201–10. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Yu KF. What is the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 24.Connors AF, Speroff T, Dawson N, et al. The effectiveness of right heart catherization in the initial care of critically ill patients. JAMA. 1996;276:889–97. doi: 10.1001/jama.276.11.889. [DOI] [PubMed] [Google Scholar]
- 25.Argenziano M, Oz MC, Rose EA. The continuing evolution of mechanical ventricular assistance. Curr Probl Surg. 1997;34:317–86. doi: 10.1016/s0011-3840(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 26.Shoemaker WC, Wo CC. Circulatory effects of whole blood, packed red cells, albumin, starch and crystalloids in resuscitation of shock and acute critical illness. Crit Care Med. 1997;74(suppl 2):69–74. doi: 10.1111/j.1423-0410.1998.tb05399.x. [DOI] [PubMed] [Google Scholar]
- 27.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 28.Knaus WA, Harrell FE, Lynn J, et al. The SUPPORT prognostic model. Objective estimates of survival for seriously ill hospitalized adults. Ann Intern Med. 1995;122:191–203. doi: 10.7326/0003-4819-122-3-199502010-00007. [DOI] [PubMed] [Google Scholar]