Abstract
BACKGROUND
The established guidelines for a diabetes foot examination include assessing circulatory, skin, and neurological status to detect problems early and reduce the likelihood of amputation. Physician adherence to the guidelines for proper examination is less than optimal.
OBJECTIVE
Our objective was to increase compliance with the performance of a proper foot examination through a predominantly physician-directed interventional campaign.
METHODS
The study consisted of 3 parts: a retrospective chart review to estimate background compliance, an educational intervention, and prospective chart review at 3 and 6 months. A properly documented foot examination was defined as assessing at least 2 of the 3 necessary components. The educational intervention consisted of 2 lectures directed at resident physicians and a quality assurance announcement at a general internal medicine staff meeting. Clinic support staff were instructed to remove the shoes and socks of all diabetic patients when they were placed in exam rooms, and signs reminding diabetics were placed in each exam room.
RESULTS
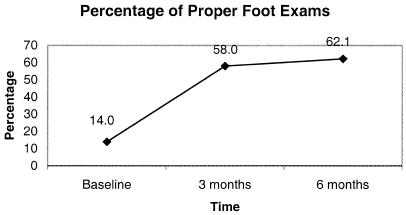
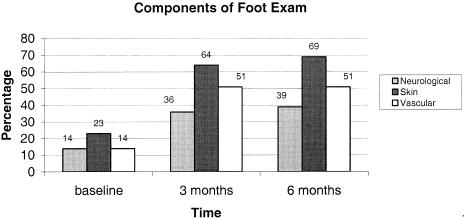
There was a significant increase in the performance of proper foot examination over the course of the study (baseline 14.0%, 3 months 58.0%, 6 months 62.1%; P < .001). Documentation of any component of a proper foot examination also increased substantially (32.6%, 67.3%, 72.5%; P < .001). Additionally, performance of each component of a proper exam increased dramatically during the study: neurological (13.5%, 35.8%, 38.5%; P < .001), skin (23.0%, 64.2%, 69.2%; P < .001), and vascular (14.0%, 51.2%, 50.5%; P < .001).
CONCLUSIONS
Patients with diabetes are unlikely to have foot examinations in their primary medical care. A simple, low-cost educational intervention significantly improved the adherence to foot examination guidelines for patients with diabetes.
Keywords: diabetes, foot ulceration, foot exam, prevention, physical education
Foot complications ranging from corns and calluses to lower-extremity amputation are a significant cause of morbidity and mortality in the 15.7 million estimated diabetics in the United States.1 Diabetes-related foot complications are a major cause of hospitalization and prolonged hospital stays. Twenty percent of all persons with diabetes in the United States are hospitalized for foot problems.2–5 In a study conducted by Smith et al., 23% of the total hospital days over a 2-year period were for treatment of diabetic foot problems.6 Foot ulcers are an extremely common complication in diabetes mellitus. In a United Kingdom survey of 6,000 patients followed in a diabetes clinic, more than 2% had an active foot ulcer, and 2.5% were status post amputation.7 Additionally, prior studies have demonstrated that 70% of diabetic foot ulcers required surgical intervention, and in more than 40% of these interventions, a toe or limb amputation was performed.3,8 Furthermore, diabetes is the leading cause of nontraumatic amputations in the United States, accounting for one half of all the nontraumatic lower extremity amputations with an estimated 67,000 diabetes-related lower limb amputations each year.9
The economic cost of diabetic foot complications is equally staggering. In 1986, care for chronic skin ulcers accounted for $150 million of the $11.6 billion direct total cost related to type 2 diabetes.9 The cost of a single lower-extremity amputation related to diabetes in 1992 ranged from $24,000 to $27,000 with an average length of hospital stay from 18.4 to 20.3 days.10 Currently, the total annual cost ascribed to diabetic foot disease is estimated to be more than $1 billion.4
The American Diabetes Association (ADA) consensus group has found that among diabetics, foot ulceration is increased in men, patients with diabetes for more than 10 years, and patients with poor glycemic control or with cardiovascular, retinal, or renal complications.11 The risk factors which predispose toward diabetic foot ulceration have also been well elucidated. These include diabetic neuropathy, peripheral vascular disease (PVD), susceptibility to infection, and biomechanical or musculoskeletal factors. Prior data suggest that the likelihood of amputation is proportional to the number of risk factors present.12 Peripheral sensory neuropathy is one of the strongest risk factors for both foot ulceration and amputation in this population.13,14 In the absence of neuropathy, people rarely develop foot ulcers. However, the underlying etiology is usually multifactorial. As demonstrated by Edmonds15 and Laing,16 foot ulcers in patients with diabetes usually have mixed ischemic and neuropathic components. Based on current ADA estimates, at least 50% of amputations that occur in diabetics can be prevented through proper foot care.2,17–19 Similarly, the factors that predispose diabetics to developing foot ulcers can be easily assessed on a focused physical exam.
