Abstract
BACKGROUND
Patient-provider communication is essential for effective care of diabetes and other chronic illnesses. However, the relative impact of general versus disease-specific communication on self-management is poorly understood, as are the determinants of these 2 communication dimensions.
DESIGN
Cross-sectional survey.
SETTING
Three VA heath care systems, 1 county health care system, and 1 university-based health care system.
PATIENTS
Seven hundred fifty-two diabetes patients were enrolled. Fifty-two percent were nonwhite, 18% had less than a high-school education, and 8% were primarily Spanish-speaking.
MEASUREMENTS AND MAIN RESULTS
Patients' assessments of providers' general and diabetes-specific communication were measured using validated scales. Self-reported foot care; and adherence to hypoglycemic medications, dietary recommendations, and exercise were measured using standard items. General and diabetes-specific communication reports were only moderately correlated (r = .35) and had differing predictors. In multivariate probit analyses, both dimensions of communication were independently associated with self-care in each of the 4 areas examined. Sociodemographically vulnerable patients (racial and language minorities and those with less education) reported communication that was as good or better than that reported by other patients. Patients receiving most of their diabetes care from their primary provider and patients with a longer primary care relationship reported better general communication. VA and county clinic patients reported better diabetes-specific communication than did university clinic patients.
CONCLUSIONS
General and diabetes-specific communication are related but unique facets of patient-provider interactions, and improving either one may improve self-management. Providers in these sites are communicating successfully with vulnerable patients. These findings reinforce the potential importance of continuity and differences among VA, county, and university health care systems as determinants of patient-provider communication.
Keywords: diabetes mellitus, self-care, communication barriers, access to care, chronic disease management, quality of care
Although technical processes of diabetes management such as glycosylated hemoglobin (A1c) monitoring have improved in many health care systems, outcomes for large numbers of patients remain suboptimal.1,2 This discordance reflects the central role that diabetes patients themselves play in determining their health status and the challenges associated with supporting their efforts to manage the complexities of their self-care.3 Patients with diabetes must modify long-standing lifestyle behaviors such as their diet and physical activity levels, maintain vigilance to identify symptoms of emerging health crises, and adhere to often complex hypoglycemic medication schedules. Not surprisingly, many patients have difficulty meeting the demands of their illness and experience poorer outcomes as a result.
When diabetes patients play central roles in setting their own self-care goals, they are more likely to adhere to treatment plans.4–6 Clinicians can contribute to this process by: providing patients with the information they need for priority setting and problem solving, assisting them in identifying realistic targets for behavior changes, and providing ongoing emotional support and encouragement. Through these efforts, clinicians can improve patients' long-term ability to maintain an effective self-management regimen and help them avoid the emotional burnout that is common among diabetes patients.7,8 More effective patient-provider communication can lead to better self-care behavior as well as improvements in health outcomes.9–15 One critical dimension of the communication process is the diabetes-specific content or information transfer that occurs during medical encounters, often considered under the broad rubric of “patient education.” Alternatively, general communication reflects more global aspects of the interaction, such as the extent to which patients have the opportunity to articulate their self-care problems, patients' preferences are considered in developing treatment plans, physicians provide clear explanations about treatments and test results, and patients receive emotional support for efforts to cope with their illness. No prior studies have examined the extent to which patients' perceptions regarding diabetes-specific and general communication are correlated or the relative influence of these 2 communication dimensions on self-care behaviors.
Regardless of which dimension of communication is considered, characteristics of patients, providers, and health systems may influence the quality of patient-provider interactions. Race and ethnicity may constitute barriers to communication with potentially deleterious effects on patients' willingness to receive necessary services and follow treatment plans. Female physicians often engage in more patient-centered communication than their male counterparts,16 and many patients are more satisfied with female providers.17–20 Continuity of care has been associated with better communication among asthma patients21 and greater patient satisfaction and quality in general.22,23 No studies have examined the role that these factors or health system differences may play in determining the communication dynamics between providers and patients with diabetes.
The purpose of this study was to examine general communication processes and diabetes-specific communication within an ethnically diverse population of diabetes patients treated in 3 different systems of care. Specifically, we asked: 1) to what extent do patient characteristics, provider characteristics, and system of care differences influence the process of general and diabetes-specific communication? and 2) how important are each of these dimensions of communication as influences on diabetes patients' self-care behaviors?
METHODS
Participants
Adults with diabetes treated in 3 VA health care systems, 1 county health care system, and 1 university-based health care system were identified as part of a study evaluating automated telephone assessments as an adjunct to diabetes management. In each system of care, we identified diabetes patients via electronic appointment lists and screened them for eligibility at the time of outpatient primary care visits (i.e., to general internal medicine and family practice clinics) and diabetes-related specialty visits (i.e., to endocrinology, diabetes education, podiatry, and ophthalmology clinics). Patients with a diagnosis of diabetes were eligible for participation if they had a fixed residence, were at least 21 years of age, and spoke either English or Spanish as their primary language. We excluded patients who were visually or hearing impaired, seriously mentally ill, had another life-threatening condition (e.g., breast cancer, HIV disease, or renal failure requiring dialysis), or planned to change health care systems in the coming year.
A total of 1,221 known eligible patients were identified. Of these, 1,015 completed the informed consent for enrollment; 167 patients later were either lost to follow-up or refused participation prior to completing their baseline telephone interview, leaving the actual response rate at 848 (70%). There were no differences between enrollees and non-enrollees with regard to primary language, gender, type of diabetes management (insulin only, pills only, or both treatments), or age when first diagnosed. Similar proportions of patients were enrolled in VA, county, and university-based clinics (72%, 70%, and 67%, respectively; P = .23). Similar proportions of whites, African Americans, and Hispanics enrolled in the study; although Asian patients and those identifying as “other race/ethnicity” were less likely to enroll (57% and 66%, respectively, P = .001). Enrollees and non-enrollees also differed somewhat by education level (P = .03), with 58% of enrollees having some college education as compared to 52% of non-enrollees. Eleven patients without baseline A1c values were excluded from the current analyses along with 85 participants who reported in their baseline interview that they had no primary care provider. These latter patients were similar to those with a primary provider in terms of their demographic characteristics and diabetes treatment, although Spanish-speakers were less likely than English-speakers to report having a primary care provider (82% vs 94%, P = .0002). The study was approved by each facility's human subjects committee, and all participants provided written informed consent.
