Abstract
OBJECTIVE
In primary prevention of atherosclerotic disease, it is difficult to decide when medical treatment should be initiated. The main goal of the study was to compare different guidelines for coronary heart disease (CHD) risk assessment and initiation of lipid-lowering therapy.
DESIGN
Cross-sectional evaluation.
SETTING
An outpatient lipid and diabetes clinic in a university hospital.
PARTICIPANTS/METHODS
Risk factor data obtained on 100 consecutive patients (58 men and 42 women) without clinical evidence of cardiovascular disease were used to compare the Framingham risk equation, the U.S. National Cholesterol Education Program (Adult Treatment Panel III) (NCEP ATP III) guidelines, the joint European Societies guidelines, the joint British guidelines, the revised Sheffield table, and the Munster Heart Study calculator (PROCAM) CHD risk assessment and lipid-lowering therapy.
RESULTS
Guidelines could be applied to different subsets of the cohort, ranging from 22% (PROCAM) to 95% of the cohort (revised Sheffield table). All guidelines (except PROCAM) could be applied to a total of 62 patients. Guidelines predicted ≥20% risk for developing CHD over 10 years in 53% (NCEP ATP III), 26% (European) and 32% (British), while Framingham predicted this risk level in 34%. CHD risk was estimated to be ≥3%/year in 5% according to Sheffield, while Framingham predicted this risk in 13%. Lipid-lowering drug therapy is recommended in 52% by NCEP ATP III, while European, British, and Sheffield guidelines recommend this in 26%, 35%, and 5%, respectively.
CONCLUSIONS
Guidelines for assessing CHD risk and lipid-lowering therapy differ greatly. Therefore, these algorithms must be used with caution.
Keywords: atherosclerosis, guideline, coronary heart disease, risk assessment, primary prevention, lipid-lowering therapy
There is no doubt that decreasing blood pressure and/or serum cholesterol is effective in the primary prevention of coronary heart disease (CHD). The most reassuring findings about the benefits and safety of lipid-lowering medical treatment in primary prevention came from the West of Scotland Coronary Prevention Study (WOSCOPS)1 and the Air Force/Texas coronary atherosclerosis prevention study (AFCAPS/TexCAPs).2 If, however, the absolute risk for atherosclerosis is low, primary prevention strategies using drug therapy must be questioned, because they may impose an unjustified financial burden, and side effects may balance benefits in the long run. It is therefore of utmost importance to identify subjects who will benefit most from primary prevention. A number of guidelines have therefore been developed to identify such subjects.
The Framingham Heart Study identified several independent risk factors for CHD, including age, gender, blood pressure, total cholesterol, high-density lipoprotein cholesterol, smoking, diabetes and electrocardiogram (ECG)-left ventricular hypertrophy.3 The U.S. National Cholesterol Education Program (Adult Treatment Panel III) (NCEP ATP III) guidelines,4 the joint European Societies guidelines,5,6 the joint British guidelines,7 and the revised Sheffield table8 use Framingham data to predict CHD risk and/or to recommend lipid-lowering therapy. The Munster Heart Study calculator, based on different epidemiological data, includes triglyceride concentration and family history, but can only be applied to men.9 All of these guidelines agree that risk factors are multiplicative, that risk is highest in subjects with several risk factors, and that specific drug therapy should only be initiated if absolute risk exceeds a certain threshold. However, these guidelines disagree on the selection and weighting of risk factors and on the risk level above which drug therapy is recommended (Appendix A). The threshold for such therapy is 20% over 10 years according to the joint European Societies guidelines and 15% over 10 years according to the joint British guidelines. The revised Sheffield table recommends lipid-lowering medical treatment if risk exceeds 3% per year. The U.S. NCEP ATP III, updated in May 2001,4 provides risk scores based on the Framingham Heart Study to estimate 10-year CHD risk as a new feature, but does not state a threshold of CHD risk above which lipid-lowering therapy should be initiated.
