Abstract
OBJECTIVE
To determine the impact that upper respiratory tract infections have on patients’ physical, social, and emotional functioning, we measured the health-related quality of life (HRQL) of adults with upper respiratory tract infections.
SETTING
Acute care clinic from November 2001 to February 2002.
DESIGN
Prospectively administered survey. To measure HRQL, we used the Acute Form of the Short Form-36, version 2 (SF-36). For all 8 SF-36 subscales, we used norm-based scoring, in which the general U.S. population has a mean of 50.
PATIENTS
Adults who had symptoms for fewer than 30 days completed the SF-36; and were diagnosed with nonspecific upper respiratory infection, viral syndrome, otitis media, sinusitis, nonstreptococcal pharyngitis, streptococcal pharyngitis, or acute bronchitis.
MEASUREMENTS and MAIN RESULTS
The sample of 318 patients was 63% female, 81% white, and had a mean age of 35 years. The primary diagnoses were nonspecific upper respiratory infection (42%), acute bronchitis (16%), sinusitis (12%), viral syndrome (9%), nonstreptococcal pharyngitis (8%), otitis media (7%), and streptococcal pharyngitis (6%). Patients had a mean general health subscale score of 50.9, which is not significantly different from the mean population value of 50 (P = .09). However, there were significant decrements in the remaining 7 subscales of the SF-36: physical functioning (45.5), role-physical (38.5), bodily pain (42.6), vitality (40.8), social functioning (37.8), role-emotional (46.8), and mental health (46.8; P < .0001 for all 7 subscales compared with normative values). Results were similar for the subset of patients with no comorbid illnesses (P < .001 for the same 7 subscales) and patients diagnosed with nonspecific upper respiratory infection (P < .001 for the same 7 subscales). These decrements were similar in magnitude, but somewhat different in subscale pattern, to those of adults with chronic lung disease, osteoarthritis, and depression.
CONCLUSIONS
Physicians should remember that adults who seek care for upper respiratory tract infections have measurable, significant decrements in HRQL. For researchers, HRQL is an attractive, potential measure of outcome in future trials of established and novel therapies for upper respiratory tract infections.
Keywords: quality of life, health status, respiratory infections, common cold
Upper respiratory tract infections are the most common symptomatic reason for seeking ambulatory care in the United States.1 Despite this, physicians feel that many, if not most, patients with upper respiratory tract infections seek care unnecessarily.2 However, virtually everyone has had to curtail his or her activities at some time due to an upper respiratory tract infection. In clinic, physicians may fail to recognize the significant impact that upper respiratory tract infections have on patients.
Similarly, in assessing the efficacy of therapy for upper respiratory tract infections, researchers have generally not examined the impact of upper respiratory tract infections. Rather, researchers have focused on upper respiratory tract symptoms.3–6 A broader, potentially more useful measure of outcome is health-related quality of life (HRQL).
Health-related quality of life is the component of overall quality of life that is determined primarily by a person's health and that can be influenced by clinical interventions.7 Health-related quality of life is self-determined and is comprised of health status, functional status, and overall quality of life.8 Included within these broad categories are physical health, psychological health, physical functioning, social functioning, role functioning, and general well-being.9,10
Health-related quality of life instruments have several advantages over symptom scores. First, HRQL instruments translate symptoms into broader concerns that are important to patients. Second, HRQL instruments measure the impact of symptoms, regardless of what specific symptoms are present. Third, generic—as opposed to disease-specific—HRQL instruments allow comparison across diagnoses, whereas symptom scores are generally limited to a single target condition.
Several studies have assessed aspects of HRQL for patients with a variety of acute upper respiratory tract infections.11–18 However, to our knowledge, no prior study has measured HRQL of adults with upper respiratory tract infections using a well-validated, generic HRQL instrument that would allow comparisons with the general population or to patients with other medical conditions. We used the SF-36 to measure the HRQL of adults with upper respiratory tract infections presenting to an acute care clinic during the winter of 2001–02.