Measures
Data for the current study were taken from the detailed telephone interview conducted with patients soon after enrollment. All interviews were conducted by trained interviewers, and participants were given the option of completing the survey in either English or Spanish.
General Communication Style
Patients' perceptions regarding the general communication process were elicited using items from the Interpersonal Processes of Care (IPC) questionnaire.24 The IPC was designed to measure multiple facets of patient-provider communication such as general clarity, explanations of conditions and prognoses, and elicitation of patients' preferences for various treatment options. Items in the IPC were selected from a larger pool generated through extensive focus groups with sociodemographically diverse patients. All items focus on treatment over the prior 12 months, and patients report the frequency of specific behaviors using a 5-point Likert scale, ranging from “always” to “never.” As in prior studies using IPC items, patients' responses in the current study were highly skewed toward positive ratings. To simplify the measure and clarify the meaning of patients' scores, we re-coded each item as a binary indicator of whether the participant selected the best possible response option or something else, and then summed the indicators to create the overall general communication scale. Analyses comparing the summary scale based on recoded items to one based on items using the original metric did not indicate any loss of reliability associated with collapsing the item responses. For example, the α reliability for the revised scale was 0.91 as compared to 0.89 using the original response set.
Although the IPC questionnaire includes 41 items and 14 suggested subscales, we found no empirical evidence that the subscales tapped unique facets of the communication experience. Consequently, we selected a subset of the original items in order to represent the overall domain as parsimoniously as possible. In so doing, we chose to maintain the integrity of the subscales, rather than sample randomly from the overall set of potential items. The subscale items that we included represent the foundational aspects of patient-provider communication25: communication with patients regarding tests and procedures (3 items, e.g., “did doctors at [usual source] explain why a test was being done?”); general explanations of self-care (7 items, e.g., “did doctors at [usual source] go over ALL of the medicines you were taking?”); elicitation of patient preferences and concerns (4 items, e.g., “did doctors at [usual source] ask how you felt about different treatments?”); and emotional support (3 items, e.g., “were doctors at [usual source] compassionate and caring?”). The summary scale created from these items was standardized to a 0–100 range, with higher numbers indicating a potentially more effective general communication process.
Diabetes-specific Communication
Diabetes-specific communication was measured using a previously unpublished scale. All items had the following root: “In the past 12 months, has someone at [usual source of care] discussed or given you information on ….” Unlike the general communication measure, which focused on communication processes applicable across clinical issues, items within the diabetes communication scale addressed specific communication content or substantive areas of diabetes education including: diet (“how to adjust your eating to improve your blood cholesterol,”“how to plan your meals to improve your blood sugar,”“how to read food labels,”“how to make appropriate food choices,” and “how specific foods affect your blood sugar”); foot care (“how to care for your feet”); physical activity (“how to exercise properly”); and other issues (“what to do for symptoms of low blood sugar,”“how and when to monitor your blood sugar,”“what is a good number for your blood sugar” and “the benefits of controlling your blood sugar”). Two items addressing hypoglycemic medication adherence (e.g., “how and when to take your diabetes medication” and “how to adjust your diabetes medications based on your own blood sugar tests”) were excluded from the scale because they overlapped with items from the general communication scale, and our goal was to measure conceptually distinct domains. All items had yes/no response options. The scale was standardized to a 0–100 range, with higher numbers indicating more extensive diabetes-specific communication.
Self-care Behavior and Other Measures
Foot self-care, hypoglycemic medication adherence, dietary behavior, and physical activity were measured using single items with Likert-type frequency response sets, modified from those in the Summary of Diabetes Self-Care Activities questionairre.26 All items focused on the frequency of self-care within the prior 7 days. Adherence to oral hypoglycemic medications and insulin was measured using 2 separate items; for patients taking both types of medication, adherence reports for oral hypoglycemics were used. In addition to their face validity, each self-care measure correlated in expected ways with patients' physiologic health status, body mass index, and physical functioning (analyses available on request). During patients' interviews, their primary care provider was identified, and information was collected regarding the provider's gender, the length of the patient-provider relationship, and whether the primary care provider was the person to whom the patient “goes for most of the care related to [his or her] diabetes.” Information about patients' race, primary language (defined as the language most often spoken in the home), educational attainment, comorbid chronic illnesses, and diabetes complications also was collected during the baseline interview. Patients' A1c levels were measured in clinic by trained research staff using fingerstick capillary blood samples and the DCA 2000 Analyzer.27
Survey Translation
The Spanish-language version of the interview included the Spanish translation of the IPC items previously validated by the scale's developers. The remaining sections of the survey were translated into Spanish by a native speaker and validated using standard procedures for back translation by a separate Spanish-speaker. Both the English and Spanish versions of the survey were extensively pretested.
Statistical Analysis
Initial analyses focused on factors predicting general and diabetes-specific communication. Multivariate ordinary least squares regression models were fit in order to identify independent patient, provider, and system-level predictors for each dimension of communication, controlling for patients' clinical characteristics (A1c levels, insulin use, presence of hypertension, history of myocardial infarction, and number of diabetes complications) and the clustering of patients by primary care provider. Robust standard errors were used to calculate 95% confidence intervals around parameter estimates.
