Abstract
Screening and treatment rates for dyslipidemia in populations at high risk for cardiovascular disease (CVD) are inappropriately low and rates among women may be lower than among men. We conducted a review of the literature for possible explanations of these observed gender differences and categorized the evidence in terms of a conceptual model that we describe. Factors related to physicians’ attitudes and knowledge, the patient's priorities and characteristics, and the health care systems in which they interact are all likely to play important roles in determining screening rates, but are not well understood. Research and interventions that simultaneously consider the influence of patient, clinician, and health system factors, and particularly research that focuses on modifiable mechanisms, will help us understand the causes of the observed gender differences and lead to improvements in cholesterol screening and management in high-risk women. For example, patient and physician preferences for lipid and other CVD risk factor management have not been well studied, particularly in relation to other gender-specific screening issues, costs of therapy, and by degree of CVD risk; better understanding of how available health plan benefits interact with these preferences could lead to structural changes in benefits that might improve screening and treatment.
Keywords: cardiac, disparity, outpatient, cholesterol, women
SUBOPTIMAL MANAGEMENT OF DYSLIPIDEMIA IN WOMEN
Cardiovascular disease (CVD) is the leading cause of death and morbidity among women; approximately 512,904 women and 455,871 men died from CVD in 1999.1 Women's cholesterol profile can be modified with statin therapy and the incidence of coronary events reduced.2–6 However, screening and treatment rates for CVD risk factors in high-risk populations may be inappropriately low, particularly in the outpatient setting.7–9
In order to identify articles that would distinguish screening and treatment of women apart from men, we searched medline for publications fitting these criteria: English language, human subjects, adults 19 years and older, and publication date during or after 1996 to allow dissemination and implementation of the ATPII guidelines released in 1993. We added the terms (gender OR women) AND (therapy OR measurement OR treatment OR management) AND (coronary OR cardiovascular OR cardiac) AND (cholesterol OR lipid OR dyslipidemia OR hypercholesterolemia), which resulted in 4019 articles; to further focus the search on management of cholesterol as opposed to pure epidemiological or mechanistic studies, we added the search term (undertreatment OR compliance OR utilization OR adherence OR bias OR disparity OR guidelines), which resulted in 541 articles. We included articles that focused on secondary prevention and outpatient management, and reported gender comparisons in screening and treatment. We excluded articles that did not have original data and did not focus on cholesterol management, resulting in 520 articles. Finally, we reviewed the reference lists of these articles and included articles that were missed in the original search (n = 3). We abstracted the screening, therapy, and successful treatment percentages for men and women and P values or confidence intervals when available.
The majority of studies that have examined screening and treatment for dyslipidemia did not report analyses by gender or the degree of disparity between men and women. The results are in Table 1. Multiple studies examining people with CVD indicate that men have cholesterol measured more often, treated more aggressively, and have lower low-density lipoprotein levels than women.7,10–14
Table 1.
Studies That Reported Results by Gender in Screening or Treatment for Dyslipidemia in Patients with Cardiovascular Disease
| Data Source | Results | Extent of Disparity |
|---|---|---|
| Randomized trial56 | Women less likely to receive lipid treatment and to be at goal than men | 35% vs 55% (P < .05); 29% vs 48% (P = .001) |
| Registry59 | Women less likely to have lipid levels measured than men | Not given |
| Registry49 | Women less likely to receive lipid treatment and to be at goal than men | 4.4% vs 4.5%; 9.5% vs 16.1% (P value not given) |
| Registry12,13 | Women less likely to be at treatment goal than men | P < .001 (percents not given) |
| Registry126 | Women less likely to receive statins than men | 8.2% vs 13.3% (P value not given) |
| Pharmacy database127 | Women less likely to receive statins than men | 13.5% vs 20.8% (P < .05) |
| Patient survey60 | Women less likely to receive lipid treatment and to be at goal than men | 29.8% vs 39.6%; 20.3% vs 21.9% (P value not given) |
| Electronic medical record54 | Women less likely to be screened/treated than men | 24.3% vs 37.3% (P < .0001) |
| Chart review48 | Women less likely to be treated than men | 33% vs 48% (P = .047) |
| Chart review95 | Women less likely to be at lipid goal than men | P < .05 (percents not given) |
| Chart review128 | Women less likely to be screened, treated, and at goal than men | 35% vs 50%; 21% vs 31%; 23% vs 33% (P < .0001) |
| Chart review97 | Women less likely to be screened and at lipid goal | 51% vs 68% (P = .001); 25% vs 34% (P = .043) |
| Randomized trial58 | No gender difference in screening or treatment | |
| Patient survey129 | No gender differences in screening or treatment | |
| Chart review57 | No gender differences in treatment | |
| Chart review130 | No gender difference in screening or treatment | |
| Pharmacy database131 | No gender difference in treatment | |
| Pharmacy database132 | No gender difference in treatment | |
| Pharmacy database133 | No gender difference in treatment | |
| Patient survey80 | Women more likely to be treated | 64% vs 81% (P < .05) |
The reasons for gender differences in management of dyslipidemia in high-risk groups are unclear. In patients with CVD and CVD risk equivalents such as diabetes, cholesterol measurement and treatment goals are identical for men and women: measurement of low-density lipoprotein cholesterol (LDL-C) and a treatment goal of less than 100 mg/dL is recommended.15 It is possible that suboptimal management of dyslipidemia in women occurs through the same mechanisms that cause gender disparities in referrals for CVD diagnostic and therapeutic procedures. Although the literature documenting such disparities is extensive, again the mechanisms remain virtually unexplained, and efforts to explain them have tended to focus on clinician factors.16–23 Elucidating the multiple factors that contribute to gender disparities may help structure quality improvement interventions for both men and women.
MODEL
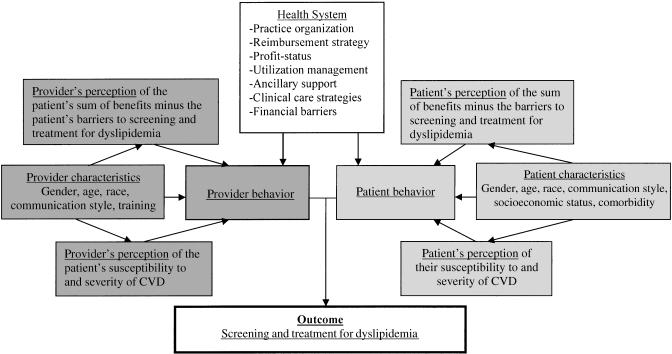
Patient, clinician, and health system factors may all contribute to suboptimal management of dyslipidemia. Drawing from the Health Belief model,24 the Landon et al. health care organization model,25 and the Jaen et al. competing demands model,26 we have developed a conceptual model (Fig. 1) for how these factors interact. A theoretical model should have face validity, provide measurable variables, and enhance understanding beyond what would be expected from consideration of individual factors affecting preventive service delivery. In our conceptual model, we have incorporated the concept of patient perception of risk and subsequent behavior from the Health Belief model; the association between detailed structural characteristics of health systems and physician behavior from Landon's model; and the role of physician characteristics and the idea of competing interests from the Jaen et al. model.