In an effort to reduce the number of preventable diabetic foot complications, the ADA18 and the Centers for Disease Control and Prevention2 have outlined recommendations for evaluating for the presence of foot ulcers and the risk factors that predispose to their formation. The ADA currently recommends a comprehensive foot exam at least each year to identify high-risk foot conditions. Diabetics with 1 or more high-risk foot conditions should be evaluated more frequently for the development of additional risk factors, and patients with neuropathy should have a visual examination of the feet at every visit with a health care professional. The exam includes the evaluation of protective sensation, foot structure and biomechanics, vascular status, and skin integrity.18 Despite the human and economic impact of diabetic foot complications and the proposed guidelines for their prevention, prior studies have failed to show significant compliance on the part of physicians in performing diabetic foot exams as a routine part of diabetic health care. Clinical practice patterns for foot examinations have been assessed on the basis of providers' self reports,20,21 chart reviews,22–27 a combination of providers' self-reports with patient corroboration and chart reviews,28 and patient surveys29 (see Table 1).
Table 1.
Prior Studies on Diabetic Foot Examination
| Foot Examination | % | N | Method of Documentation | Setting |
|---|---|---|---|---|
| In past year | ||||
| Comprehensive foot examination | 52 | 6,959 | CR | Indian Health Service22* |
| <10 | 350 | CR | Academic, inner-city clinic24 | |
| Unspecified foot examination | 68 | 352 | CR & PI | Academic, inner-city clinic27* |
| <6 | NR | NR | UCLA study23 | |
| 55 | 544 | CR | 8 Community clinics28 | |
| 41 | 237 | CR | Control community clinics25 | |
| 94 | 399 | CR | Intervention community clinics25* | |
| 67 | 590 | CR | 7 Diabetes miniclinics, UK26† | |
| 49 | 65 | SR & PI | Diabetes clinic, U.S.33† | |
| Periodic foot examination | 81 | 5,640 | SR | Postprogram, Clinical Education Program21 |
| Every 4–6 mo | 53 | 1,502 | SR | National Survey, NIDDK20 |
| Foot doctor in past 2 y | 78 | 393 | PI | 8 Communities in Michigan29 |
Interventional study.
Diabetes clinic(s) (all others primary care clinics).
CR, chart review; PI, patient interview; SR, self-reporting by physicians; NR, not reported; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases.
A 1989 National Institutes of Health survey mailed to primary care physicians found that over 80% of the 1,502 respondents reported performing a “foot exam for infection and ulceration” and “peripheral circulatory and neurological exam” in patients with type 2 diabetes mellitus 1 or more times per year.20 However, studies based on chart review alone reveal a finding of more concern, that as few as 6% of patients have undergone a foot examination in the past year (see Table 1). Only 2 of the studies actually specified the components of a comprehensive foot exam and performed chart reviews to determine compliance.22,24
There have been a several studies attempting to show the effect of various interventions on the performance of diabetic foot exams.22,25,27 These studies demonstrated that patient and physician education on diabetes foot complications along with reminders for both patients and physicians dramatically improve compliance rates up to 52% to 94%.22,25,27 In the current study, we attempt to determine the effect of a primarily physician-directed intervention on the documentation of proper diabetic foot exams.
METHODS
Setting
The study took place at the outpatient internal medicine clinic at Wilford Hall Medical Center (WHMC) in San Antonio, Texas. This is an Air Force hospital that is the largest training site for internal medicine in the Air Force. There are 54 residents in our training program and 16 general internal medicine staff physicians. Residents are supervised by both general internists and subspecialty physicians in the clinic. There are 45 subspecialty physicians in our institution that assist with resident supervision in the continuity clinic. The clinic serves as the primary care provider for military retirees and their spouses. Patients are seen by internal medicine residents and staff as well as rotating medical students under staff supervision. All patient encounters by residents are reviewed with staff physicians practicing either general internal medicine or the various internal medicine subspecialties. Practice patterns of only staff general internists and residents in internal medicine were analyzed in this study.