In the second phase of the analysis, we examined the independent influence of each communication dimension on the 4 self-care behaviors: foot care, hypoglycemic medication adherence, diet, and exercise. Communication effects were estimated using multivariate models that controlled for potential confounding by patients' sociodemographic characteristics (race, gender, age, and educational attainment), clinical characteristics (as described above), and treatment context (site of care, provider gender, continuity, and length of primary care relationship). Ordinal logit models (fit using the ologit procedure, Stata Statistical Software, Release 7.0. [Stata Corp., College Station, Tex]) were used to accommodate the ordered response sets for each self-care item, and standard errors were adjusted for the clustering of patients by provider.28 For each self-care behavior, we calculated model-based predicted probabilities for the distribution of responses across potential patient subgroups defined by combinations of various levels of general communication and diabetes-specific communication scores. The 4 prediction points on each communication scale were chosen to represent equidistant cutpoints covering the range of potential scores, i.e., lowest or “worst” possible score, 33rd percentile, 66th percentile, and highest or “best” possible score. Thus, we calculated the probability of self-care among patients with the best-possible general and diabetes-specific communication, best general communication and moderately good diabetes-specific communication, etc. Predicted probabilities for reporting optimal adherence within each self-care area are presented in Figure 1A–1D. Other findings from the multivariate modeling are presented in the text, and all output from these analyses are available from the first author on request.
FIGURE 1.
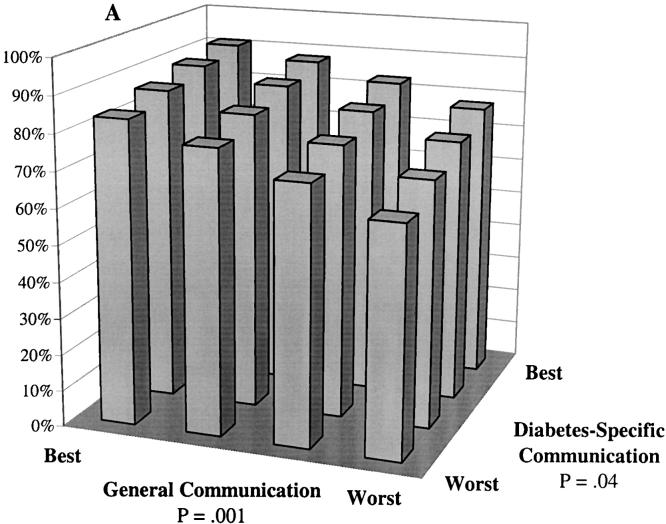
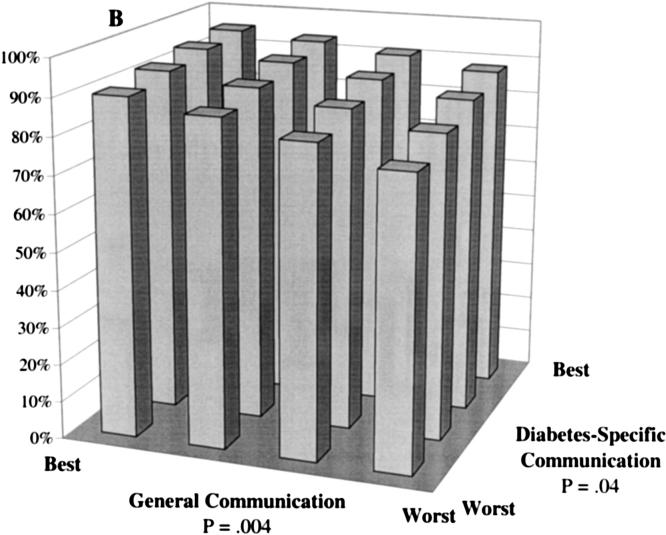
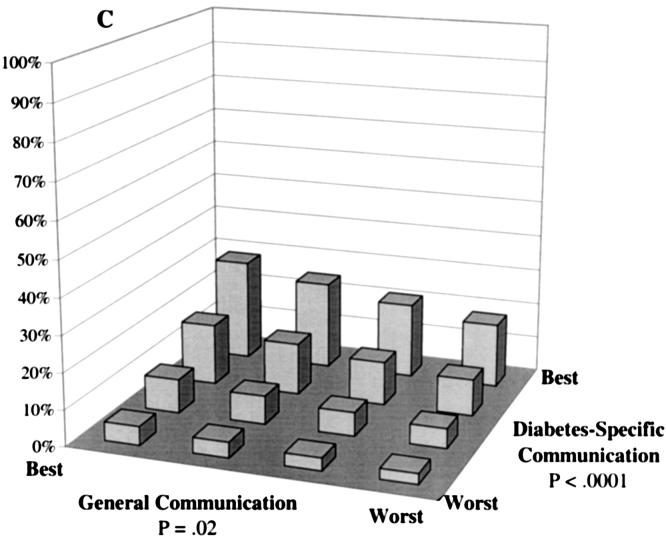
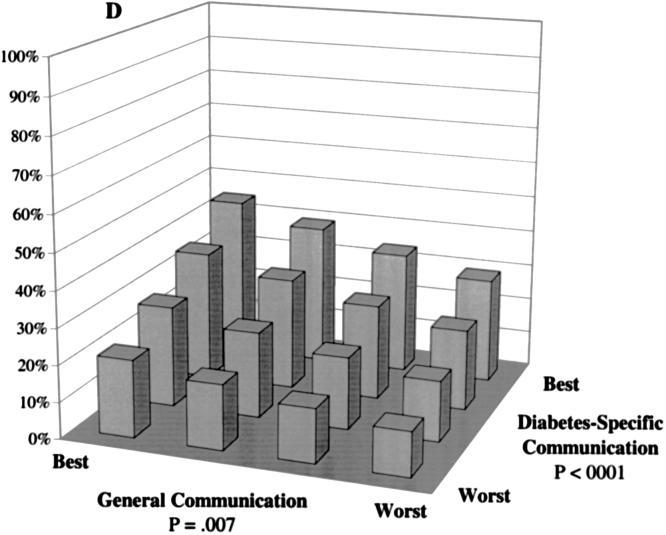
Predicted probability of optimal self-care at differing levels of general communication and diabetes-specific communication. Probabilities were calculated based on multivariate, ordinal probit models controlling for patients' sociodemographic characteristics (race, language, gender, age, and educational attainment), clinical characteristics (insulin use, A1c level, hypertension, history of myocardial infarction, and number of diabetes complications), and characteristics of the treatment context (whether the primary provider provides most of the diabetes care, provider gender, length of the primary care relationship, and site of care). A: The probability of “daily” or “almost daily” foot checks. B: The probability of “always” taking diabetes medications as prescribed. C: The probability of “always” following a recommended diet. D: The probability of “daily” exercise.