FIGURE 1.
Conceptual model of patient, clinician, and health system factors affecting management of cholesterol in high-risk patients.
The Health Belief model argues that health behaviors are related to personal beliefs about susceptibility to disease, seriousness of disease, benefit of intervention, and risk of intervention. In this model, individuals who do not believe they are at high risk of disease are unlikely to pursue preventive health behavior even if the benefits of the behavior are high and the risks are low, and individuals who believe they are at high risk of disease may pursue preventive health behavior even if the benefits are low and the risks are high.24 This model has proven useful in understanding and predicting many preventive health behaviors, including diet and exercise. However, the model is not as useful in examining the barriers to acting on such beliefs, i.e., barriers related to the structural organization of the health care system and barriers related to specific aspects of the health care visit, such as limited time. In the Health Belief model, the barriers are limited primarily to the patient's perceived barriers to behavior change.
In contrast, the Landon et al. model of health care organization focuses on characteristics of the health care system that can influence health care delivery.25 In the Landon et al. model, disease processes and outcomes can be influenced by financial incentives, management strategies such as utilization review, structure of care such as the location of the practice site and staffing patterns, and finally normative influences such as the culture of the organization. The strengths of this model are that it details health plan and provider group characteristics that are probably influential but have not been the focus of extensive research. Such a model is extremely useful in conceptualizing changes to health care organizations in order to improve care, but does not necessarily address the patient's and provider's perceptions of risk or barriers nor their interaction with the health system.
Finally, the Jaen et al. health care model is posited on a theory of competing interests.26 In the Jaen et al. model, the patient, the physician, and the practice environment are separate domains that interact during the health care visit. The model emphasizes the physician's role in delivering preventive services, specifically, physician's skills and attitudes. It also puts forth the idea of competing or alternative demands for the physician's time as a physician barrier. This model is extremely useful for illustrating the physician's perceptions of barriers to provision of health services and is also valuable in that it empathizes with the clinician and pinpoints a potentially reversible barrier rather than placing blame on the individual clinician's character. As a result of research showing that physician-level variation is small compared with patient and health system variation, we believe that the physician's behavior is more heavily influenced by the environment of the health system, e.g., variable such as “lack of time” may be more of a health system characteristic than a physician-level characteristic, and that women's agendas for screening play a more important role.
Our model postulates that perceptions of the risks and barriers to screening and treatment of CVD risk factors will affect the clinician's behavior and the patient's behavior during the health care visit. We further hypothesize that these perceptions can be partially predicted from patient characteristics such as gender. In addition, the health system structure affects screening and treatment of CVD risk factors by affecting clinician behavior and patient behavior. We use this model in framing the following review of patient, clinician, and health system variables that may contribute to gender differences in management and will refer back to it throughout the paper. Although conceptualized for CVD risk factor management, this model may easily be applied to understand gender differences in the management of other diseases as well.
POSSIBLE PATIENT FACTORS
Multiple studies suggest that both mutable and immutable patient-related factors, such as access to care, competing health issues and prioritization of these issues, communication and decision-making preferences, demographics (e.g., race and age), and disease severity, may play a role in gender disparities for hyperlipidemia treatment (Table 2).
Table 2.
Possible Mechanisms for Gender Disparities in Patients with Cardiovascular Disease
| References | |
|---|---|
| Possible patient factors | |
| Decreased access to care among uninsured women | 28,29 |
| Cost barriers greater in women | 27,34 |
| Lower socioeconomic status* | 14,35 |
| Women's prioritization of cholesterol below gender-specific screening* | 36,37 |
| Women's perception of CVD risk compared with risk of other diseases* | 38 |
| Women's different communication and decision-making preferences | 41,42,44 |
| Women's increased age* | 48,132 |
| African-American race | 8,50 |
| Increased comorbidity | 1,56 |
| Women's lower rate of revascularization | 48,58 |
| Substitution of hormone replacement therapy for lipid-specific therapy* | 61 |
| Possible provider factors | |
| Perception of CVD risk inappropriately low; treatment threshold in women inappropriately high* | 49,60,77,78 |
| Perception of CVD risk higher for revascularized patients, but women undergo revascularization less | 22,58 |
| Cardiologists specialty protective, but women may see them less frequently* | 80,81,84,85 |
| Providers prioritize cholesterol management below other gender-specific screening* | 87 |
| Providers overestimate the amount of care they provide for women more than men | 108 |
| Women physician's different communication and decision-making preferences | 43 |
| Younger physicians with more recent training more likely to enforce prevention, less likely to see women | 93,94 |
| Possible system factors | |
| Case-management programs and lipid clinics may reduce gender disparities* | 97,99 |
| Cardiac rehabilitation programs underused by women* | 100,134 |
| Measurement factors | 107 |
| Other health system factors such as profit status, model type, referral management? | – |
This factor has already been demonstrated to differ between men and women with cardiovascular disease (CVD) for dyslipidemia screening, treatment, or goals.
Access to Care
Management of dyslipidemia depends on adequate access to clinicians, and it is possible that women in certain high-risk groups have decreased access compared with men. Women,27 particularly uninsured women,28 cite greater cost barriers for access to care than men, which in turn are associated with decreased preventive services such as cholesterol screening. In addition, the quality of this care may be affected by cost barriers. Quality care is characterized by high continuity, reasonable availability, and good clinician communication and predicts use of preventive services in women29 independent of a regular source of care and insurance status.30,31 Although these analyses did not stratify by coronary risk, they did focus on a middle-aged population for examination of cholesterol screening.