METHODS
Patients, Setting, and Survey
The Institutional Review Board of Massachusetts General Hospital approved the study protocol. Data for this analysis were collected as part of a larger study of antibiotic prescribing. The study was conducted from November 27, 2001 to February 28, 2002 in the Massachusetts General Hospital Medical Walk-In Unit (Boston, Mass). The Medical Walk-In Unit is an adult acute care clinic, open 7 days a week, where most patients are seen on a first-come, first-served basis.
At check-in, the clinic secretary asked patients about the presence of respiratory symptoms such as runny nose, congestion, ear pain, cough, sore throat, or any other symptom that would make a patient think they might have a cold, the flu, bronchitis, sinusitis, or pneumonia. If the patient had respiratory symptoms, the secretary offered the patient a survey to complete in the waiting room. Patients could also receive a survey by responding to flyers posted in the waiting room or by being asked by the triage nurses. Patients filled out the survey and returned them to a lockbox in the waiting room prior to their visit with the physician.
Patients filled out the Acute Form (1-week recall) of the Medical Outcomes Trust Short Form-36, version 2. We obtained permission to use and score the SF-36, which is copyrighted (2000), from QualityMetric Incorporated.19 Patients also answered questions about demographics and their health history.
Data and Statistical Analyses
For the present analysis, we included patients who had a physician-diagnosed upper respiratory tract infection. Because we wanted patients to respond to questions about health status prior to their visit with the doctor, we anticipated a need to exclude patients without a primary diagnosis of a common upper respiratory infection. We included diagnoses for several common upper respiratory tract infections because the specific diagnosis may be more related to treatment (e.g., use of antibiotics) than the actual symptom complex.
Included diagnoses were nonspecific upper respiratory infection, viral syndrome, influenza, otitis media, sinusitis, nonstreptococcal pharyngitis, streptococcal pharyngitis, tonsillitis, infectious mononucleosis, acute bronchitis, and acute cough. Patients with influenza (3 patients) and infectious mononucleosis (1 patient) were included in the viral syndrome category; patients with cough (10 patients) were included in the acute bronchitis category. We excluded patients who reported symptoms for more than 30 days.
We also excluded patients who answered fewer than half the questions in any SF-36 subscale. Otherwise, as recommended by the Medical Outcomes Trust, missing values were entered as the average of the remaining completed questions in a subscale.19
Results from the SF-36 were reported using 8 subscales. The physical functioning subscale measures the ability of patients to perform a variety of tasks, from vigorous (such as running) to basic (such as bathing or dressing oneself). The role-physical subscale measures limitations in work or other activities resulting from physical problems. The bodily pain subscale measures patients’ degree of pain and limitations in activity due to pain. The general health subscale measures patients’ self-evaluation of overall health. The vitality subscale measures the presence or absence of pep and energy. The social functioning subscale measures the degree to which physical or emotional problems interfere with social activities. The role-emotional subscale measures limitations in work or other activity limitations due to emotional problems. The mental health subscale measures feelings of nervousness or depression.
In the Acute Form of the SF-36, questions that comprise the general health subscale are not prefaced with a time limitation. Questions that comprise the physical functioning subscale ask about the ability to perform a range of activities currently. Questions that make up the remaining 6 subscales ask patients to consider the previous 1 week. In addition to the 8 subscales, patients answer a health transition item about how their health, in general, has changed over the previous week.
To score the SF-36, we used norm-based scoring, in which the mean for the general U.S. population is 50, with a standard deviation of 10.19 The scale has no units and scores can theoretically range from 0 to 100. Higher scores indicate better health. Cronbach's α, a measure of internal consistency, for this patient population ranged from 0.79 to 0.95 across the 8 subscales of the SF-36.20
We used Student's t test to compare the subscale scores from our sample with normative values based on a sample of 7,581 adults from the 1998 National Survey of Functional Health Status.19 To examine whether results were caused by comorbid illnesses, we compared scores from the subset of patients who reported no comorbid illnesses with normative values. We also hypothesized that patients with nonspecific upper respiratory tract infection may have better HRQL compared with patients with other diagnoses in this study. We compared scores from the subset of patients who were diagnosed with nonspecific upper respiratory tract infections with normative values.