RESULTS
Participant Characteristics
A total of 752 patients participated in the study. Because participants were identified from 3 differing health system types, a wide range of sociodemographic characteristics was represented in the sample (Table 1). Sixty-two percent of participants used oral hypoglycemic medication only, 16% used both pills and insulin, and the remaining 22% used insulin only. There were no significant differences across systems of care in the type of hypoglycemic medications used, patients' duration of diabetes, or the number of recently diagnosed patients. Study participants were seen by 328 primary care providers; 175 primary providers contributed 1 patient to the study, 127 contributed 2 to 5 patients, and 26 contributed between 6 and 16 patients.
Table 1.
Patient Characteristics by Site of Care
| VA Clinics | County Clinics | University Clinics | P Value | |
|---|---|---|---|---|
| N | 326 | 177 | 249 | 752 |
| Race, % | ||||
| White | 72.7 | 19.2 | 35.7 | <.0001 |
| African American | 12.9 | 29.9 | 20.9 | |
| Hispanic | 4.3 | 29.4 | 14.1 | |
| Other | 10.1 | 21.5 | 29.3 | |
| Primary language, % | ||||
| English | 98.5 | 58.2 | 81.5 | <.0001 |
| Spanish | 0.6 | 27.7 | 4.8 | |
| Other | 0.9 | 14.1 | 13.3 | |
| Male, % | 95.4 | 47.5 | 42.2 | <.0001 |
| Age, y | ||||
| <50 | 9.5 | 23.7 | 29.7 | <.0001 |
| 50–59 | 32.5 | 30.5 | 27.3 | |
| 60+ | 58.0 | 45.8 | 43.0 | |
| Education, % | ||||
| <High school | 15.0 | 33.3 | 13.4 | <.0001 |
| High school degree to some college | 67.8 | 54.8 | 44.9 | |
| College degree+ | 17.2 | 11.9 | 41.7 | |
| PCP is primary diabetes provider, % | 73.7 | 81.4 | 65.7 | .002 |
| Female PCP | 52.5 | 59.3 | 54.6 | .33 |
| Years with PCP | ||||
| <1 | 34.3 | 33.7 | 23.4 | <.0001 |
| 1 to <2 | 19.7 | 29.1 | 18.0 | |
| 2 to <5 | 33.0 | 26.9 | 32.8 | |
| 5+ | 13.0 | 10.3 | 25.8 | |
| Insulin use, % | 37.1 | 40.7 | 33.9 | .36 |
| A1C, mean %± SD | 7.6 ± 1.5 | 8.5 ± 1.9 | 8.2 ± 1.8 | <.0001 |
| History of HTN, % | 78.2 | 74.6 | 69.9 | .08 |
| History of MI, % | 27.2 | 18.1 | 10.9 | <.0001 |
| ≥1 Diabetes complication*, % | 52.3 | 47.3 | 41.7 | .02 |
Kidney disease, retinopathy, or neuropathy.
PCP, primary care provider; HTN, hypertension; MI, myocardial infarction.
Reliability of the Communication Measures
Inter-item correlations for the 17-item IPC scale ranged between 0.2 and 0.4 (all P < .001). Cronbach's α for the summary measure was 0.90, and the scale was equally reliable within subgroups of patients defined by race, educational attainment, and diabetes severity measures (A1c levels, insulin use, and diabetes complications). Inter-item correlations for the 11-item diabetes-specific communication measure ranged from 0.24 to 0.70 (all P <.0001), and the overall scale had an α of 0.88.
Predictors of General Communication and Diabetes-specific Communication
While general communication and diabetes-specific communication ratings were moderately correlated (r = .35, P < .0001), 34% of participants had scores above the median on one dimension but below the median on the other, and 15% of respondents were in the highest tercile on one dimension but the lowest tercile on the other.
In bivariate analyses (Table 2), we found that African-American and Hispanic participants reported significantly better general communication on average than participants who were white or of some other race/ethnicity (P < .0001). Spanish-speakers reported better general communication than patients whose primary language was English or something else (P < .0001). Patients with fewer years of formal education reported both better general and diabetes-specific communication. General communication also was better among patients who reported that their primary care provider was also their main diabetes provider, as well as among patients who had been with the same provider longer (both P < .001). Patients treated in university-based clinics or VA clinics reported poorer general communication than county clinic patients, and diabetes-specific communication was poorer among university clinic patients than among patients in either VA or county systems of care (P < .0001).
Table 2.