Cost Barriers
Women who experience cost barriers for clinician access may also experience cost barriers for laboratory testing and medication copayment, and decreased copayment has been associated with improved performance of preventive interventions.32 These cost barriers may have been partially alleviated by Medicare or Medicaid. Among Medicare beneficiaries, women with known coronary disease may be more likely to be on statin therapy than men,33 although in another analysis women reported greater difficulty than men in obtaining medical care and prescribed medications, and women have reported delaying care owing to cost and dissatisfaction with the ease of getting to a physician more often than men.27 Medicaid patients had less frequent cholesterol screening than those with private insurance, but Medicaid patients did not cite specific barriers as a result of Medicaid, suggesting that the poorer Medicaid population also faced cost barriers other than their insurance status.34 Not surprisingly, the few studies examining gender, socioeconomic status, and cholesterol levels in the United States suggest that lower socioeconomic status is associated with adverse lipid levels to a greater extent in women than in men.14,35
Competing Health Issues and Communication/Decision-Making Styles
Direct observation of primary care visits has demonstrated that women receive gender-specific screening, specifically mammography, more often than cholesterol screening.36,37 Since these studies have not examined women at high risk for CVD separately, it is possible that high-risk women are managed differently than low-risk women. However, across all age groups and ethnicities, women are more concerned about breast cancer than coronary disease,38 which might lead to their initiating discussion of this topic. Since the issues addressed in the outpatient visit are largely driven by patient concerns and needs,39,40 and the length of the visit is fixed, this competing concern could decrease the attention paid to cholesterol management.26 Time constraints might be further compounded by women's different communication and decision-making style,41,42 which tends to be associated with longer office visits.43,44
Demographics
Differences in lipid management between men and women may partially reflect a disparity in other factors, such as age. Multiple studies have documented that elderly patients are not treated as aggressively as younger patients after a myocardial infarction45–47 despite recommendations for management of dyslipidemia extend into older age groups.15 However, age does not explain the entire disparity, as gender discrepancies in lipid management persist in studies of older patients.48,49 Similarly, a greater proportion of women with dyslipidemia are from minority populations, and gender differences may represent racial differences to some extent.50,51 However, managed care registries have documented gender differences in lipid management that persist after adjustment for race; gender differences may be greater in minority populations than in white populations.52–54 Also, difficulties with access to care tend to be greater in African-American women compared with African-American men, and white women compared with white men.27 Examination of U.S. population-based data indicates that gender, race, and socioeconomic status probably exert independent effects on lipid levels.14,35,55
Disease Severity
Since women with CVD tend to have a greater number of comorbid conditions than men with CVD,1 comorbidity may partially explain differences in cholesterol management.56 Despite the effectiveness of therapy in women overall,5 in actual practice sicker patients may be prescribed therapy less often or be able to comply with therapy less often than healthier patients. Given the excellent clinical risk models that currently exist for CVD, adjustment for disease severity differences is possible, but may require information that is not always available, and therefore it is not always performed.
The effect of comorbidity upon cholesterol management is complex and probably goes beyond the number of comorbid conditions. Analyses of dyslipidemic patients admitted for myocardial infarction suggest that patients with no comorbidity or severe comorbidity may be treated less often than patients with moderate comorbidity.57 In addition, specific comorbid conditions such as hypertension may increase awareness of CVD. After myocardial infarction, patients with hypertension tend to be treated more often for hypercholesterolemia than patients without hypertension,33 despite evidence of treatment for cholesterol-lowering therapy regardless of blood pressure status in this group of patients. A similar association between presence of hypertension and cholesterol screening exists in a broader population,30 suggesting that these 2 CVD risk factors are probably linked cognitively or in a more systematic fashion, i.e., through prompts or guidelines.
Similarly, procedures such as revascularization increase the likelihood of appropriate lipid therapy.48,58 This could reflect increased recognition by clinicians of CVD status, better underlying health of patients who underwent revascularization compared with those who did not, the increased likeliness of these patients to comply with therapy, or other factors associated with revascularization. Of note, women veterans with CVD were still less likely than male veterans with CVD to have their cholesterol measured even after adjustment for age, coronary procedures, angina, and other CVD risk factors.59
Other Confounders
Other confounders for gender probably exist. In 2 studies that also adjusted for other CVD risk factors, body mass index, years of education, current employment, and cardiology visit, female gender no longer predicted underuse of lipid therapy in women with CVD.33,60 In addition, women may be treated less often for hypercholesterolemia than men because of the substitution of hormone replacement therapy (HRT) for specific lipid-lowering therapy, since HRT is associated with lowered LDL-C levels.61 Unfortunately, hormone replacement in and of itself cannot lower LDL-C to goal in women with established coronary disease,62 and previously held beliefs about the cardiovascular indications for HRT have been discredited.63,64 Finally, women tend to be affected by disorders that are not always included in risk-adjustment models but that nonetheless may affect management of CVD risk factors. Specifically, women suffer from higher rates of disability,65 obesity,66 and anxiety and affective disorders67 that have been demonstrated to adversely affect health services such as cancer screening68,69 and work-up of pain,70 although the association between these disorders and gender differences in the management of CVD has not been studied.
POSSIBLE CLINICIAN FACTORS
It is logical that individual clinician practices influence screening and treatment of cholesterol71 and contribute to differences in hyperlipidemia management between men and women at high risk for CVD. Studies that account for patient case-mix and clustering of patients have concluded that variation between individual clinician practices accounts for less than 5% of variation in practice.72–76 Nonetheless, if clinicians on the whole are treating women less aggressively than men, then clinician factors could be important. Such clinician factors include perception of the patient's CVD risks and the benefits of treatment, confidence in the ability to manage cholesterol disorders, prioritization of other preventive services over CVD risk factor management, and communication and decision-making styles. (Table 2).
Misperceptions of Risk
Gender differences in lipid management could reflect different clinician treatment thresholds for men and women that are dictated by factors other than coronary disease.49 In turn, these thresholds may be affected by perceptions of coronary risk that are inappropriately low for women. Perception of risk could be affected by the lower prevalence of coronary disease in women compared with men, although coronary disease is still common in older women.77,78 It could also be affected by other factors that increased the salience of coronary disease to the clinician, such as revascularization58; since men undergo revascularization at higher rates than women,16,22,79 this factor could increase clinician awareness of CVD in men more often than in women.
Perception of risk could also explain why specialists with heightened awareness of coronary disease would be more likely to treat dyslipidemia.80,81 Specialty training may also reflect ability or confidence in one's ability to treat82,83 and the degree to which cholesterol testing is included in the particular specialist's role in the patient's health care. A significant number of women receive care from a generalist physician only, and therefore may overall be less likely to receive preventive testing.84,85 Visits to obstetrician-gynecologists may actually lead to increased cholesterol screening, although this finding may be primarily in populations of low-risk women,85 has not been consistently documented,86 and subsequent treatment rates were not examined.
Prioritization
Physicians, along with their female patients, may prioritize gender-specific screening ahead of cholesterol management. When presented with a vignette presenting 53-year-old woman, clinicians ranked cancer screening ahead of cholesterol testing in importance.87 This may be because of misperceptions about the risk of coronary disease in relation to breast cancer, or driven by other provider concerns such as liability. Missed cases of breast cancer are the most common cause of litigation in the United States,88,89 and this concern may drive certain clinicians to focus on the breast examination and mammography discussions at the expense of other health issues.