Recent studies have shown that these guidelines do not necessarily provide the same recommendations for the same patient. These studies were performed in the United Kingdom and only included some guidelines.10,11 We now assess the comparability of 6 different guidelines and algorithms (Framingham risk equation, U.S. NCEP ATP III guidelines, joint European Societies guidelines, joint British guidelines, revised Sheffield table, and Munster Heart Study calculator [PROCAM]) in a group of patients who presented to an outpatient lipid clinic in Germany but did not have preexisting evidence of atherosclerosis. Comparability was evaluated (a) regarding risk assessment (using the Framingham risk equation as the gold standard) and (b) regarding treatment recommendations.
METHODS
One hundred consecutive patients without any clinical evidence of cardiovascular disease and without any lipid-lowering medication referred to our outpatient lipid and diabetes clinic were included in this study. Lack of hyperlipidemia was not a reason for exclusion. Each patient was interviewed using a standardized questionnaire concerning hypertension, antihypertensive treatment, diabetes mellitus, smoking, and family history of coronary heart disease, and was examined by a physician.
Blood pressure was measured after a 5-minute rest to the nearest 5 mmHg by mercury sphygmomanometry. Diabetes mellitus was defined according to World Health Organization criteria.12 A resting ECG was obtained from each patient assessing left ventricular hypertrophy by use of the Sokolow-Lyon voltage criteria.13 Total plasma cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured by enzymatic methods after a 12-hour fast. Low-density lipoprotein cholesterol was calculated using the Friedewald formula14 if plasma triglycerides were <400 mg/dL, or were determined by preparative ultracentrifugation if triglyceride concentration exceeded 400 mg/dL.
Published versions of different guidelines were applied to this set of data to evaluate whether CHD risk estimates agree or differ between various guidelines and to estimate whether guidelines differ in their recommendation concerning lipid-lowering therapy. The U.S. NCEP ATP III guidelines,4 the joint European Societies guidelines,5,6 the joint British guidelines7 and the Munster Heart Study calculator (PROCAM) (http://www.chd-taskforce.com) were used to estimate a coronary heart disease risk of ≥20% over 10 years. The revised Sheffield table8 was used to predict risk of ≥3% over the next year. The results were compared to the risk calculated with the Framingham equation,3 which was used as a gold standard, because the Framingham Heart Study generated the original data upon which many of the other guidelines are based.
Furthermore, the U.S. NCEP algorithm ATP III, the joint European Societies guidelines, the joint British guidelines, and the revised Sheffield table were compared regarding the recommendation for lipid-lowering therapy (the Framingham risk equation and the Munster Heart Study calculator [PROCAM] do not provide recommendations regarding lipid-lowering therapy).
Most algorithms only allow the evaluation of patients with specific characteristics (for example: in PROCAM only men aged 40 to 65 years can be evaluated, and in the Joint European guidelines, total cholesterol must be between 125 and 325 mg/dL). If patients have parameters outside the specific range of the individual algorithms, the algorithm cannot be applied. Patients in whom any algorithm could not be applied (except PROCAM) were excluded.
Agreement between different guidelines was evaluated using calculation of kappa (κ). Strength of agreement was considered poor for κ < 0.20, fair for κ = 0.21 to 0.40, moderate for κ = 0.41 to 0.60, good for κ = 0.61 to 0.80, and very good for κ = 0.81 to 1.00.15
Patients gave informed consent. A potential conflict of interest does not exist.
RESULTS
The characteristics of the patients enrolled in this study are shown in Table 1. There was a high proportion of patients with diabetes (35%; type 1, N = 7; type 2, N = 28) and with a family history of premature CHD (45%).
Table 1.