Bivariate Relationships Between Patient, Provider, and System Characteristics, and Perceived Communication
| General Communication | P Value | Diabetes-specific Communication | P Value | |
|---|---|---|---|---|
| Race | ||||
| White | 50.0 ± 27.2 | <.0001 | 76.6 ± 27.9 | .17 |
| American African | 63.2 ± 25.8 | 82.1 ± 25.9 | ||
| Hispanic | 61.3 ± 28.9 | 77.5 ± 30.1 | ||
| Other | 51.9 ± 29.6 | 80.4 ± 27.8 | ||
| Primary language | ||||
| English | 52.9 ± 28.0 | <.0001 | 77.6 ± 28.1 | .24 |
| Spanish | 71.3 ± 25.3 | 83.5 ± 26.2 | ||
| Other | 53.1 ± 27.2 | 82.0 ± 26.2 | ||
| Gender | .29 | .43 | ||
| Male | 56.0 ± 29.2 | 77.4 ± 28.4 | ||
| Female | 53.7 ± 27.6 | 79.1 ± 27.6 | ||
| Age, y | .19 | .45 | ||
| <50 | 54.1 ± 28.6 | 80.4 ± 27.0 | ||
| 50–59 | 51.8 ± 29.9 | 79.3 ± 27.3 | ||
| 60+ | 56.2 ± 26.9 | 77.3 ± 28.4 | ||
| Education | ||||
| <High school | 64.2 ± 26.6 | <.0001 | 81.5 ± 26.5 | .02 |
| High school degree to some college | 53.9 ± 28.2 | 79.3 ± 27.4 | ||
| College degree+ | 48.5 ± 27.3 | 73.7 ± 28.7 | ||
| PCP is primary diabetes provider | ||||
| Yes | 57.0 ± 27.5 | .0002 | 81.0 ± 26.1 | .20 |
| No | 48.4 ± 29.2 | 78.0 ± 28.2 | ||
| Provider gender | ||||
| Male | 52.7 ± 28.0 | .13 | 77.7 ± 27.6 | .46 |
| Female | 55.9 ± 28.3 | 79.2 ± 28.0 | ||
| Years with PCP | .001 | .06 | ||
| <1 | 49.0 ± 28.1 | 74.8 ± 30.5 | ||
| 1 to <2 | 54.5 ± 28.9 | 80.8 ± 25.0 | ||
| 2 to <5 | 57.8 ± 28.4 | 80.1 ± 27.5 | ||
| 5+ | 59.8 ± 25.5 | 81.6 ± 24.6 | ||
| Clinic type | <.0001 | <.0001 | ||
| VA | 51.4 ± 27.8 | 81.6 ± 25.5 | ||
| County | 62.4 ± 27.1 | 83.2 ± 24.5 | ||
| University | 52.8 ± 28.5 | 71.2 ± 31.3 |
Note: Communication scores range from 0 to 100 with higher scores indicating better communication. Table entries are mean scores ± SD.
PCP, primary care provider.
Multivariate regression models predicting patients' general and diabetes-specific communication scores indicated that African-American participants and Spanish-speakers reported better general communication (as indicated by positive βs in Table 3) than patients of other racial and language groups. Patients with lower education levels also reported better general communication, as did those whose primary care provider was their main diabetes provider and those who had seen the same primary care provider for a longer time period. With regard to diabetes-specific communication, both African Americans and patients of other races reported better communication than those who were white or Hispanic. Patients who were younger, had less formal education, and had seen the same primary care provider for a longer period of time also reported better diabetes-specific communication. Patients treated in VA or county clinics reported significantly better diabetes communication than those treated in university-based clinics.
Table 3.
Regression Models Predicting General Communication and Diabetes-specific Communication
| General Communication | Diabetes-specific Communication | |
|---|---|---|
| African-American* | 10.4¶ | 8.5¶ |
| 95% CI | 4.7 to 16.1 | 3.5 to 13.6 |
| Hispanic* | 0.8 | −0.7 |
| 95% CI | −7.6 to 9.2 | −9.0 to 7.6 |
| Other non-white race* | 2.1 | 9.5¶ |
| 95% CI | −3.9 to 8.1 | 4.1 to 14.8 |
| Primarily Spanish-speaking† | 15.4¶ | 7.0 |
| 95% CI | 6.2 to 24.6 | −2.1 to 16.2 |
| Female | −2.7 | 0.9 |
| 95% CI | −7.5 to 2.0 | −4.8 to 6.5 |
| Age | 0.01 | −0.2# |
| 95% CI | −0.2 to 0.2 | −.4 to −0.07 |
| Education‡ | −6.0¶ | −3.8** |
| 95% CI | −9.3 to −2.8 | −7.1 to −0.5 |
| PCP is primary diabetes provider | 7.4# | 2.9 |
| 95% CI | 2.2 to 12.6 | −1.9 to 7.7 |
| Female PCP | 2.4 | 3.4 |
| 95% CI | −1.6 to 6.5 | −0.8 to 7.6 |
| Length of PCP relationship§ | 4.1¶ | 3.4¶ |
| 95% CI | 2.4 to 5.8 | 1.6 to 5.3 |
| VA clinics‖ | −0.9 | 15.4¶ |
| 95% CI | −6.4 to 4.5 | 9.0 to 21.8 |
| County clinics‖ | 3.1 | 10.6¶ |
| 95% CI | −1.8 to 8.1 | 5.1 to 16.1 |
| Adjusted R2 | .13 | .11 |
Note: Cell entries are β's from each multivariate ordinary least squares regression model and their 95% confidence intervals (95% CIs). Communication scores range from 0–100; higher scores indicate better communication. Each model controlled for the clustering of patients by primary care provider and the following clinical characteristics: insulin use, A1c, hypertension, history of myocardial infarction, and the number of diabetes complications.
Referent, white.
Referent, English or other primary language.
1 = less than high school degree, 2 = high school degree to some college, 3 = college degree or more.
1 = less than one year, 2 = one to less than two years, 3 = two to less than five years, 4 = five or more years.
Referent, university clinics.
P <.001.
.001 < P <.01.
.01 < P <.05.
PCP, primary care provider.
Because 3 VA systems of care were represented in the study, overall system effects could mask important local differences in patients' communication experience. In auxiliary analyses, we tested for differences across VAs by replacing the overall VA-system indicator variable with 3 separate indicators for patients enrolled in each VA facility. Coefficients associated with each of the 3 VA systems of care were similar in magnitude, and all 3 indicated that care in that facility was associated with significantly (P < .05) better diabetes-specific communication than that perceived by university clinic patients.