Communication and Decision-Making Styles
Women patients tend to prefer women physicians, who may also prioritize gender-specific screening over cholesterol screening, although studies to date have demonstrated that women physicians generally perform many preventive services at comparable rates or more often than male physicians.85,90–92 High-risk populations for CVD were not examined separately. Women physicians also tend to have more participatory and social communication styles than male physicians, which can be associated with longer visit length and contribute to time constraints.41,43 Younger physicians93 and physicians with fewer years in training94 tend to provide increased preventive services including cholesterol screening, but it is not known whether these physicians see women less often or provide different care to women. Finally, individual clinicians may attract different patient populations and tailor or impose their unique practice styles upon that population.95
POSSIBLE HEALTH SYSTEM FACTORS
Although variation attributable to the patient tends to account for the majority of variation in treatment, the health system as represented by the facility can account for variation in practice as well.76 As a result, in part, of the large numbers of facilities needed for an adequately powered analysis, specific system-level factors have not been examined for their effect on gender differences in screening or treatment for dyslipidemia (Table 2). There is some evidence that the presence of disease management programs can significantly influence screening and treatment rates. Case management,96 lipid clinics,97 and multidisciplinary CVD programs in general98 have been effective in decreasing lipid levels in patients with known coronary disease. LaBresh et al. and Bramlet et al. found that men with CVD were more likely to respond to lipid-lowering therapy than women with CVD with standard care; however, gender differences were absent in patients who were referred to nurse management.97,99 Women may also have lower participation rates in cardiac rehabilitation programs after myocardial infarction,100 but it is not clear how much of this is because of patient preferences101 or gender biases in referral practices.102 To our knowledge, other gender differences in the associations between other health system factors (Fig. 1) and lipid management strategies have not been reported.
METHODS OF MEASUREMENT
The severity of the problem depends partially on the method of measurement.103 The use of medical records and claims data may not adequately record services provided, particularly discussion of issues surrounding cholesterol screening and management.103–106 Quality measurement studies that have trained experienced actors to serve as standardized patients for several common conditions have captured a greater number of services provided in the visit.94,107 These studies found that medical record abstraction underestimated compliance with preventive measures by as much as 26% and that patients did not recall a significant portion of what they had been told during the visit.94,107 On the other hand, surveys of providers tend to overestimate provider compliance with cholesterol guidelines.108
The instrument used to measure treatment may also affect the estimates of the relative importance of patient, clinician, and health system factors from analyses of larger databases. Larger amounts of variation in clinician practice is seen when processes of care that are linked to intermediate outcomes are examined instead of outcomes alone. For example, measurement of lipid level alone demonstrates that patient and health system factors are the primary determinants of lipid level. However, clinician variability in practice is more pronounced for an indicator that measures whether a statin was prescribed for an elevated lipid level.76 Therefore, the extent of the problem may vary depending on the source and construction of information used. Whether or not the method of measurement affects lipid management in men and women differently has not been examined.
LIMITATIONS OF SOLUTIONS FOR SUBOPTIMAL SCREENING AND TREATMENT
To date, proposed solutions to improve management of CVD risk factors for both men and women have included educating clinicians and increasing clinician awareness and accountability through feedback reports.71 Unfortunately, profiling for resource utilization and clinician “accountability” purposes has not been shown to affect prescribing behavior or lab test ordering,109–111 perhaps because these “report cards” do not accurately reflect a clinician's case-mix and because of limited power to detect differences among clinicians.75 Also, clinicians tend not to think of screening and treatment failures as clinician-based problems.71
Health system level interventions such as case-management, computerized reminders, and patient education programs have demonstrated success in improving process or outcomes in high-risk populations,96,98,112–116 but the patient population, practice setting, and programs vary widely across studies. In a meta-analysis of interventions to improve CVD risk factor management, programs that targeted several levels of care, including the structural organization of care and patient education, tended to be the most successful.117 To our knowledge, only the previously mentioned studies by LaBresh et al. and Bramlet et al. have compared the effects of these programs on lipid levels between women and men.97,99
CONCLUSIONS
Despite the availability of effective medical therapies to reduce CVD mortality, current literature suggests that women with CVD experience suboptimal cholesterol management. The reasons underlying the gender difference and poor management overall are not well understood. Therefore, it is unclear how to reduce such gender disparities, and these disparities may translate to significantly higher rates of CVD events and mortality for women. The gap between research and actual practice has led Healthy People 2010 to support the study of the management of dyslipidemia,118 and the National Heart, Lung, and Blood Institute to declare the study of the translation of research results into practice as part of its strategic plan for 2002–06.119
Further research on disparities in lipid mismanagement should focus on modifiable mechanisms. Women's and men's preferences for lipid and other CVD risk factor management have not been well studied, particularly in relation to other gender-specific screening issues, cost of therapy, and by degree of CVD risk. Understanding clinician prioritization of cholesterol screening and management, and gender-specific thresholds in management, could provide further insight into “clinical inertia.”71 Better understanding of how the structure of health care organizations, particularly specialty referral, utilization management, and payment arrangements, affect screening and treatment in women and men separately might also provide insight into differences in management. For example, understanding of how available health plan benefits interact with patient and physician preferences for cholesterol management could lead to structural changes in benefits that might improve screening and treatment. In general, we found in our review that there is weak and often inconsistent evidence for the importance of a wide variety of variables throughout the major domains of our conceptual model. Yet there are no studies that consider more than a few variables or domains in any single analysis. What is most critical to this research agenda is that patient, clinician, and health system defects be considered simultaneously in order to clarify which factors are most influential and modifiable.
There are a number of reasons to pursue this research agenda. Investigation of gender disparities in CVD risk and lipid management may shed light on gender disparities in other disease areas. The area of lipid management has a well-developed evidence base supporting a set of widely accepted and specific guidelines, thus reducing reasonable variations in practice. The presence of information that enables accurate assessment of CVD risk in men and women can reduce concern about confounding by disease severity. Finally, it seems likely that insights about possible mechanisms of disparities outlined in our model for CVD may be generalizable to other diseases,120–125 particularly those managed in the outpatient setting.
Although clinicians may not be able to single-handedly change adherence patterns, they can be aware of issues of screening and treatment during the health care visit. When managing a woman at high risk for CVD, clinicians should respect the patient's agenda but also attempt to negotiate that agenda so that interventions such as screening and treatment of cholesterol occur. The time for such negotiation can occur by delegating discussions to ancillary staff or automating testing procedures, decreasing the amount of time spent on other screening recommendations for which the patient is at lower risk, or having the patient return for another visit. Clinicians need to be aware of the services their health system or insurance plan offers to help manage dyslipidemia in the face of competing time constraints, such as wellness clinics, preventive cardiology services, nutritional counseling, exercise programs, case management programs, and social workers who can educate patients about their eligibility for health care benefits. Finally, they should be sympathetic to the barriers that women, particularly those of lower socioeconomic status, face in successfully implementing such goals.
Acknowledgments
Dr. Kim is supported by an American Diabetes Association Junior Faculty Award. Dr. Kerr is supported by an Advanced Research Career Development Award from the Department of Veterans Affairs Health Services Research and Development Service. Dr. Hofer is supported by grant 1P20HS011540-01 from the Agency for Health Research and Quality.