We also compared subscale scores from our sample with scores from adults with self-reported chronic lung disease, osteoarthritis, and depression drawn from the general U.S. population.19 Patients with chronic lung disease, osteoarthritis, and depression were also evaluated with the Acute Form (1-week recall) of the SF-36. The number of patients assessed with these chronic conditions varied by condition and across subscales: lung disease, 328–337 patients; osteoarthritis, 998–1,009 patients; and depression, 923–940 patients.
All analyses were carried out using SAS 8.1 (SAS Institute, Cary, NC). P values were two-tailed and P values ≤ .05 were considered significant.
RESULTS
Characteristics of the Patients
During the study period, 421 surveys were returned, representing approximately 7% of all patients presenting to the clinic for any reason. Of 421 patients who returned the surveys, 318 met our inclusion and exclusion criteria (Fig. 1). For 9 patients, there was no primary diagnosis listed and 63 patients were excluded for having a primary diagnosis that was not an upper respiratory tract infection. The most commonly excluded diagnoses were pneumonia (8 visits), asthma, (3 visits) urinary tract infection, (3 visits) conjunctivitis, (2 visits) hypertension, (2 visits) otitis externa, (2 visits) and tympanic membrane perforation. (2 visits)
FIGURE 1.
Exclusion and inclusion of patients. SF-36, Short Form-36.
There was incomplete SF-36 data for 21 patients. There were no identifiable differences between patients who did and did not have complete SF-36 data in age, sex, race, education, insurance status, income, employment, having a primary care doctor, heart disease, lung disease, diabetes, cancer, smoking, duration of symptoms, missing work, or overall symptom bother (data not shown).
The sample of patients who met our inclusion and exclusion criteria had a mean age of 35 and was 63% women (Table 1). Patients generally did not take daily medicines or report comorbid illnesses: 7% reported heart disease and 11% reported lung disease. The mean duration of symptoms was 8 days (median 6; interquartile range, 4 to 10 days). The primary diagnoses were nonspecific upper respiratory infection (42%), acute bronchitis (16%), sinusitis (12%), viral syndrome (9%), nonstreptococcal pharyngitis (8%), otitis media (7%), and streptococcal pharyngitis (6%).
Table 1.
Characteristics of 318 Adults with Upper Respiratory Tract Infection
| Characteristic | N* | Value (%) |
|---|---|---|
| Age, mean in years (SD; range) | 318 | 35 (12; 18 to 73) |
| Female | 318 | 199 (63) |
| Race/ethnicity | 307 | |
| White | 249 (81) | |
| Black | 20 (7) | |
| Latino | 14 (5) | |
| Asian | 18 (6) | |
| Other | 6 (2) | |
| Some college | 306 | 286 (93) |
| Insurance | 318 | |
| Private | 220 (69) | |
| Medicare | 17 (5) | |
| Medicaid | 15 (5) | |
| Other | 66 (20) | |
| Household income | 295 | |
| Less than $50,000 | 139 (47) | |
| More than $50, 000 | 156 (53) | |
| Employed | 306 | 256 (83) |
| Primary care doctor | 305 | 220 (72) |
| Comorbidities | ||
| Diseases of heart | 306 | 20 (7) |
| Diseases of lung | 306 | 33 (11) |
| Diabetes | 303 | 3 (1) |
| Cancer† | 305 | 12 (4) |
| Number of daily medicines, median (interquartile range) | 298 | 0 (0, 1) |
| Smoking status | 299 | |
| Current | 32 (11) | |
| Former | 73 (24) | |
| Never | 194 (64) |
Number may be less than 318 because of nonresponse.