Patient Identification and Recruitment
Subjects with clinically apparent type 2 diabetes were identified by chart review. All ambulatory general internal medicine clinic patients at WHMC with type 2 diabetes were eligible for inclusion. Clinic notes from routine appointments were assessed to determine if a diabetic foot exam was performed. Acute care/walk-in patients, patients seen and examined by medical students, patients with a documented proper diabetic exam or hospitalization within the previous 3 months, and patients of the investigators or staffed with the investigators were excluded from analysis.
Study Design
The study consisted of 3 parts: a retrospective chart review, an educational intervention, and a prospective analysis of physician behaviors following the intervention at 3 and 6 months. The retrospective phase consisted of chart review of 556 randomly selected diabetic patient visits from 1995 to 1998. For the purposes of this study, the documentation of a proper foot exam was defined as including the assessment of at least 2 of the 3 following elements in a lower-extremity examination: vascular/circulation exam, neurological status, and skin condition.2,22–24 The intervention phase was multifaceted. Two short presentations on the importance of foot examination as part of routine diabetic care were given at separate morning report sessions during the month of September 1998. In addition, a handout on the importance of diabetic foot exams was distributed to all residents at that time. It was also announced during a general internal medicine staff meeting that a “QA review” was to be performed on the adequacy of diabetic foot care at some unspecified time. Medicine clinic support staff were instructed to have diabetic patients remove their shoes and socks when they were placed in an exam room. Notices were also placed on the back of clinic doors reminding diabetic patients to remove their shoes and socks for foot examination. The final phase of the study assessed impact on physician behavior by chart review focusing on documented foot examinations. Prospective chart review was conducted at 3 and 6 months to assess the early and late impact of the physician and support staff directed intervention. All chart review was performed by 3 of the 4 physician investigators listed in the author section. All charts of diabetic patients seen in the internal medicine clinic were reviewed each day during the 3- and 6-month phases of the study to determine physician compliance with and documentation of foot examination.
Statistical Analysis
One-way analysis of variance and/or χ2 tests were used to compare the demographic makeup of all 3 groups for which data were collected (pre, 3 months, 6 months). Pearson's χ2 analysis was then employed to determine the impact of the educational intervention on the performance of diabetic foot examination at 3 and 6 months. Ninety-five percent confidence intervals were calculated for baseline comparisons as well as at the 3- and 6-month intervention phases.
RESULTS
Patient Characteristics
The patient demographics are outlined in Table 2. Patient visits were reviewed at baseline (N = 556), then at 3 (N = 162) and 6 months (N = 182) post-intervention and were well matched with respect to age, gender, duration of diabetes, presence of diabetes-related micro- and macro-vascular complications, prior history of foot ulcers, and modality of treatment. Significant differences were noted in diabetic control as reflected by hemoglobin A1c values, with a significantly higher proportion of patients having better control at 3 and 6 months. There was also a significant difference in type of visit (new versus follow-up) and staff appointments, as shown in Table 2. The increased proportion of new patients and staff appointments in the prospective arm of the study is due to the implementation of a Medicare HMO demonstration project. This coincided with the 3-month follow-up portion of the study. However, by 6 months, the proportion of new patient and staff appointments had fallen back to baseline levels. This incorporation of a managed-care panel into our patient population may have also accounted for the improvements in glycemic control noted at 3- and 6-month follow-up. It is important to note that chart documentation requirements did not change with the implementation of the HMO demonstration project.
Table 2.
Patient and Physician Characteristics*
| Patient Visit Characteristics | Pre-intervention, % (n) | 3 Months Post-intervention, % (n) | 6 Months Post-intervention, % (n) | P Value† |
|---|---|---|---|---|
| Age >50 | 95.0 (528) | 98.1 (159) | 95.6 (174) | .22 |
| Male | 54.1 (301) | 54.3 (88) | 58.2 (106) | .61 |
| Duration ≥5 y | 70.3 (391) | 79.0 (128) | 74.7 (136) | .07 |
| Neuropathy | 23.9 (133) | 24.1 (39) | 27.5 (50) | .60 |
| Retinopathy | 27.7 (154) | 29.0 (47) | 30.8 (56) | .69 |
| Nephropathy | 30.4 (169) | 34.6 (56) | 39.6 (72) | .06 |
| PVD | 10.3 (57) | 7.4 (12) | 9.9 (18) | .55 |
| Ulcer Hx | 2.3 (13) | 3.1 (5) | 3.8 (7) | .53 |
| A1c <8 | 59.9 (333) | 69.8 (113) | 72.0 (131) | .003 |
| Treatment | .111 | |||
| Diet | 12.2 (68) | 14.2 (23) | 14.8 (27) | |
| Insulin | 43.9 (244) | 37.0 (60) | 35.7 (65) | |
| Oral agents only | 43.9 (244) | 48.8 (79) | 49.5 (90) | |
| Type of visit | .001 | |||
| New | 22.3 (124) | 34.0 (55) | 17.0 (31) | |
| Follow-up | 77.7 (432) | 66.0 (107) | 83.0 (151) | |
| Level of training | .016 | |||
| PG-1 | 26.1 (145) | 26.5 (43) | 21.4 (39) | |
| PG-2/3 | 47.7 (265) | 36.4 (59) | 52.8 (96) | |
| Staff | 26.3 (146) | 37.0 (60) | 25.8 (47) |
All percentages rounded to the nearest decimal point.