Characteristics of Subjects
| Patients (N = 100) | Men (N = 58) | Women (N = 42) | |
|---|---|---|---|
| Mean age, y ± SD | 47.4 ± 14.4 | 45.2 ± 15.0 | 50.4 ± 13.0 |
| Systolic blood pressure ≥140 mmHg, % | 43 | 40 | 49 |
| Diastolic blood pressure ≥90 mmHg, % | 35 | 38 | 32 |
| Smoking, % | 24 | 29 | 17 |
| Diabetes, % | 35 | 33 | 39 |
| Family history of premature CHD, % | 45 | 41 | 51 |
| Left ventricular hypertrophy on ECG, % | 7 | 9 | 5 |
| Mean total cholesterol, mg/dL ± SD | 244.2 ± 105.6 | 245.0 ± 130.3 | 243.1 ± 55.5 |
| Mean triglyceride, mg/dL ± SD | 389.7 ± 883.5 | 557.6 ± 1127.3 | 157.8 ± 104.3 |
| Mean HDL cholesterol, mg/dL ± SD | 45.8 ± 18.8 | 37.0 ± 13.1 | 58.0 ± 18.8 |
| Mean LDL cholesterol, mg/dL ± SD | 133.4 ± 53.1 | 116.6 ± 50.6 | 156.5 ± 47.5 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The Framingham risk equation was applicable to 87 patients, the U.S. NCEP ATP III algorithm to 93 patients, the joint European Societies guidelines to 81, the joint British guidelines to 72 patients, and the revised Sheffield table to 95 patients. The Munster Heart Study calculator (PROCAM) was applicable to only 22 patients, because the PROCAM study applies only to men aged 40 to 65 years.
All guidelines (except PROCAM) could be applied to a total of 62 persons. Of these 62 patients, 21 (34%) showed a CHD risk ≥20% over the next 10 years according to the Framingham equation. The NCEP ATP III risk scores do not include diabetes mellitus as a risk factor but consider diabetes mellitus as a CHD risk equivalent corresponding to a CHD risk >20% over 10 years. Therefore, 33 patients (53%) showed a CHD risk ≥20% over 10 years according to the U.S. NCEP ATP III guidelines. Only 19 of 21 patients identified by the Framingham risk equation are included in these 33 patients. Sixteen patients (26%) showed a risk ≥20% over 10 years according to the joint European Societies guidelines and 20 (32%) according to the joint British guidelines. The 21 patients identified by the Framingham equation include all patients identified by the joint European Societies guidelines and the joint British guidelines. The revised Sheffield table estimated a coronary heart disease risk exceeding 15% over the next 5 years in 3 persons (5%), while Framingham predicted this risk in 8 patients (13%). The PROCAM study shows a coronary heart disease risk of more than 20% over 10 years in 3 of 22 men (Framingham equation ≥20%: 10/22).
Comparison of the different guidelines to the gold standard (Framingham risk equation) regarding risk prediction revealed fair agreement between NCEP ATP III guidelines and the Framingham risk equation (κ = 0.22), good agreement between the European Societies guidelines and the Framingham risk equation (κ = 0.64), very good agreement between the joint British guidelines and the Framingham equation (κ = 0.81), and poor agreement between the revised Sheffield table and the Framingham equation (κ < 0.20).
NCEP ATP III recommended lipid-lowering drug treatment in 32 patients (52%). Medical treatment was recommended by the joint European Societies guidelines in 16 (26%) and by the joint British guidelines in 22 patients (35%), while only 3 patients (5%) met the revised Sheffield table criteria for lipid-lowering medication (Fig. 1). Only 11 and 14 patients requiring drug therapy according to the joint European Societies guidelines and the joint British guidelines, respectively, would receive lipid-lowering medication according to NCEP ATP III.
FIGURE 1.
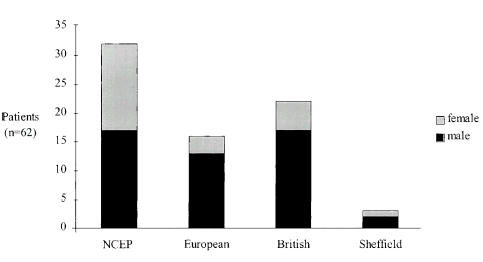
Number of patients in which lipid-lowering drug therapy is recommended (according to different guidelines).