The Relationship Between Communication and Self-care Behavior
Foot Care
Multivariate probit models indicated that both general communication and diabetes-specific communication were independently associated with patients' frequency of foot self-care (Fig. 1A). Controlling for patient characteristics, provider characteristics, and system of care differences, the predicted probability of daily or almost daily foot checks increased from 63% for a patient with both poor general communication and poor diabetes-specific communication to 91% for a patient with the best communication of both types. Another way to appreciate the importance of a given communication dimension is to consider the potential impact of improving communication in that domain while assuming communication in the other domain remains consistently poor. Among patients with poor diabetes-specific communication, the probability of daily foot checks increased from 63% to 84% across the range of general communication scores, and a similar increase was predicted when one improves diabetes-specific communication among patients with poor general communication reports. Other results from the model (data not shown) indicate that the predicted probability of foot checks occurring less than once a week decreases from 18% for patients with poor communication of both types to 4% (more than a 4-fold change) among patients with the best possible general and diabetes-specific communication.
Hypoglycemic Medication Adherence
Both general communication and diabetes-specific communication were independently associated with patients' self-reported level of adherence to hypoglycemic medications (Fig. 1B). Controlling for covariates, the predicted probability of consistently taking these medications as prescribed increased from 77% for a patient with poor general and diabetes-specific communication to 95% among patients with the best scores on both dimensions. Among patients in the lowest category for general communication, improvements in diabetes-specific communication were associated with an 11% absolute improvement in adherence (from 77% to 88%). Among patients with the lowest diabetes-specific communication score, the model predicted a 13% absolute improvement in adherence across the range of general communication scores (from 77% to 90%).
Dietary Adherence
Although overall adherence levels were much lower for dietary than other self-care behaviors, dietary behavior also was independently associated with both general communication and diabetes-specific communication measures (Fig.1C). Controlling for covariates, patients' predicted probability of following their recommended diet daily increased from 3% among patients with poor communication on both dimensions to 28% among patients with the best possible combination of communication scores—more than a 9-fold improvement. In contrast, patients' predicted probability of following their recommended diet “rarely” or “never” decreased from 60% to 12% over this same range (data not shown).
Exercise
Patients' probability of daily exercise also was strongly associated with both their general and diabetes-specific communication reports (Fig. 1D). The model predicted more than a tripling in the proportion of patients who exercise daily between patients with the lowest possible and highest possible communication scores (from 12% to 45%). Among patients with the lowest general communication scores, the probability of daily exercise increased from 12% to 29% across levels of diabetes-specific communication, and increased from 12% to 21% across general communication levels among individuals with the worst diabetes-specific communication. In contrast, the probability of never exercising decreased from 51% among patients with the worst possible combination of scores to 15% among patients with the best combination (data not shown).
DISCUSSION
We found that 2 dimensions of patient-provider communication, general communication and diabetes-specific communication, are related but distinct facets of patients' communication experience, with differing predictors and independent impacts on diabetes self-care practices. Although these 2 facets of the patient-provider relationship are correlated, many patients reported that their providers were effective communicators in general, yet weak with regard to diabetes-specific communication; other patients found general communication to be poor although diabetes-specific information was well-conveyed. While the data suggest that improving both general and diabetes-specific communication is ideal, better self-care may result from improving only 1 of these dimensions.
Another important finding was that among these individuals with an identified primary care provider, sociodemographically vulnerable patients reported communication that was as good as or better than that reported by their less-vulnerable counterparts. African-American participants reported better general communication than participants who were white. In prior studies among managed care enrollees, African Americans also reported higher satisfaction than white enrollees both with their overall treatment and with providers' psychosocial and lifestyle health promotion practices.28 In contrast, other studies have found that racial and ethnic minority patients are more likely than are white patients to report difficulty communicating with their physicians,29 and to rate their visits with physicians as less participatory than whites.30–32 In the context of such differing findings, we can only conclude that the relationship between race/ethnicity and patient-provider communication is variable, may be strongly influenced by factors that currently are poorly understood, and thus requires more targeted investigation. In particular, more conclusive studies including community-based samples would provide important information regarding the extent to which traditionally disenfranchised patients with communication problems are under-represented in outpatient clinics (and thus, in the current study).
Hispanic participants in our study did not differ from whites in their general communication perceptions, a finding that is consistent with research suggesting little difference between Latinos and whites in their levels of satisfaction with provider communication.28,32 Another prior study found that among patients with diabetes or hypertension, Hispanic ethnicity was associated with a more favorable health outlook relative to non-Hispanic whites, comparable satisfaction with care, and comparable outcomes.33 Spanish-speakers in our study actually reported better general communication than English-speakers. Although this seems counter-intuitive, a study of the quality of care for non–English-speakers found that the availability of language-concordant diabetes care was not associated with improved glucose control or receipt of recommended tests.34 Nevertheless, the ways in which language affects treatment are poorly understood, and measures to definitively understand these findings are beyond the scope of the current study.35
One potential explanation for our finding of relatively positive general communication assessments among sociodemographically vulnerable patients is that these patients have lower expectations of their patient-provider relationship or greater discomfort with criticizing their providers. Other studies have shown that differential expectations, standards, or propensity to provide socially desirable responses can substantially confound reporting of patients' satisfaction with care.36,37 However, the instrument that we used to measure general communication was designed based on extensive focus groups of individuals with a wide range of socioeconomic characteristics, most of whom were drawn from the same geographic region in which the current study took place. Individual items in this instrument were designed specifically to avoid biases related to expectations by focusing on objective reports of communication events rather than more subjective evaluations regarding the quality or value of these events to the patient.24 Moreover, one study suggests that differences in expectations between socioeconomically vulnerable patients and others may be over-estimated, and that VA patients' expectations regarding medical care are no different from those of primary care patients treated in nearby university-affiliated private clinics.38
A more straightforward explanation for the relatively favorable reports of sociodemographically vulnerable participants in this study is that providers in these clinics spend more time counseling patients who they perceive to need additional attention. Providers in these sites may have been especially sensitized to the needs of vulnerable populations, and clinicians with a high level of commitment to underserved patients may have gravitated to these settings. Nevertheless, a number of alternative explanations for the observed associations, including patients' influence on providers' communication style or competing demands brought by patients' to the clinical encounter should be explored in subsequent studies.