χ2 result from comparison of groups.
PVD, peripheral vascular disease.
At baseline, the documentation of a proper diabetic foot exam at our institution was poor (14.0%). Similarly, the performance of any portion of a proper foot exam was seen in only 32.6% of patient visits. These figures are consistent with other published reports. In subgroup analysis, the variables of patient age, gender, modality of therapy, most recent hemoglobin A1c, and type of reviewing staff had no significant impact on the performance of proper foot exams. The effect of level of training and the likelihood of performance of a diabetic foot exam was also assessed (Table 3). As compared to PG-2/3 residents, PG-1 residents and staff where more likely to perform a proper foot exam (6.8% vs 22.8% and 18.5%, respectively; P < .0001 by Fisher's exact test). Interestingly, the presence of risk factors associated with the development of foot ulcers, namely microangiopathic disease of the eyes, kidneys, and nerves, had no affect on the likelihood of the performance of a proper foot exam during an individual patient visit (see Table 4). An even more surprising finding at baseline is that during patient visits in diabetics with a history of PVD or previous foot ulcer, when taken as a combined variable, there was no greater incidence of documented proper foot exams than during patient visits in diabetics without these high-risk conditions (14.0% vs 14.3%; P = .95 by χ2 analysis). At baseline, new-patient appointments had a statistically higher incidence of proper exams than did follow-up patient appointments (21.0% vs 12.0%; P = .018 by Fisher's exact test), but this highlights the fact that 79% of new-patient appointments did not include a proper diabetic foot exam.
Table 3.
Level of Training and Performance of a Proper Diabetic Foot Exam at Baseline With Exact 95% Confidence Intervals*
| Patient Visits with Any Foot Exam | Patient Visits with Proper Foot Exam | |||||
|---|---|---|---|---|---|---|
| Level of Training | Total Number of Patient Visits, n | n (%) | 95% CI | n (%) | 95% CI | P Value† |
| PG-1 | 145 | 76 (52.4) | 52.0 to 52.8 | 33 (22.8) | 22.4 to 23.2 | <.0001 |
| PG-2/3 | 265 | 54 (20.4) | 20.2 to 20.7 | 18 (6.8) | 6.6 to 7.1 | Reference |
| Staff | 146 | 51 (34.9) | 34.6 to 35.4 | 27 (18.5) | 18.2 to 19.0 | <.0001 |
| Total | 556 | 181 (32.6) | 32.4 to 32.7 | 78 (14.0) | 13.9 to 14.2 | |
All percentages rounded to the nearest decimal point.
By Fisher's exact test from comparison of groups.
Table 4.
Risk Factors for Foot Ulceration and the Likelihood of Foot Examination at Baseline*
| Risk Factor for Foot Ulceration | Total Number of Patient Visits | Patient Visits With Proper Foot Exam, n | Percentage With Proper Foot Exam | P Value |
|---|---|---|---|---|
| Microangiopathy–none | 289 | 35 | 12.1 | |
| 1 Organ system affected | 121 | 21 | 17.4 | |
| 2 Organ systems affected | 93 | 13 | 14.0 | .55† |
| 3 Organ systems affected | 53 | 9 | 17.0 | |
| No known peripheral vascular disease or history of a foot ulcer | 493 | 69 | 14.0 | .95† |
| Known peripheral vascular disease or history of a foot ulcer | 63 | 9 | 14.3 |
All percentages rounded to the nearest decimal point.
χ2 result from comparison of groups.