Stratification by gender did not reveal significant differences between males and females concerning risk estimates. However, lipid-lowering drug treatment was recommended by NCEP ATP III in 15 of 25 females (60%) but in only 17 of 37 males (46%), while the other guidelines recommended lipid-lowering therapy more often in men (European 35%, British 46%, Sheffield 5%) than in women (European 12%, British 20%, Sheffield 4%).
We also evaluated the comparability of guidelines in the subgroup of patients without diabetes, which revealed similar results, although agreement in risk estimate between NCEP ATP III and Framingham was somewhat improved (κ = 0.33). It remained good between the European guidelines and Framingham (κ = 0.63), very good between the British guidelines and Framingham (κ = 0.90), and poor between the Sheffield table and Framingham (κ < 0.20).
DISCUSSION
This study shows that algorithms for CHD risk assessment and guidelines for lipid-lowering drug therapy vary widely. Patients who are estimated to be at considerable risk for developing CHD according to one guideline are considered low-risk according to other guidelines. Compared to the original Framingham risk equation, which was used a gold standard, the joint British guidelines perform most closely. Guidelines also differ in their recommendation concerning lipid-lowering drug therapy. Finally, there are a number of patients for whom current guidelines cannot be applied, because guidelines can only be used if raw data are within prespecified ranges (Appendix A).
Our results, obtained in a specialized segment of the German population, confirm previous studies performed in the U.K.10,11 and extend the evidence to high-risk patients in Germany. In these previous studies, guidelines were also not applicable to all subjects and resulted in different risk estimates and different recommendations concerning lipid-lowering drug therapy.
If algorithms are to be useful, they should be widely applicable. In contrast to our study and the study by Durrington et al.,10 patients with parameters outside the range of validity were placed in the nearest applicable section of the algorithms in the study by Wierzbicki et al.11 Therefore, applicability of algorithms in our study was less than that described by Wierzbicki et al.11 However, the Framingham risk equation, the U.S. NCEP ATP III algorithm, and the revised Sheffield table were still widely applicable (>87%). The Munster Heart Study Calculator performs worst in this respect, because it could be applied only to 22% of the subjects (middle-aged men). This, however, is related to our study population. If PROCAM is applied to other relevant segments of the population, applicability will increase.
Guidelines are based on epidemiological studies. There are only a very limited number of epidemiological studies available that are large and long enough to allow CHD risk to be estimated. The U.S. NCEP ATP III algorithm, the joint European Societies guidelines, the joint British guidelines, and the revised Sheffield table are all based on data from the Framingham Heart Study. The Munster Heart Study (PROCAM) Calculator in contrast is based on a separate set of epidemiological data. Thus, although most guidelines are based on the same epidemiological data, risk estimates differ. If the original Framingham risk equation is used as a gold standard for calculating coronary heart disease risk, the joint British guidelines reflect risk assessment best (κ = 0.81). The revised Sheffield table, although it is also based on the Framingham data, compared poorly with the Framingham equation (κ < 0.20). This is not related to the fact that this guideline was applied to a German cohort, because a very similar finding was described by Durrington et al.10 and Wierzbicki et al.11 in U.K. subjects.
The PROCAM calculator is based on a separate set of epidemiological data, and risk estimates differed considerably from the results of the Framingham risk equation. Possible explanations for the observed differences relate to the fact that the epidemiological data used to develop the algorithm were collected in different societies and at different times. The Munster Heart Study was initiated in 1979, recruiting subjects from Munster and the Northern Ruhr Area (Germany), whereas the Framingham Heart Study was started about 30 years earlier.
Furthermore, even if the same epidemiological data are used, the guidelines depend very much on the model used to analyze the raw data. The model will, to a certain point, determine which parameters remain in the analysis and which will be excluded. In the end, all guidelines for assessing CHD risk represent simplifications with a certain selection and weighting of risk factors. Appendix A, comparing the different guidelines, for example, shows that diabetic patients are handled quite differently in different guidelines.