VA and county clinic patients reported better diabetes-specific communication than did their counterparts treated in university-based clinics. In addition to differences across settings in organizational culture, system-specific support services such as the availability of diabetes educators and the time allotted for outpatient encounters may have contributed to the differences we observed. Our findings regarding the impact on communication of continuity (measured both in terms of having diabetes care provided by one's primary care provider and by the length of the primary care relationship) suggest that familiarity may improve providers' proficiency with a given patient. At the system level, sites that see a higher volume of chronically ill and sociodemographically vulnerable patients, such as the VA and county clinics, may become more effective at supporting meaningful interactions, a possibility that would parallel the volume-outcome relationship observed for many technical processes of care.39
Although intriguing, these system-level differences should be viewed with caution. Patients were seen by more than 300 primary care providers and were enrolled from multiple clinics in each system type; however, the study included patients from only 1 university-based health system, 1 county health system, and 3 VA health systems. The communication experience among non–diabetes patients in these systems of care or diabetes patients who were excluded from the current study (e.g., patients with end-stage renal disease, visual impairments, or a plan to change health systems within the coming year) may differ.
Both general communication processes and diabetes-specific communication were independently associated with patients' self-care, even when controlling for multiple indicators of patients' sociodemographic characteristics, health status, and other characteristics of their health care context. This finding should provide further support for including provider training in communication as a legitimate and important component of medical education and bolster efforts to develop novel strategies for increasing patients' access to effective diabetes education. Nevertheless, analyses of the relationship between communication and self-care should be interpreted with several caveats in mind. Diabetes self-care behavior is notoriously difficult to measure, and self-report data may reflect both biases as well as random errors.40,41 Patients who are more adherent with their self-care plans may be more likely to recall conversations regarding their diabetes self-care. Also, it is well known that patients tend to over-report their adherence to self-care activities, e.g., by overestimating their adherence to medication regimens, physical activity levels, and intake of healthy foods.42–44 This over-reporting may differ across sociodemographic groups or may be more common among patients who receive more intensive counseling regarding these issues. In the current study, survey questions regarding patients' self-care behaviors, general communication, and diabetes-specific communication were asked at different points in the interview in order to minimize the likelihood of socially desirable responses.
In summary, we found that African Americans, Spanish-speakers, and less-educated patients reported patient-provider communication that was as good as or better than that reported by less-vulnerable patients. We also found that aspects of the care process such as whether patients' primary care provider served as their main diabetes provider, the length of the patient-provider relationship, and patients' system of care also influenced communication. Both general and diabetes-specific dimensions of communication were associated with self-care behaviors. Our findings support the traditional advice to physicians to “treat the whole patient, not just their disease,” insofar as success across both dimensions of communication was associated with better self-care in a variety of critical areas.
Acknowledgments
This study was supported by grants from the Department of Veterans Affairs and the Agency for Healthcare Research and Quality. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
REFERENCES
- 1.Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care. 2000;23:754–8. doi: 10.2337/diacare.23.6.754. [DOI] [PubMed] [Google Scholar]
- 2.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 3.Goldman DP, Smith JP. Can patient self-management help explain the SES health gradient? Proc Natl Acad Sci USA. 2002;99:10929–34. doi: 10.1073/pnas.162086599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasgow RE, Anderson RM. In diabetes care, moving from compliance to adherence is not enough. Something entirely different is needed. Diabetes Care. 1999;22:2090–2. doi: 10.2337/diacare.22.12.2090. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RM, Funnell MM. Compliance and adherence are dysfunctional concepts in diabetes care. Diabetes Educ. 2000;26:597–604. doi: 10.1177/014572170002600405. [DOI] [PubMed] [Google Scholar]
- 6.Olivarius NF, Beck-Nielsen H, Andreasen AH, Horder M, Pedersen PA. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ. 2001;323:946–7. doi: 10.1136/bmj.323.7319.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charman D. Burnout and diabetes: reflections from working with educators and patients. J Clin Psychol. 2000;56:607–17. doi: 10.1002/(sici)1097-4679(200005)56:5<607::aid-jclp3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Hoover JW. Patient burnout, and other reasons for noncompliance. Diabetes Educ. 1983;9:41–3. doi: 10.1177/014572178300900308. [DOI] [PubMed] [Google Scholar]
- 9.Heisler M, Bouknight RR, Hayward RA. The relative importance of physician communication, participatory decision-making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17:243–52. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMatteo MR. The physician-patient relationship: effects on the quality of health care. Clin Obstet Gynecol. 1994;37:149–61. doi: 10.1097/00003081-199403000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield S, Kaplan S, Ware JE., Jr Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102:520–8. doi: 10.7326/0003-4819-102-4-520. [DOI] [PubMed] [Google Scholar]