Effect of Intervention
Figure 1 demonstrates the statistically significant and persistent increase in the percentage of physicians documenting proper foot examinations at 3 and 6 months as compared to baseline. The attributable improvement for performance of a proper exam at the 3-month interval was 44% (95% confidence interval [95% CI], 35.9% to 52.1%) and 48.1% (95% CI, 40.0% to 55.7%) at the 6-month interval. Additionally, the performance of any 1 of the 3 components of a proper exam increased dramatically over the course of the study from 32.6% at baseline to 67.3% (P < .001) at 3 months and 72.5% (P < .001) at 6 months, an attributable improvement of 34.7% (95% CI, 26.5% to 42.9%) and 39.9% (95% CI, 32.4% to 47.5%), respectively. Moreover, the performance of each component of the exam increased substantially over the course of the study (see Figure 2). Age, gender, type of visit, presence and number of microangiopathic complications, history of PVD or foot ulcers did not affect the probability of the documentation of a proper exam at 3- and 6-month follow-up. It appears that the educational campaign improved the performance of a proper foot exam regardless of patient characteristics or appointment type. However, level of training was again found to have an impact on the likelihood of foot examination during the late follow-up phase of the study (see Table 5). At the 3-month interval, all groups were equally likely to perform a proper of foot exam. However, at the 6-month follow-up point, PG-1 residents and staff were more likely to document a proper foot exam as compared to PG 2/3 residents (71.8% and 74.5% vs 52.1%; P = .0138 by Fisher's exact test).
FIGURE 1.
Improvement in performance of a proper foot exam over the course of the study (Baseline, 3 and 6 months post intervention). All percentages rounded to the nearest decimal point; P < .001 for comparison to baseline foot exam by χ2 analysis.
FIGURE 2.
The improvement in all 3 components of diabetic foot examination over the course of the study (Baseline, 3 and 6 months post intervention). All percentages rounded to the nearest whole number; P < .001 for comparison to baseline for each component of the foot exam by χ2 analysis.
Table 5.
Level of Training and the Performance of Proper Foot Examination at Baseline and After the Educational Intervention*
| Level of Training | Baseline, % | 3 Months, % | 6 Months, % |
|---|---|---|---|
| PG-1 | 22.8 | 60.5 | 71.8 |
| PG-2/3 | 6.8 (P < .0001)† | 59.3 (P = .844)† | 52.1 (P = .0138)† |
| Staff | 18.5 | 55.0 | 74.5 |
All percentages rounded to the nearest decimal point.
Fisher's exact test result from comparison of groups.
DISCUSSION
Patients, physicians, and health care systems can be influential factors in the implementation of preventive care recommendations.30–32 Given that previous chart-based interventional studies22,25,27 were directed at patients and healthcare systems, we directed our educational program predominantly at physicians serving as primary care providers in our general internal medicine clinic. Previous studies26,33 suggest that the rate of foot examination is considerably higher among patients who are seen in diabetes clinics as compared to primary care clinics. It is important to note that primary care providers render >75% of all care for patients with diabetes.22,23 Therefore, we felt that an educational campaign directed at primary care providers would have the most impact.
As with prior studies, the analysis of practice patterns at the onset of our study shows relatively poor performance on the part of physicians in providing adequate diabetic foot exams, with only 14% of physicians documenting proper exams. There was a significant increase in documented proper foot exams at 3 and 6 months after a multifaceted intervention, which exceeded the impact shown in prior studies. Unlike previous studies, the intervention made in the current trial focused primarily on physician education through formal teaching sessions with an ancillary focus on support staff, which may account for the more dramatic impact on physician behavior. Significant improvements were made in all 3 components of the diabetic foot exam, and the percentage of physicians in compliance remained persistently elevated even at the 6-month end point. Finally, the improvements in documented diabetic foot exams were brought about with minimal time investment and cost. Less than $10 was needed to prepare information handouts for physicians and support staff and to create reminder signs for diabetic patients that were placed in each exam room.
The dramatic response rate to the educational campaign occurred for several reasons. We emphasized the remarkable incidence, prevalence, morbidity and even mortality, along with the economic impact of diabetes-related foot disease as a means to encourage performance of foot exams by primary care physicians. Another factor that contributed to our dramatic response was involving the support staff in the educational campaign. Instructing support staff to have diabetic patients remove their shoes and socks along with the reminder signs for patients in the exam rooms almost certainly had a considerable effect. On the basis of a study by Cohen,30 diabetics who present with bare feet in the exam room are 3 to 4 times more likely to have their feet examined as those wearing shoes and socks. We realize that even with our intervention, 38% of diabetics did not have foot examinations at routine appointments. However, based on current ADA recommendations, only those patients with peripheral neuropathy or other high-risk conditions require more frequent examinations than simply on an annual basis. Stamps in the progress notes providing space for documentation of the diabetic foot exam, flagging charts, additional educational seminars for physicians, patients, and support staff, and providing a Semmes-Weinstein monofilament or a 128-Hz tuning fork in each exam room would be additional methods of further improving compliance.