The threshold above which lipid-lowering medical therapy is recommended varies between ≥15% over 10 years to ≥3% per year. The U.S. NCEP guidelines do not state a threshold of CHD risk. Clinical assessment of previous NCEP guidelines demonstrated that people at substantially lower risk thresholds would be treated compared to the European or British recommendations.16–18 This is confirmed in our study and extended to the NCEP ATP III guidelines. Depending on the guideline, between 52% (U.S. NCEP ATP III) and 5% (revised Sheffield table) of the patients should be treated with lipid-lowering drugs. While the European guidelines recommend lipid-lowering drug therapy more often in men, NCEP ATP III recommends such therapy more often in women. The discrepancy between the U.S. NCEP ATP III guidelines on the one hand and the European guidelines on the other hand is related to several facts. Most importantly, ATP III defines diabetes as a CHD risk equivalent (i.e., diabetic patients carry a risk for major coronary events >20% per 10 years). Consensus recommendations such as the U.S. NCEP ATP III guidelines are not based only on one set of data (such as Framingham), but incorporate results from other studies that emphasize, for example, the CHD risk of diabetic patients and clearly demonstrate how this risk can be reduced by lipid-lowering therapy.19,20 Therefore the presence/absence of diabetes has a strong impact on risk estimation by NCEP AT III. However, even when the analysis is restricted to nondiabetic persons, agreement between NCEP ATP III and the Framingham risk equation remains only fair.
Because of the obvious discrepancy between guidelines, it is difficult to recommend which guideline to follow. Compared to the original Framingham risk equation, the Joint British guidelines perform best in this cohort. However, the epidemiological basis may have changed. NCEP ATP III incorporates new evidence best and has the most aggressive approach, but is not in very good agreement with the Framingham data. In individual patients, when in doubt about the risk and particularly about initiating lipid-lowering drug therapy, other tests, such as the flow-mediated dilation of the brachial artery and the carotid intima-media thickness, which have been shown to predict CHD, may provide additional information.21–26 These tests indicate whether early forms of atherosclerosis are present. However, the usefulness of these parameters is still under debate, and it is unclear in which patients these exams should be performed and how results should be incorporated into existing guidelines. To optimize guidelines in the long run, new epidemiological information from studies such as the MONICA Augsburg cohort study27 or the Women's Health Initiative28 must be incorporated. Although these newer studies generally confirm the risk factors established in the Framingham study, the importance of individual risk factors is different (for example, the risk associated with diabetes is higher in MONICA than in Framingham). There is, however, neither an established method for incorporating such new information into existing guidelines nor an easy way to validate the modified guidelines.
The limitations of this study should be noted. First, the sample size is small, which reduces the power of the study. Any results relating to subgroups (gender, diabetic/nondiabetic patients) must be interpreted with caution. In addition, the single-site study design makes the study sensitive to local differences in the study population. Furthermore, our results may be limited by a selection and referral bias. Since the study was performed in an outpatient lipid and diabetes clinic of a university hospital, we had a high proportion of diabetic patients and patients with a positive family history for premature CHD. Thus, our findings are restricted to this segment of the population. It may well be that guidelines agree to a much higher extent and are more widely applicable in other segments of the population.
This study confirms that guidelines for assessing CHD risk and lipid-lowering drug therapy differ significantly, despite the fact that most guidelines are based on the same epidemiological data. However, despite these differences the guidelines agree that risk factors are multiplicative and that risk is highest in subjects with several risk factors. The observed differences also provide the impetus for developing new consensus recommendations that take into account the wide range of opinions that exist in the community.