REFERENCES
- 1.American Heart Association. American Heart Association 2002 Heart and Stroke Statistical Update. Dallas, TX: American Heart Association; 2001. pp. 1–38. [Google Scholar]
- 2.Miettinen T, Pyorala K, Olsson A, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris. Circulation. 1997;96:4211–8. doi: 10.1161/01.cir.96.12.4211. [DOI] [PubMed] [Google Scholar]
- 3.McPherson R, Genest J, Angus C, Murray P. The Women's Atorvastatin Trial on Cholesterol (WATCH): frequency of achieving NCEP-II target LDL-C levels in women with and without established CVD. Am Heart J. 2001;141:949–56. doi: 10.1067/mhj.2001.115588. [DOI] [PubMed] [Google Scholar]
- 4.LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 5.Lewis S, Sacks F, Mitchell J, et al. Effect of pravastatin on cardiovascular events in women after myocardial infarction. The Cholesterol and Recurrent Events (CARE) trial. J Am Coll Cardiol. 1998;32:140–6. doi: 10.1016/s0735-1097(98)00202-2. [DOI] [PubMed] [Google Scholar]
- 6.Waters D, Higginson L, Gladstone P, Boccuzzi S, Cook T, Lesperance J. Effects of cholesterol lowering on the progression of coronary atherosclerosis in women: a Canadian Coronary Atherosclerosis Intervention Trial (CCAIT) substudy. Circulation. 1995;92:2404–10. doi: 10.1161/01.cir.92.9.2404. [DOI] [PubMed] [Google Scholar]
- 7.Meigs J, Stafford R. Cardiovascular disease prevention practices by U.S. physicians for patients with diabetes. J Gen Intern Med. 2000;15:220–8. doi: 10.1111/j.1525-1497.2000.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi A, Suri M, Guterman L, Hopkins L. Ineffective secondary prevention in survivors of cardiovascular events in the U.S. population. Report from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2001;161:1621–8. doi: 10.1001/archinte.161.13.1621. [DOI] [PubMed] [Google Scholar]
- 9.Saadine J, Engelgau M, Beckles G, Gregg E, Thompson T, Venkat-Narayan K. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 10.Brown D, Giles W, Greenlund K, Croft J. Disparities in cholesterol screening: falling short of a national health objective. Prev Med. 2001;33:517–22. doi: 10.1006/pmed.2001.0928. [DOI] [PubMed] [Google Scholar]
- 11.Davis K, Cogswell M, Lee S, Rothenberg R, Koplan J. Lipid screening in a managed care population. Public Health Rep. 1998;113:346–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson T. The undertreatment of LDL-cholesterol: addressing the challenge. Int J Cardiol. 2000. pp. S23–28. [DOI] [PubMed]
- 13.Pearson T, Laurora I, Chu H, Kafonek S. The Lipid Treatment Assessment Project (L-TAP) Arch Intern Med. 2000;160:459–67. doi: 10.1001/archinte.160.4.459. [DOI] [PubMed] [Google Scholar]
- 14.Gardner C, Winkleby M, Fortmann S. Population frequency distribution of non-high-density lipoprotein cholesterol (Third National Health and Nutrition Examination Survey, 1988–94) Am J Cardiol. 2000;86:299–304. doi: 10.1016/s0002-9149(00)00918-8. [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Ayanian J, Epstein A. Differences in the use of procedures between women and men hospitalized for coronary heart disease. N Engl J Med. 1991;325:221. doi: 10.1056/NEJM199107253250401. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub W, Kosinski A, Wenger N. Is there a bias against performing coronary revascularization in women? Am J Cardiol. 1996;78:1154–60. [PubMed] [Google Scholar]
- 18.Schulman K, Berlin J, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340:618–26. doi: 10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 19.Roger V, Farkouh M, Weston S, et al. Sex differences in evaluation and outcome of unstable angina. JAMA. 2000;283:646–52. doi: 10.1001/jama.283.5.646. [DOI] [PubMed] [Google Scholar]
- 20.Rathore S, Chen J, Wang Y, Radford M, Vaccarino V, Krumholz H. Sex differences in cardiac catheterization: the role of physician gender. JAMA. 2001;286:2849–56. doi: 10.1001/jama.286.22.2849. [DOI] [PubMed] [Google Scholar]
- 21.Alter D, Naylor C, Austin P, Tu J. Biology or bias. practice patterns and long-term outcomes for men and women with acute myocardial infarction. J Am Coll Cardiol. 2002;39:1909–16. doi: 10.1016/s0735-1097(02)01892-2. [DOI] [PubMed] [Google Scholar]
- 22.Ghali W, Faris P, Galbraith P, et al. Sex differences in access to coronary revascularization after cardiac catheterization: importance of detailed clinical data. Ann Intern Med. 2002;136:723–32. doi: 10.7326/0003-4819-136-10-200205210-00007. [DOI] [PubMed] [Google Scholar]
- 23.Shaw L, Miller D, Romeis J, et al. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1991;120:559. doi: 10.7326/0003-4819-120-7-199404010-00005. [DOI] [PubMed] [Google Scholar]
- 24.Janz N, Becker M. The Health Belief Model: a decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 25.Landon B, Wilson I, Cleary P. A conceptual model of the effects of health care organizations on the quality of medical care. JAMA. 1998;279:1377–82. doi: 10.1001/jama.279.17.1377. [DOI] [PubMed] [Google Scholar]
- 26.Jaen C, Stange K, Nutting P. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166–71. [PubMed] [Google Scholar]
- 27.Janes G, Blackman D, Bolen J, et al. Surveillance for use of preventive health-care services by older adults, 1995–97. Morb Mortal Wkly Rep CDC Surveill Summ. 1999;48:51–88. [PubMed] [Google Scholar]
- 28.Ayanian J, Weissman J, Schneider E, Ginsburg J, Zaslavsky A. Unmet health needs of uninsured adults in the United States. JAMA. 1998;284:2061–9. doi: 10.1001/jama.284.16.2061. [DOI] [PubMed] [Google Scholar]
- 29.Bindman A, Grumbach K, Osmond D, Vranizan K, Stewart A. Primary care and receipt of preventive services. J Gen Intern Med. 1996;11:269–76. doi: 10.1007/BF02598266. [DOI] [PubMed] [Google Scholar]
- 30.Corbie-Smith G, Flagg E, Doyle J, O'Brien M. Influence of usual source of care on differences by race/ethnicity in receipt of preventive services. J Gen Intern Med. 2002;17:458–64. doi: 10.1046/j.1525-1497.2002.10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainous A, Hueston W, Love M, Griffith C. Access to care for the uninsured: is access to a physician enough? Am J Public Health. 1999;89:910–2. doi: 10.2105/ajph.89.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lurie N, Manning W, Peterson C, Goldberg G, Phelps C, Lillard L. Preventive care: do we practice what we preach? Am J Public Health. 1987;77:801–4. doi: 10.2105/ajph.77.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayanian J, Landon B, Landrum M, Grana J, McNeil B. Use of cholesterol-lowering therapy and related beliefs among middle-aged adults after myocardial infarction. J Gen Intern Med. 2002;17:95–7. doi: 10.1046/j.1525-1497.2002.10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hueston W, Spencer E, Kuehn R. Differences in the frequency of cholesterol screening in patients with Medicaid compared with private insurance. Arch Fam Med. 1995;4:331–4. doi: 10.1001/archfami.4.4.331. [DOI] [PubMed] [Google Scholar]