Only 2 previous studies have addressed the components of a proper foot examination, and physician compliance rates in these studies ranged from <10% to 52%.22,24 In 1986, the Indian Health Service (IHS)22 established diabetes care standards and an assessment process to evaluate adherence to those standards using medical record review. We chose a similar process to document adherence to proper foot examination in our study. It is encouraging to see that physician performance of foot examination actually increased over time in our study. This has the potential to be a durable response given the 52% compliance rate in the IHS model 6 years after the implementation of their program.22 Furthermore, Wylie-Rosett et al.24 have demonstrated that having a foot examination in the preceding year doubles the chance of having a foot examination in the following year. Both of these studies support the cautious optimism we have for continued physician compliance with diabetic foot examination at our institution. Finally, we felt that discussing the components of a proper exam with physicians would further emphasize the risk factors and pathophysiology for foot complications in diabetes, which would then translate into increased examination, improved patient education, and potentially increased referrals to foot specialists.
Previous intervention studies by Deeb et al.25 and Litzelman et al.27 indicate that a systematic approach to patient care improves rates of foot examination. Our study took this systematic approach one step further by directly involving physicians. It is difficult to assess which component of the intervention had the most significant impact on physician behavior. Additionally, given the limited follow-up time and design of our study, we were unable to ascertain if this behavioral change led to improvements in long-term patient outcomes. However, several uncontrolled studies have found that the implementation of improved foot care programs can significantly reduce lower-extremity complications in patients with diabetes, demonstrating a 44% to 85% reduction in the rate of lower-extremity amputation.19,34–36 Involving physicians in the interventional process will improve patient education in the long term. As Reiber et al. 37 have shown, patients with diabetes who had not received outpatient education were more than 3 times likely to have a lower-limb amputation. We did not follow referral patterns to podiatry, orthopedics, or vascular surgery, but based on 2 previous studies by Wylie-Rosett et al.24 and Bailey et al.,33 it appears that foot examinations by primary care providers and subsequent referrals are closely related.
Some potential limitations of our study deserve consideration. We believe that our results can be reproduced in nonmilitary, nonhospital-based patient care settings. As demonstrated by Jackson et al.,38,39 patient characteristics, diagnoses, and procedures, in military and civilian internal medicine are more similar than they are different, whether the context of care occurs in the inpatient or the outpatient setting. Similarly, previous studies22,25,27 have found that physician performance and documentation of proper foot examination can be improved with an educational intervention regardless of patient, physician, or clinic characteristics. Performance and documentation of proper foot examination are not necessarily concordant. It is likely that foot examination was performed in the pre-intervention and post-intervention phases and not documented. However, the fact that only 21% of new-patient appointments had a documented foot exam and that follow-up appointments had an even lower documentation rate suggests that performance and documentation may be closely related.
The effect on level of training and the likelihood of performance/documentation of a diabetic foot examination during a patient visit is another interesting finding in our study. At baseline, PG-1 residents and staff were more likely to perform foot exams than were PG-2/3 residents. At the 3-month interval, all groups performed foot examination to a similar degree; however, at the 6-month follow-up period, PG-1 residents and staff were more likely to perform foot examination than were PG-2/3 residents. On the basis of these data, it appears that residents may actually be less likely to perform a diabetic foot examination as they advance through residency training. Similarly, we do not feel that the marked improvement in performance of foot examination was related to improved practice patterns as residents gained experience in the outpatient setting, since this was not apparent during our baseline retrospective review or at the 6-month follow-up period. It appears that the improvement in performance/documentation of diabetic foot exams was due solely to the educational intervention. Future studies should focus on techniques to ensure that a high level of foot examination is maintained throughout residency training.
This study confirms the relatively poor compliance of primary care physicians in performing adequate diabetic foot screening. Our results add evidence to support the role of continued interventional campaigns to improve the likelihood of a proper diabetic foot examination. In particular, a predominantly physician-directed intervention appears to be an important component in improving comprehensive diabetic care. In conclusion, further investigation is needed to determine the impact of such an intervention on the long-term morbidity and mortality of diabetic foot disease.
Acknowledgments
We are indebted to Drs. Robert O'Connell and Ernest Mazzaferri for their thoughtful comments and advice concerning the manuscript. We would also like to thank our Internal Medicine nurses and clinic support staff for their assistance with our diabetic educational campaign.