Excludes skin cancer.
Health-Related Quality of Life
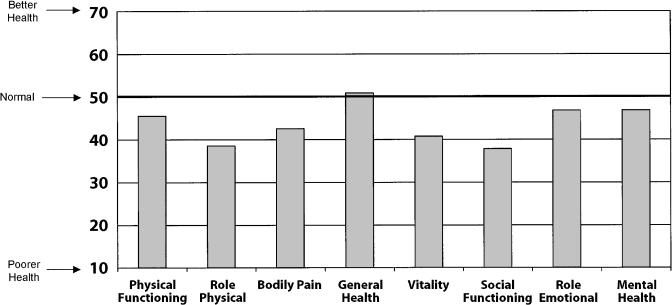
Adults with upper respiratory tract infections had no significant decrement in general health compared with normative values. The mean score for the general health subscale was 50.9 (SD ± 8.8; P = .09 compared with normative values; Fig. 2). However, there were significant decrements compared with normative values in the remaining 7 subscales of the SF-36: physical functioning (45.5, SD ± 10.6), role-physical (38.5, SD ± 10.9), bodily pain (42.6, SD ± 9.0), vitality (40.8, SD ± 10.4), social functioning (37.8, SD ± 11.9), role-emotional (46.8, SD ± 12.3), and mental health (46.8, SD ± 10.0; P < .0001 for all 7 subscales compared with normative values).
FIGURE 2.
Health status of 318 adults with upper respiratory tract infections *P < .0001 for comparison between scores for upper respiratory tract patients and normative scores for all subscales except for general health.
In rating how their general health had changed over the previous week, 8% of patients felt it was much better, 18% said it was somewhat better, 33% said it was about the same, 22% said it was somewhat worse, and 19% said it was much worse.
For the subset of 258 patients who reported no comorbid conditions, the general health subscale was significantly higher than normative values (mean 52.0, SD ± 8.3; P < .001). There were significant decrements in the remaining 7 subscales compared with normative values: physical functioning (46.2, SD ± 9.8), role-physical (39.1, SD ± 10.8), bodily pain (43.1, SD ± 8.9), vitality (41.0, SD ± 10.5), social functioning (38.2, SD ± 11.8), role-emotional (44.4, SD ± 11.9), and mental health (47.2, SD ± 9.9; P < .001 for all 7 subscales compared with normative values).
For the subset of 135 patients diagnosed with nonspecific upper respiratory infection, there was no significant difference between the mean of the general health subscale and normative values (mean 51.1, SD ± 8.7; P = .16). There were significant decrements in the remaining 7 subscales compared with normative values: physical functioning (46.2, SD ± 9.8), role-physical (39.4, SD ± 10.5), bodily pain (42.9, SD ± 9.0), vitality (40.0, SD ± 10.8), social functioning (37.8, SD ± 12.3), role-emotional (46.3, SD ± 12.2), and mental health (46.5, SD ± 11.0; P < .001 for all 7 subscales compared with normative values).
Comparison with Other Conditions
Comparison of the present sample with adults with self-reported lung disease, osteoarthritis, and depression showed a similar magnitude of decrement in many SF-36 subscales (Fig. 3). However, there were some significant differences. Adults with lung disease, osteoarthritis, and depression had significantly lower scores in the general health and role=emotional subscales than patients with upper respiratory tract infections. Adults with depression had significantly lower mental health subscale scores than patients with upper respiratory tract infections. Adults with osteoarthritis had significantly lower scores on the bodily pain subscale than patients with upper respiratory tract infections.
FIGURE 3.
Comparison of Short Form-36 results between patients with upper respiratory tract infections and adults with self-reported lung disease, osteoarthritis, and depression. URI, upper respiratory tract infection (n = 318). *P < .001 and †P < .05 for comparison with URI.