- 12.Sherbourne CD, Hays RD, Ordway L, DiMatteo MR, Kravitz RL. Antecedents of adherence to medical recommendations: results from the Medical Outcomes Study. J Behav Med. 1992;15:447–68. doi: 10.1007/BF00844941. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan SH, Greenfield S, Ware JE., Jr Assessing the effects of physician-patient interactions on the outcomes of chronic disease [published erratum] appears in Med Care. 1989;27:110–27. doi: 10.1097/00005650-198903001-00010. [DOI] [PubMed] [Google Scholar]
- 14.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152:1423–33. [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment. Results of a randomized controlled trial. Diabetes Care. 1995;18:943–9. doi: 10.2337/diacare.18.7.943. [DOI] [PubMed] [Google Scholar]
- 16.Roter DL, Hall JA, Aoki Y. Physician gender effects in medical communication: a meta-analytic review. JAMA. 2002;288:756–67. doi: 10.1001/jama.288.6.756. [DOI] [PubMed] [Google Scholar]
- 17.Sprague-Jones J. Gender effects in physician-patient interaction. In: Lipkin M, Putnam SM, Lazare A, editors. The Medical Interview: Clinical Care, Education, and Research. New York: Springer-Verlag; 1995. pp. 163–71. [Google Scholar]
- 18.Arnold RM, Martin SC, Parker RM. Taking care of patients—does it matter whether the physician is a woman? West J Med. 1988;149:729–33. [PMC free article] [PubMed] [Google Scholar]
- 19.Zare N, Sorenson JR, Heeren T. Sex of provider as a variable in effective genetic counseling. Soc Sci Med. 1984;19:671–5. doi: 10.1016/0277-9536(84)90238-7. [DOI] [PubMed] [Google Scholar]
- 20.Linn LS, Cope DW, Leake B. The effect of gender and training of residents on satisfaction ratings by patients. J Med Educ. 1984;59:964–6. doi: 10.1097/00001888-198412000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Love MM, Mainous AG, III, Talbert JC, Hager GL. Continuity of care and the physician-patient relationship: the importance of continuity for adult patients with asthma. J Fam Pract. 2000;49:998–1004. [PubMed] [Google Scholar]
- 22.Howie JG, Heaney DJ, Maxwell M, Walker JJ, Freeman GK, Rai H. Quality at general practice consultations: cross sectional survey. BMJ. 1999;319:738–43. doi: 10.1136/bmj.319.7212.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hjortdahl P, Laerum E. Continuity of care in general practice: effect on patient satisfaction. BMJ. 1992;304:1287–90. doi: 10.1136/bmj.304.6837.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. Milbank Q. 1999;77:305–39. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipkin M, Putnam SM, Lazare A. The Medical Interview: Clinical Care, Education, and Research. New York: Springer-Verlag; 1995. Three functions of the medical interview; pp. 3–19. In: [Google Scholar]
- 26.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 27.Arsie MP, Marchioro L, Lapolla A, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol. 2000;37:1–7. doi: 10.1007/s005920070028. [DOI] [PubMed] [Google Scholar]
- 28.Murray-Garcia JL, Selby JV, Schmittdiel J, Grumbach K, Quesenberry CP., Jr Racial and ethnic differences in a patient survey: patients' values, ratings, and reports regarding physician primary care performance in a large health maintenance organization. Med Care. 2000;38:300–10. doi: 10.1097/00005650-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Collins TC, Clark JA, Petersen LA, Kressin NR. Racial differences in how patients perceive physician communication regarding cardiac testing. Medical Care. 2002;40(1 Suppl):I27–34. doi: 10.1097/00005650-200201001-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282:583–9. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 31.Meredith LS, Siu AL. Variation and quality of self-report health data. Asians and Pacific Islanders compared with other ethnic groups. Med Care. 1995;33:1120–31. doi: 10.1097/00005650-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Harpole LH, Orav EJ, Hickey M, Posther KE, Brennan TA. Patient satisfaction in the ambulatory setting. Influence of data collection methods and sociodemographic factors. J Gen Intern Med. 1996;11:431–4. doi: 10.1007/BF02600192. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Stable EJ, Napoles-Springer A, Miromontes JM. The effects of ethnicity and language on medical outcomes of patients with hypertension and diabetes. Med Care. 1997;35:1212–9. doi: 10.1097/00005650-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Tocher TM, Larson E. The quality of diabetes care for non-English-speaking patients. West J Med. 1998;168:504–11. [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Stable EJ, Napoles-Springer A. Interpreters and communication in the clinical encounter. Am J Med. 2000;108:509–10. doi: 10.1016/s0002-9343(00)00317-x. [DOI] [PubMed] [Google Scholar]
- 36.Scott A, Smith RD. Keeping the customer satisfied: issues in the interpretation and use of patient satisfaction surveys. Int J Qual Health Care. 1994;6:353–9. doi: 10.1093/intqhc/6.4.353. [DOI] [PubMed] [Google Scholar]
- 37.Sitzia J, Wood N. Patient satisfaction: review of issues and concepts. Soc Sci Med. 1997;45:1829–43. doi: 10.1016/s0277-9536(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 38.Zemenuck JK, Hayward RA, Skarupski KA, Katz SJ. Patients' desires and expectations for medical care: a challenge to improving patient satisfaction. Am J Med Qual. 1999;14:21–7. doi: 10.1177/106286069901400104. [DOI] [PubMed] [Google Scholar]
- 39.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 40.Glasgow RE, Wilson W, McCaul KD. Regimen adherence: a problematic construct in diabetes research. Diabetes Care. 1985;8:300–1. doi: 10.2337/diacare.8.3.300. [DOI] [PubMed] [Google Scholar]
- 41.McNabb WL. Adherence in diabetes: can we define it and can we measure it? Diabetes Care. 1997;20:215–8. doi: 10.2337/diacare.20.2.215. [DOI] [PubMed] [Google Scholar]
- 42.Johnson SB. Methodologic issues in diabetes research: measuring adherence. Diabetes Care. 1992;15:1658–67. doi: 10.2337/diacare.15.11.1658. [DOI] [PubMed] [Google Scholar]
- 43.Stewart M. The validity of an interview to assess patients' drug taking. Am J Prev Med. 1987;25:63–76. [PubMed] [Google Scholar]
- 44.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]