No grant support was obtained to perform this study.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as reflecting the views of the Department of the Air Force or the Department of Defense.
REFERENCES
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes in the United States. Atlanta, Ga: U.S. Department of Health and Human Services; 1997. [Google Scholar]
- 2.Centers for Disease Control and Prevention. The Prevention and Treatment of Complications of Diabetes. A Guide for Primary Care Practitioners. Atlanta, Ga: U.S. Department of Heath and Human Services; 1991. [Google Scholar]
- 3.Grunfeld C. Diabetic foot ulcers: etiology, treatment, and prevention. Adv Intern Med. 1992;37:103–32. [PubMed] [Google Scholar]
- 4.Levin ME, O'Neal LW, Bowker JH, editors. The Diabetic Foot. 5th ed. St. Louis: Mosby Year Book; 1993. pp. xxi–xxii. [Google Scholar]
- 5.Levin ME. Endocrinology and Metabolism Clinics of North America. vol. 25. Philadelphia: W.B. Saunders; 1996. Foot lesions in patients with diabetes mellitus; pp. 447–62. [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Weinberger M, Katz B. A controlled trial to increase office visits and reduce hospitalizations of diabetic patients. J Gen Intern Med. 1987;2:232–38. doi: 10.1007/BF02596446. [DOI] [PubMed] [Google Scholar]
- 7.Macleod AF, Williams DRR, Sonksen PH, et al. Risk factors for foot ulceration in hospital clinic attendees. Diabetologia. 1991;34(suppl 2):A39. [Google Scholar]
- 8.Sathe SR. Managing the diabetic foot in developing countries. IDF Bull. 1993;38:16–8. [Google Scholar]
- 9.U.S. Deptartment of Health and Human Services. Diabetes Surveillance: Annual 1990 Report. Atlanta, Ga: Centers for Disease Control, Division of Diabetes Translation; 1990. pp. 23–25. 93–95. [Google Scholar]
- 10.Reiber GE, Boyko EJ, Smith DG. Diabetes in America. 2nd ed. Bethesda, Md: National Institute of Health; 1995. Lower extremity foot ulcers and amputations in diabetes. [Google Scholar]
- 11.American Diabetes Association. Preventive foot care in people with diabetes. Diabetes Care. 1999;22(Suppl 1):554–55. doi: 10.2337/diacare.26.2007.s78. [DOI] [PubMed] [Google Scholar]
- 12.Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischili JG. Practical criteria for screening patients at high risk for foot ulceration. Arch Intern Med. 1998;158:157–62. doi: 10.1001/archinte.158.2.157. [DOI] [PubMed] [Google Scholar]
- 13.McNeely MJ, Bayko EJ, Ahronhi JE, et al. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. Diabetes Care. 1995;18:216–9. doi: 10.2337/diacare.18.2.216. [DOI] [PubMed] [Google Scholar]
- 14.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 15.Edmonds ME. Experience in a multi-disciplinary diabetic foot clinic. In: Conor H, Boulton AJM, Ward JD, editors. The Foot in Diabetics. Chichester, U.K.: Wiley; 1987. pp. 121–33. [Google Scholar]
- 16.Laing P. The development and complications of diabetic foot ulcers. Am J Surg. 1998;176(Suppl 1):115–95. doi: 10.1016/s0002-9610(98)00182-2. [DOI] [PubMed] [Google Scholar]
- 17.Prevention and Early Intervention for Diabetes Foot Problems. A Research Review. Available at http://ndep.nih.gov/materials/pubs/feet/feet2001.pdf. Accessed February, 2003.