DISCUSSION
We found that this sample of adults seeking care for upper respiratory tract infections had normal general health, but had significant decrements in 7 other domains of HRQL captured by the SF-36. We found similar significant decrements in patients without comorbid illnesses and in patients diagnosed with nonspecific upper respiratory tract infections. To our knowledge, no study has previously measured the HRQL of patients with upper respiratory tract infections using such a well-validated, generic HRQL instrument.
The pattern we found is consistent with generally healthy adults who had a recent, acute change in HRQL. The questions that comprise the general health subscale are not prefaced with a time constraint in the Acute Form of the SF-36. In contrast, questions that make up the physical functioning subscale ask respondents to consider current limitations in activities. Questions that comprise the remaining 6 subscales have respondents consider the previous week.
These acute decrements are of a magnitude comparable to those of adults with other chronic medical conditions. For example, patients with upper respiratory tract infections have role-physical and bodily pain subscale scores that are similar to those of adults with chronic lung disease. Patients with upper respiratory tract infections have vitality and social functioning subscale scores that are similar to those of adults with depression.
Despite these similarities, there are important differences between the scores of patients with upper respiratory tract infections and adults with other chronic medical conditions. First, decrements in HRQL are presumably transient in patients with upper respiratory tract infections. Second, patterns differ according to illness. For example, adults with osteoarthritis, a condition characterized by pain, have more bodily pain than patients with upper respiratory tract infections. Adults with depression have significantly lower mental health subscale scores than patients with upper respiratory tract infections. Such differences support the validity of the SF-36 for crosscondition comparisons.
Certain aspects of this study should have biased our results toward a null finding. First, the mean age of patients in this study was 35 years. Because the mean age of patients who comprised the normative sample was 51 years old,19 we would have expected our sample to have had scores greater than 50, the national mean. Second, ours was a nonconsecutive sample. We hypothesized, and anecdotally noted, that patients who chose not to participate in the study declined to participate because they did not feel well enough. As such, we measured HRQL in a group of patients who may have been “less sick” than the full population of patients who presented to our clinic with upper respiratory tract infections.
Our conclusions should be considered in light of the limitations of this study. First, this study was performed at a single, urban, academic, acute care clinic. Second, patients who choose to come to an acute care clinic for upper respiratory tract infections may be different from patients who choose not to seek medical care. However, those who seek care will naturally be the population of interest in studies assessing the efficacy of treatment for upper respiratory tract infections. Third, the study was cross-sectional. Future research is needed to take longitudinal measurements of patients with upper respiratory tract infections to ensure that HRQL returns to near normative values over time. Studies are needed to show that HRQL measures are valid, reliable, and responsive to change for use with upper respiratory tract infections.21
Validated, reliable, and responsive measures of HRQL could add valuable outcome information to symptom scores that have been generally used in studies of upper respiratory tract infections.3–6 Simple symptom scores can potentially give misleading results because they are too narrowly focused. For example, if symptoms improve, but not to the point where a patient is able to return to work or if more general symptoms persist (e.g., fatigue), a symptom score would show improvement where an HRQL measure would not. Similarly, if a patient's symptoms resolve, but the patient is unable to return to work for a new reason (e.g., adverse effect of treatment), again, a symptom score would show improvement where an HRQL measure would not.
For now, physicians should remember that adults who seek care for upper respiratory tract infections have significant, measurable decrements in HRQL. Physicians should provide relief in the form of analgesics, antipyretics, antitussives, decongestants, and β-agonists when appropriate to reduce symptoms and potentially improve HRQL.