APPENDIX A
Characteristics of Guidelines
| Guideline | Framingham | U.S. NCEP ATP III | Joint European | Joint British | Revised Sheffield | PROCAM |
|---|---|---|---|---|---|---|
| Database | Framingham Heart Study | Framingham Heart Study | Framingham Heart Study | Framingham Heart Study | Framingham Heart Study | Munster Heart Study |
| Parameters considered for risk estimation | Sex | Sex | Sex | Sex | Sex | Age |
| Age | Age | Age | Age | Age | SBP | |
| SBP | SBP | SBP | SBP | Hypertension | Smoking | |
| Smoking | Smoking | Smoking | Smoking | Smoking | Diabetes mellitus | |
| Diabetes mellitus | Diabetes mellitus | Diabetes mellitus | Diabetes mellitus | Diabetes mellitus | LDL cholesterol | |
| Total cholesterol | Total cholesterol | Total cholesterol | Ratio of total to HDL cholesterol | Total cholesterol | HDL cholesterol | |
| HDL cholesterol | HDL cholesterol | HDL cholesterol | ECG-LVH | Trigycerides | ||
| ECG-LVH | Family history of heart attack | |||||
| Applicability of guidelines | Age: 30–74 y | Age: 20–79 y | Age: 30–70 y | Age: 35–74 y | Age: ≤70 y | Sex: men |
| SBP: 110–190 mg/dL | SBP: 110–190 mmHg | Age: 40–65 y | ||||
| TC: 125–325 mg/dL | TC:HDL ratio: 3:11 | SBP: 100–225 mmHG | ||||
| LDL-C: 75–250 mg/dL | ||||||
| HDL-C: 25–75 mg/dL | ||||||
| TG: 50–400 mg/dL | ||||||
| Handling of diabetic patients | No separate chart for CHD risk estimation | Considered as high-risk patients (CHD risk >20%/10 y) | Separate chart for CHD risk estimation | Separate chart for CHD risk estimation | No separate chart for CHD risk estimation | No separate chart for CHD risk estimation |
| Recommendations concerning lipid-lowering therapy | No | Yes (depending on LDL-C, CHD risk and number of risk factors) | Yes (CHD risk ≥20% over 10 y and TC ≥190 mg/dL and/or LDL-C ≥115 mg/dL) | Yes (CHD risk ≥15% over 10 y and TC ≥5 mmol/L) | Yes (CHD risk ≥3%/y) | No |
SBP, systolic blood pressure; ECG-LVH, electrocardiogram-center ventricular hypertrophy; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
REFERENCES
- 1.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastation in men with hypercholesterolaemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 2.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of the AFCAPS/TexCAPS. Air Force/Texas coronary atherosclerosis prevention study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KM, Odell PM, Wilson PWF, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1990;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Pyrola K, de Backer G, Poole-Wilson P, Wood D on Behalf of the Task Force. Prevention of coronary heart disease in clinical practice: recommendations of the task force of the European Society of Cardiology, European Atherosclerosis Society, and European Society of Hypertension. Eur Heart J. 1994;15:1300–31. doi: 10.1093/oxfordjournals.eurheartj.a060388. [DOI] [PubMed] [Google Scholar]
- 6.Pyrola K, Wood D. Prevention of coronary heart disease in clinical practice. European recommendations revised and reinforced. Eur Heart J. 1998;19:1413–5. doi: 10.1053/euhj.1998.1244. [DOI] [PubMed] [Google Scholar]
- 7.Wood D, Durrington P, Poulter N, G McInnes, Rees A, Wray R on Behalf of the Societies. Joint British recommendations on prevention of coronary heart disease in clinical practise. Heart. 1998;80(suppl 2):1–29. [Google Scholar]
- 8.Ramsay LE, Haq IU, Jackson PR, Yeo WW, Pickin DM, Payne JN. Targeting lipid-lowering drug therapy for primary prevention of coronary disease: an updated Sheffield table. Lancet. 1996;348:387–8. doi: 10.1016/s0140-6736(96)05516-x. [DOI] [PubMed] [Google Scholar]