- 18.American Diabetes Association. Clinical practice recommendations 2002. Diabetes Care. 2002;25(Suppl 1):1–147. doi: 10.2337/diacare.25.2007.s1. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstock JA. Lower extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1989;12:24–31. doi: 10.2337/diacare.12.1.24. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Diabetes and Digestive and Kidney Diseases. Survey of Physician Practices Related to the Treatment of People with Diabetes Mellitus. Primary Care Physicians. Rockville Md: Prospect Associates; 1990. [Google Scholar]
- 21.Mazze R, Deeb L, Palumbo PJ. Altering physicians' practice patterns: a nationwide educational experiment: evaluation of the Clinical Education Program of the American Diabetes Association. Diabetes Care. 1986;9:420–5. doi: 10.2337/diacare.9.4.420. [DOI] [PubMed] [Google Scholar]
- 22.Mayfield JA, Rith-Najarian SJ, Acton KJ, et al. Assessment of diabetes care by medical record review: The Indian Health Service model. Diabetes Care. 1994;17:918–23. doi: 10.2337/diacare.17.8.918. [DOI] [PubMed] [Google Scholar]
- 23.Bloomgarden ZT. Diabetes and managed care. Diabetes Care. 1994;17:1552–54. doi: 10.2337/diacare.17.12.1552. [DOI] [PubMed] [Google Scholar]
- 24.Wylie-Rosett J, Walker EA, Shamoon H, Engel S, Basch C, Zybert P. Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med. 1995;4:46–50. doi: 10.1001/archfami.4.1.46. [DOI] [PubMed] [Google Scholar]
- 25.Deeb LC, Pettijohn FP, Shirah JK, Freeman G. Intervention among primary care practitioners to improve care for preventable complications of diabetes. Diabetes Care. 1988;11:275–80. doi: 10.2337/diacare.11.3.275. [DOI] [PubMed] [Google Scholar]
- 26.Willliams DRR, Munroe C, Hospedales CJ, Greenwood RH. A three-year evaluation of the quality of diabetes care in the Norwich community care scheme. Diabet Med. 1990;7:74–9. doi: 10.1111/j.1464-5491.1990.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 27.Litzelman DK, Slemenda CW, Langefeld CD, et al. Reduction of lower extremity clinical abnormalities in patients with non-insulin-dependent diabetes mellitus: a randomized, controlled trial. Ann Intern Med. 1993;119:36–41. doi: 10.7326/0003-4819-119-1-199307010-00006. [DOI] [PubMed] [Google Scholar]
- 28.Payne TH, Gabella BA, Michael SL, et al. Preventive care in diabetes mellitus: current practices in an urban health care system. Diabetes Care. 1989;12:745–47. doi: 10.2337/diacare.12.10.745. [DOI] [PubMed] [Google Scholar]
- 29.Hiss RG, editor. Michigan Diabetes Research and Training Center. Diabetes in Communities. Ann Arbor: University of Michigan; 1992. pp. 1–136. [Google Scholar]
- 30.Cohen SJ. Potential barriers to diabetes care. Diabetes Care. 1983;6:499–500. doi: 10.2337/diacare.6.5.499. [DOI] [PubMed] [Google Scholar]
- 31.Carter WB, Belcher DW, Inui TS. Implementing preventive care in clinical practice. II. Problems for managers, clinicians and patients. Med Care Rev. 1981;38:195–216. doi: 10.1177/107755878103800401. [DOI] [PubMed] [Google Scholar]
- 32.Green LW, Krenter MW. Health Promotion Planning: An Educational and Environmental Approach. 2nd ed. Mountain View, Calif: Mayfield Publishing Co; 1991. [Google Scholar]
- 33.Bailey TS, Yu HM, Rayfield EJ. Pattern of foot examination in a diabetes clinic. Am J Med. 1985;78:371–74. doi: 10.1016/0002-9343(85)90326-2. [DOI] [PubMed] [Google Scholar]
- 34.Davidson JK, Alogna M, Goldsmith M, Borden J. Assessment of program effectiveness of Grady-Memorial Hospital-Atlanta. In: Steiner G, Lawrence PA, editors. Educating Diabetic Patients. New York: Springer-Verlag; 1981. pp. 329–48. [Google Scholar]
- 35.Runyan JW., Jr The Memphis Chronic Disease Program. Comparisons in outcome and the nurse's extended role. JAMA. 1975;231:264–7. [PubMed] [Google Scholar]
- 36.Assal JP, Muhlhauser I, Pernat A, Gfeller R, Jorgens V, Berger M. Patient education as the basis for diabetes care in clinical practice. Diabetologia. 1985;28:602–13. doi: 10.1007/BF00281995. [DOI] [PubMed] [Google Scholar]
- 37.Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus: a case-control study. Ann Intern Med. 1992;117:97–105. doi: 10.7326/0003-4819-117-2-97. [DOI] [PubMed] [Google Scholar]
- 38.Jackson JL, Strong J, Cheng EY, Meyer G. Patients, diagnoses, and procedures in a military internal medicine clinic: comparison with civilian practices. Mil Med. 1999;164:194–7. [PubMed] [Google Scholar]
- 39.Jackson JL, Cheng EY, Jones DL, Meyer G. Comparison of discharge diagnoses and inpatient procedures between military and civilian health care systems. Mil Med. 1999;164:701–4. [PubMed] [Google Scholar]