- 35.Luepker R, Rosamond W, Murphy R, et al. Socioeconomic status and coronary heart disease risk factor trends. The Minnesota Heart Survey Circulation. 1993;88:2172–9. doi: 10.1161/01.cir.88.5.2172. [DOI] [PubMed] [Google Scholar]
- 36.Cooper G, Goodwin M, Stange K. The delivery of preventive services for patient symptoms. Am J Prev Med. 2001;21:177–81. doi: 10.1016/s0749-3797(01)00348-8. [DOI] [PubMed] [Google Scholar]
- 37.Stange K, Flocke S, Goodwin M, Kelly R, Zyzanski S. Direct observation of rates of preventive service delivery in community family practice. Prev Med. 2000;31:167–76. doi: 10.1006/pmed.2000.0700. [DOI] [PubMed] [Google Scholar]
- 38.Mosca L, Jones W, King K, Ouyang P, Redberg R, Hill M. Awareness, perception, and knowledge of heart disease risk and prevention among women in the United States. American Heart Association Women's Heart Disease and Stroke Campaign Task Force. Arch Fam Med. 2000;9:506–15. doi: 10.1001/archfami.9.6.506. [DOI] [PubMed] [Google Scholar]
- 39.Marvel M, Epstein R, Flowers K, Beckman H. Soliciting the patient's agenda: have we improved? JAMA. 1999;281:283–7. doi: 10.1001/jama.281.3.283. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan C, Siegel B, Madill J, Epstein A. Communication and the medical interview: strategies for learning and teaching. J Gen Intern Med. 1997. pp. S49–55. [DOI] [PMC free article] [PubMed]
- 41.Hall J, Roter D. Patient gender and communication with physicians: results of a community-based study. Womens Health. 1995;1:77–95. [PubMed] [Google Scholar]
- 42.Elderkin-Thompson V, Waitzkin H. Differences in clinical communication by gender. J Gen Intern Med. 1999;14:112–21. doi: 10.1046/j.1525-1497.1999.00296.x. [DOI] [PubMed] [Google Scholar]
- 43.Roter D, Hall J, Aoki Y. Physician gender effects in medical communication: a meta-analytic review. JAMA. pp. 756–64. [DOI] [PubMed]
- 44.Kaplan S, Gandek B, Greenfield S, Rogers W, Ware J. Patient and visit characteristics related to physicians’ participatory decision-making style. Med Care. 1995;33:1176–87. doi: 10.1097/00005650-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Ayanian J, Landrum M, McNeil B. Use of cholesterol-lowering therapy by elderly adults after myocardial infarction. Arch Intern Med. 2002;162:1013–9. doi: 10.1001/archinte.162.9.1013. [DOI] [PubMed] [Google Scholar]
- 46.Lemaitre R, Furberg C, Newman A, et al. Time trends in the use of cholesterol-lowering agents in older adults: the Cardiovascular Health Study. Arch Intern Med. 1998;158:1761–8. doi: 10.1001/archinte.158.16.1761. [DOI] [PubMed] [Google Scholar]
- 47.Aronow W. Underutilization of lipid-lowering drugs in older persons with prior myocardial infarction and a serum low-density lipoprotein cholesterol > 125 mg/dl. Am J Cardiol. 1998;82:668–9. doi: 10.1016/s0002-9149(98)00401-9. A6,A8. [DOI] [PubMed] [Google Scholar]
- 48.Di Cecco R, Patel U, Upshur R. Is there a clinically significant gender bias in post-myocardial pharmacological management in the older (>60) population of a primary care practice? BMC Fam Pract. 2002;3:8. doi: 10.1186/1471-2296-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laubach E, Otto C, Schwandt P. Toward better therapy of hypercholesterolemia. Arch Intern Med. 2000;160:2685–6. doi: 10.1001/archinte.160.17.2685. [DOI] [PubMed] [Google Scholar]
- 50.Harris M. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24:454–9. doi: 10.2337/diacare.24.3.454. [DOI] [PubMed] [Google Scholar]
- 51.Nelson K, Norris K, Mangione C. Disparities in the diagnosis and pharmacologic treatment of high serum cholesterol by race and ethnicity. Arch Intern Med. 2002;162:929–35. doi: 10.1001/archinte.162.8.929. [DOI] [PubMed] [Google Scholar]
- 52.Wisdom K, Fryzek J, Havstad S, Anderson R, Dreiling M, Tilley B. Comparison of laboratory test frequency and test results between African-Americans and Caucasians with diabetes: opportunity for improvement: findings from a large urban health maintenance organization. Diabetes Care. 1997;20:971–7. doi: 10.2337/diacare.20.6.971. [DOI] [PubMed] [Google Scholar]
- 53.Cook C, Erdman D, Ryan G, et al. The pattern of dyslipidemia among urban African-Americans with type 2 diabetes. Diabetes Care. 2000;23:319–24. doi: 10.2337/diacare.23.3.319. [DOI] [PubMed] [Google Scholar]
- 54.Maviglia S, Teich J, Fiskio J, Bates D. Using an electronic medical record to identify opportunities to improve compliance with cholesterol guidelines. J Gen Intern Med. 2001;16:531–7. doi: 10.1046/j.1525-1497.2001.016008531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winkleby M, Cubbin C, Ahn D, Kraemer H. Pathways by which SES and ethnicity influence cardiovascular risk factors. Ann N Y Acad Sci. 1999;896:191–209. doi: 10.1111/j.1749-6632.1999.tb08116.x. [DOI] [PubMed] [Google Scholar]
- 56.Miller M, Byington R, Hunninghake D, Pitt B, Furberg C. Sex bias and underutilization of lipid-lowering therapy in patients with coronary artery disease at academic medical centers in the United States and Canada. Arch Intern Med. 2000;160:343–7. doi: 10.1001/archinte.160.3.343. [DOI] [PubMed] [Google Scholar]
- 57.Majumdar S, Gurwitz J, Soumerai S. Undertreatment of hyperlipidemia in the secondary prevention of coronary artery disease. J Gen Intern Med. 1999;14:711–7. doi: 10.1046/j.1525-1497.1999.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McBride P, Schrott H, Plane M, Underbakke G, Brown R. Primary care practice adherence to National Cholesterol Program guidelines for patients with coronary heart disease. Arch Intern Med. 1998;158:1238–44. doi: 10.1001/archinte.158.11.1238. [DOI] [PubMed] [Google Scholar]
- 59.Sloan K, Sales A, Willems J, et al. Frequency of serum low-density lipoprotein cholesterol measurement and frequency of results < 100 mg/dl among patients who had coronary events (Northwest VA Network Study) Am J Cardiol. 2001;88:1143–6. doi: 10.1016/s0002-9149(01)02050-1. [DOI] [PubMed] [Google Scholar]
- 60.Vanuzzo D, Pilotto L, Ambrosio G, et al. Potential for cholesterol lowering in secondary prevention of coronary heart disease in Europe: findings from EUROASPIRE study. Atherosclerosis. 2000;153:505–17. doi: 10.1016/s0021-9150(00)00596-7. [DOI] [PubMed] [Google Scholar]
- 62.The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 63.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart Estrogen/Progestin Replacement Study (HERS) Res Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 64.Womens Health Initiative. Primary prevention with estrogen/progestin. JAMA. 2002.