- 9.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the Prospective Cardiovascular Munster (PROCAM) Study. Circulation. 2002;105:310–5. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 10.Durrington PN, Prais H, Bhatnagar D, et al. Indications for cholesterol-lowering medication: comparison of risk-assessment methods. Lancet. 1999;353:278–81. doi: 10.1016/s0140-6736(98)04027-6. [DOI] [PubMed] [Google Scholar]
- 11.Wierzbicki AS, Reynolds TM, Gill K, Alg S, Crook MA. A comparison of algorithms for initiation of lipid-lowering therapy in primary prevention of coronary heart disease. J Cardiovasc Risk. 2000;7:63–71. doi: 10.1177/204748730000700111. [DOI] [PubMed] [Google Scholar]
- 12.WHO Expert Committee on Diabetes Mellitus: second report. World Health Organ Tech Rep Ser. 1980;646:1–80. [PubMed] [Google Scholar]
- 13.Jern S. Assessment of left ventricular hypertrophy in patients with essential hypertension. Blood Press Supp. 1997;2:16–23. [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of LDL-cholesterol in plasma, without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall/CRC; 1997. pp. 403–5. [Google Scholar]
- 16.WJ McIsaac, Naylor D, Basinski A. Mismatch of coronary risk and treatment intensity under the National Cholesterol Education Program guidelines. J Gen Intern Med. 1991;6:518–23. doi: 10.1007/BF02598220. [DOI] [PubMed] [Google Scholar]
- 17.Bush TL, Riedel D. Screening for total cholesterol: do the National Cholesterol Education Program`s recommendations detect individuals at high risk of coronary heart disease? Circulation. 1991;83:1287–93. doi: 10.1161/01.cir.83.4.1287. [DOI] [PubMed] [Google Scholar]
- 18.Grover SA, Coupal L, Hu X-P. Identifying adults at increased risk of coronary heart disease: how well do the current cholesterol guidelines work? JAMA. 1995;274:801–6. [PubMed] [Google Scholar]
- 19.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 20.Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation. 2000;102:1893–900. doi: 10.1161/01.cir.102.16.1893. [DOI] [PubMed] [Google Scholar]
- 21.Teragawa H, Kato M, Kurokawa J, Yamagata T, Matsuura H, Chayama K. Usefulness of flow-mediated dilation of the brachial artery and/or the intima-media thickness of the carotid artery in predicting coronary narrowing in patients suspected of having coronary artery disease. Am J Cardiol. 2001;88:1147–51. doi: 10.1016/s0002-9149(01)02051-3. [DOI] [PubMed] [Google Scholar]
- 22.Davis PH, Dawson JD, Mahoney LT, Lauer RM. Increased carotid intimal-medial thickness and coronary calcification are related in young and middle-aged adults. The Muscatine study. Circulation. 1999;100:838–42. doi: 10.1161/01.cir.100.8.838. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98:1504–9. doi: 10.1161/01.cir.98.15.1504. [DOI] [PubMed] [Google Scholar]
- 24.Hulthe J, Wikstrand J, Emanuelsson H, Wiklund O, de Feyter PJ, Wendelhag I. Atherosclerotic changes in the carotid artery bulb as measured by B-mode ultrasound are associated with the extent of coronary atherosclerosis. Stroke. 1997;28:1189–94. doi: 10.1161/01.str.28.6.1189. [DOI] [PubMed] [Google Scholar]
- 25.Adams MR, Nakagomi A, Keech A, et al. Carotid intima-media thickness is only weakly correlated with the extent and severity of coronary artery disease. Circulation. 1995;92:2127–34. doi: 10.1161/01.cir.92.8.2127. [DOI] [PubMed] [Google Scholar]
- 26.Crouse JR, III, Craven TE, Hagaman AP, Bond MG. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92:1141–7. doi: 10.1161/01.cir.92.5.1141. [DOI] [PubMed] [Google Scholar]
- 27.Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 28.Pradhan AD, Manson JE, Rossouw JE, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative Observational Study. JAMA. 2002;288:980–7. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]