REFERENCES
- 1.Cherry DK, Burt CW, Woodwell DA. National Ambulatory Medical Care Survey: 1999 Summary Advance Data from Vital and Health Statistics No 322. Hyattsville, Md: National Center for Health Statistics; 2001. [Google Scholar]
- 2.Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ. 1998;317:637–42. doi: 10.1136/bmj.317.7159.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gwaltney JM, Jr, Park J, Paul RA, Edelman DA, O'Connor RR, Turner RB. Randomized controlled trial of clemastine fumarate for treatment of experimental rhinovirus colds. Clin Infect Dis. 1996;22:656–62. doi: 10.1093/clinids/22.4.656. [DOI] [PubMed] [Google Scholar]
- 4.Macknin ML. Zinc gluconate lozenges for treating the common cold in children: a randomized controlled trial. JAMA. 1998;279:1962–7. doi: 10.1001/jama.279.24.1962. [DOI] [PubMed] [Google Scholar]
- 5.Turner RB, Wecker MT, Pohl G, et al. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection: a randomized clinical trial. JAMA. 1999;281:1797–804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 6.Gwaltney JM, Jr, Winther B, Patrie JT, Hendley JO. Combined antiviral-antimediator treatment for the common cold. J Infect Dis. 2002;186:147–54. doi: 10.1086/341455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juniper EF. Quality of life in adults and children with asthma and rhinitis. Allergy. 1997;52:971–7. doi: 10.1111/j.1398-9995.1997.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 8.Curtis JR, Martin DP, Martin TR. Patient-assessed health outcomes in chronic lung disease: what are they, how do they help us, and where do we go from here? Am J Respir Crit Care Med. 1997;156(4 Part 1):1032–9. doi: 10.1164/ajrccm.156.4.97-02011. [DOI] [PubMed] [Google Scholar]
- 9.Spilker B, Revicki DA. Taxonomy of quality of life. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd edn. Philadelphia, PA: Lippincott-Raven;; 1996. pp. 25–31. [Google Scholar]
- 10.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 11.Evans AT, Husain S, Durairaj L, Sadowski LS, Charles-Damte M, Wang Y. Azithromycin for acute bronchitis: a randomised, double-blind, controlled trial. Lancet. 2002;359:1648–54. doi: 10.1016/S0140-6736(02)08597-5. [DOI] [PubMed] [Google Scholar]
- 12.Barrett B, Locken K, Maberry R, et al. The Wisconsin Upper Respiratory Symptom Survey (WURSS): a new research instrument for assessing the common cold. J Family Prac. 2002;51:265. [PubMed] [Google Scholar]
- 13.French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of a cough-specific quality-of-life questionnaire. Chest. 2002;121:1123–31. doi: 10.1378/chest.121.4.1123. [DOI] [PubMed] [Google Scholar]
- 14.Aoki FY, Fleming DM, Griffin AD, Lacey LA, Edmundson S. Impact of zanamivir treatment on productivity, health status and healthcare resource use in patients with influenza. Zanamivir Study Group. Pharmacoeconomics. 2000;17:187–95. doi: 10.2165/00019053-200017020-00007. [DOI] [PubMed] [Google Scholar]
- 15.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 16.Verheij T, Hermans J, Kaptein A, Mulder J. Acute bronchitis: course of symptoms and restrictions in patients’ daily activities. Scand J Primary Health Care. 1995;13:8–12. doi: 10.3109/02813439508996728. [DOI] [PubMed] [Google Scholar]
- 17.Witsell DL, Dolor RJ, Bolte JM, Stinnett SS. Exploring health-related quality of life in patients with diseases of the ear, nose, and throat: a multicenter observational study. Otolaryngol Head Neck Surg. 2001;125:288–98. doi: 10.1067/mhn.2001.118693. [DOI] [PubMed] [Google Scholar]
- 18.Meyboom-de Jong BM, Smith RJ. How do we classify functional status? Family Med. 1992;24:128–33. [PubMed] [Google Scholar]
- 19.Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. 3rd edn. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 20.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 21.Lohr KN, Aaronson NK, Alonso J, et al. Scientific Advisory Committee Instrument Review Criteria. Boston, MA: Medical Outcomes Trust; 1997. [Google Scholar]