- 65.Iezzoni L, McCarthy E, Davis R, Siebens H. Mobility difficulties are not only a problem of old age. J Gen Intern Med. 2001;16:235–43. doi: 10.1046/j.1525-1497.2001.016004235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McTigue K, Garrett J, Popkin B. The natural history of the development of obesity in a cohort of young U.S. adults between 1981 and 1998. Ann Intern Med. 2002;136:857–64. doi: 10.7326/0003-4819-136-12-200206180-00006. [DOI] [PubMed] [Google Scholar]
- 67.Kessler R, McGonagle K, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 68.Iezzoni L, McCarthy E, Davis R, Siebens H. Mobility impairments and use of screening and preventive services. Am J Public Health. 2000;90:955–61. doi: 10.2105/ajph.90.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wee C, McCarthy E, Davis R, Phillips R. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med. 2000;132:697–704. doi: 10.7326/0003-4819-132-9-200005020-00003. [DOI] [PubMed] [Google Scholar]
- 70.Garber M, Bergus G, Dawson J, Wood G, Levy B, Levin I. Effect of a patient's psychiatric history on physicians’ estimation of probability of disease. J Gen Intern Med. 2000;15:204–6. doi: 10.1046/j.1525-1497.2000.04399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips L, Branch W, Cook C, et al. Clinical inertia. Ann Intern Med. 2001;135:824–34. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 72.Greenfield S, Kaplan S, Kahn R, Ninomiya J, Griffith J. Profiling care by different groups of physicians. effects of patient case-mix (bias) and physician-level clustering on quality assessment results. Ann Intern Med. 2002;136:111–21. doi: 10.7326/0003-4819-136-2-200201150-00008. [DOI] [PubMed] [Google Scholar]
- 73.Orav E, Wright E, Palmer R, Hargraves J. Issues of variability and bias affecting multisite measurement of quality of care. Med Care. 1996. pp. S87–101. [DOI] [PubMed]
- 74.Sixma H, Spreeuwenberg P, van der Pasch M. Patient satisfaction with the general practitioner: a two-level analysis. Med Care. 1998;36:212–29. doi: 10.1097/00005650-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Hofer T, Hayward R, Greenfield S, Wagner E, Kaplan S, Manning W. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA. 1999;281:2098–105. doi: 10.1001/jama.281.22.2098. [DOI] [PubMed] [Google Scholar]
- 76.Krein S, Hofer T, Kerr E, Hayward R. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups and healthcare facilities. Health Serv Res. 2002;27:1159–80. doi: 10.1111/1475-6773.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grover S, Lowensteyn I, Esrey K, et al. Do doctors accurately assess coronary risk in their patients? Preliminary results of the coronary health assessment study. BMJ. 1995;310:975–8. doi: 10.1136/bmj.310.6985.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birdwell B, Herbers J, Kroenke K. Evaluating chest pain. Arch Intern Med. 1993;153:1991–5. doi: 10.1001/archinte.153.17.1991. [DOI] [PubMed] [Google Scholar]
- 79.Roger V, Jacobsen S, Weston S, et al. Sex differences in evaluation and outcome after stress testing. Mayo Clin Proc. 2002;77:638–45. doi: 10.4065/77.7.638. [DOI] [PubMed] [Google Scholar]
- 80.Ayanian J, Landrum M, Guadagnoli E, Gaccione P. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N Engl J Med. 2002;347:1678–86. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- 81.Stafford R, Blumenthal D. Specialty differences in cardiovascular disease prevention practices. J Am Coll Cardiol. 1998;32:1238–43. doi: 10.1016/s0735-1097(98)00380-5. [DOI] [PubMed] [Google Scholar]
- 82.Hyman D, Maibach W, Flora J, Fortmann S. Cholesterol treatment practices of primary care physicians. Public Health Rep. 1992;107:441–8. [PMC free article] [PubMed] [Google Scholar]
- 83.Marcelino J, Feingold K. Inadequate treatment with HMG-CoA reductase inhibitors by health care providers. Am J Med. 1996;100:605–10. doi: 10.1016/s0002-9343(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 84.Wyn R, Brown E, Yu H. Women's Use of Preventive Services: The Commonwealth Fund Survey. Baltimore, MD: Johns Hopkins University Press; 1996. [Google Scholar]
- 85.Henderson J, Weisman C, Grason H. Are two doctors better than one? Women's physician use and appropriate care. Womens Health Issues. 2002;12:138–49. doi: 10.1016/s1049-3867(02)00134-2. [DOI] [PubMed] [Google Scholar]
- 86.Giles W, Anda R, Jones D, Serdula M, Merritt R, DeStefano F. Recent trends in the identification and treatment of high blood cholesterol by physicians. Progress and missed opportunities. JAMA. 1993;269:1133–8. [PubMed] [Google Scholar]
- 87.Stange K, Fedirko T, Zyzanski S, Jaen C. How do family physicians prioritize delivery of multiple preventive services? J Fam Pract. 1994;38:231–7. [PubMed] [Google Scholar]
- 88.Osuch J, Bonham V, Morris L. Primary care guide to managing a breast mass: step-by-step work-up. Medscape Womens Health. 1998;3:4. [PubMed] [Google Scholar]
- 89.Barratt A, Cockburn J, Furnival C, McBride A, Mallon L. Perceived sensitivity of mammographic screening: women's views on test accuracy and financial compensation for missed cancers. J Epidemiol Community Health. 1999;53:716–20. doi: 10.1136/jech.53.11.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henderson J, Weisman C. Physician gender effects on preventive screening and counseling: an analysis of male and female patients’ health care experiences. Med Care. 2001;39:1281–92. doi: 10.1097/00005650-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 91.Cassard S, Weisman C, Plichta S, Johnson T. Physician gender and women's preventive services. J Womens Health. 1997;6:199–207. doi: 10.1089/jwh.1997.6.199. [DOI] [PubMed] [Google Scholar]
- 92.Franks P, Clancy C. Physician gender bias in clinical decision making: screening for cancer in primary care. Med Care. 1993;31:213–8. doi: 10.1097/00005650-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz J, Lewis C, Clancy C, Kinosian M, Radany M, Koplan J. Internists’ practices in health promotion and disease prevention. A Survey. Ann Intern Med. 1991;114:46–53. doi: 10.7326/0003-4819-114-1-46. [DOI] [PubMed] [Google Scholar]
- 94.Dresselhaus T, Peabody J, Lee M, Wang M, Luck J. Measuring compliance with preventive care guidelines. J Gen Intern Med. 2000;15:782–8. doi: 10.1046/j.1525-1497.2000.91007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harnick D, Cohen J, Schechter C, Fuster V, Smith D. Effects of practice setting on quality of lipid-lowering management in patients with coronary artery disease. Am J Cardiol. 1998;81:1416–20. doi: 10.1016/s0002-9149(98)00209-4. [DOI] [PubMed] [Google Scholar]
- 96.DeBusk R, Miller N, Superko H, et al. Case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120:721–9. doi: 10.7326/0003-4819-120-9-199405010-00001. [DOI] [PubMed] [Google Scholar]
- 97.Bramlet D, King H, Young L, Witt J, Stoukides C, Kaul A. Management of hypercholesterolemia: practice patterns for primary care providers and cardiologists. Am J Cardiol. 1997. pp. 39H–44H. [DOI] [PubMed]
- 98.McAlister F, Lawson F, Teo K, Armstrong P. Randomised trials of secondary prevention programs in coronary heart disease: systematic review. BMJ. 2001;323:957–62. doi: 10.1136/bmj.323.7319.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LaBresh K, Owen P, Alteri C, et al. Secondary prevention in a cardiology group practice and hospital setting after a heart-care initiative. Am J Cardiol. 2000;85:23A–29A. doi: 10.1016/s0002-9149(99)00935-2. [DOI] [PubMed] [Google Scholar]
- 100.Evenson K, Rosamond W, Luepker R. Predictors of outpatient cardiac rehabilitation utilization: the Minnesota Heart Surgery Registry. J Cardiopulm Rehabil. 1998;18:192–8. doi: 10.1097/00008483-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 101.Blackburn G, Foody J, Sprecher D, Park E, Apperson-Hansen C, Pashkow F. Cardiac rehabilitation participation patterns in a large, tertiary care center: evidence for selection bias. J Cardiopulm Rehabil. 2000;20:189–95. doi: 10.1097/00008483-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Mosca L, Han R, Filip J. Barriers for physicians to refer to cardiac rehabilitation and impact of a critical care pathway on rates of participation. Circulation. 1998. pp. I–811. Abstract.
- 103.Stange K, Zyzanski S, Smith T, et al. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patients visits. Med Care. 1998;36:851–67. doi: 10.1097/00005650-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Kerr E, Krein S, Vijan S, Hofer T, Hayward R. Avoiding pitfalls in chronic disease quality management: a case for the next generation of technical quality measures. Am J Manag Care. 2001;7:1033–43. [PubMed] [Google Scholar]
- 105.Luck J, Peabody J, Dresselhaus T, Lee M, Glassman P. How well does chart abstraction measure quality? A prospective comparison of standardized patients with the medical record. Am J Med. 2000;108:642–9. doi: 10.1016/s0002-9343(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 106.Bloom S, Harris J, Thompson B, Ahmed F, Thompson J. Tracking clinical preventive service use: a comparison of the Health Plan Employer Data and Information Set with the Behavioral Risk Factor Surveillance System. Med Care. 2000;38:187–94. doi: 10.1097/00005650-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 107.Peabody J, Luck J, Glassman P, Dresselhaus T, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–22. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 108.Headrick L, Speroff T, Pelecanos H, Cebul R. Efforts to improve compliance with the National Cholesterol Education Program guidelines. Results of a randomized controlled trial. Arch Intern Med. 1992;152:2490–6. [PubMed] [Google Scholar]
- 109.Schechtman J, Kanwal N, Schroth W, Elinsky E. The effect of an education and feedback intervention on group-model and network-model health maintenance organization physician prescribing behavior. Med Care. 1995;33:139–44. [PubMed] [Google Scholar]
- 110.Mainous A, Hueston W, Love M, Evans M, Finger R. An evaluation of statewide strategies to reduce antibiotic overuse. Fam Med. 2000;32:22–9. [PubMed] [Google Scholar]
- 111.Balas E, Boren S, Brown G, Ewigman B, Mitchell J, Perkoff G. Effect of physician profiling on utilization: meta-analysis of randomized clinical trials. J Gen Intern Med. 1996;11:584–90. doi: 10.1007/BF02599025. [DOI] [PubMed] [Google Scholar]
- 112.Renders C, Valk G, Franse L, Schellveis F, Van Eijk J, van der Wal G. Long-term effectiveness of a quality improvement program for patients with type 2 diabetes in general practice. Diabetes Care. 2001;24:1365–70. doi: 10.2337/diacare.24.8.1365. [DOI] [PubMed] [Google Scholar]
- 113.Baker A, Lafata J, Ward R, Whitehouse F, Divine G. A web-based diabetes care management support system. Jt Comm J Qual Improv. 2001;27:179–90. doi: 10.1016/s1070-3241(01)27016-3. [DOI] [PubMed] [Google Scholar]
- 114.Peters A, Davidson M. Application of a diabetes managed care program. The feasibility of using nurses and a computer system to provide effective care. Diabetes Care. 1998;21:1037–43. doi: 10.2337/diacare.21.7.1037. [DOI] [PubMed] [Google Scholar]
- 115.Rubin R, Kietrich K, Hawk A. Clinical and economic impact of implementing a comprehensive diabetes management program in managed care. J Clin Endocrinol Metab. 1998;83:2635–42. doi: 10.1210/jcem.83.8.5075. [DOI] [PubMed] [Google Scholar]
- 116.Domurat E. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente Diabetes Care System. Am J Manag Care. 1999;5:1299–307. [PubMed] [Google Scholar]
- 117.Renders C, Valk G, Griffin S, Wagner E, Eijk van J, Assendelft W. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24:1821–33. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 118.Centers for Disease Control and Prevention and Health. Heart Disease and StrokeHealthy People 2010-Conference Edition 1999. Bethesda, MD: United States Public Health Service; 1999. [Google Scholar]
- 119.National Heart Lung and Blood Institute. Strategic Plan FY 2002–06. Rockville, MD: National Institutes of Health; 2002. http://www.nhlbi.nih.gov/resources/docs/plan/index.htm/; accessed on April 1, 2003. [Google Scholar]
- 120.Mangione C, Reynolds E. Disparities in health and health care. J Gen Intern Med. 2001;16:276–80. doi: 10.1046/j.1525-1497.2001.016004276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weisse C, Sorum P, Sanders K, Syat B. Do gender and race affect decisions about pain management? J Gen Intern Med. 2001;16:211–7. doi: 10.1046/j.1525-1497.2001.016004211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chapman K, Tashkin D, Pye D. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691–5. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]
- 123.Watson R, Stein A, Dwamena F, et al. Do race and gender influence the use of invasive procedures? J Gen Intern Med. 2001;16:227–34. doi: 10.1046/j.1525-1497.2001.016004227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raine R. Does gender bias exist in the use of specialist health care? J Health Serv Res Policy. 2000;5:237–49. doi: 10.1177/135581960000500409. [DOI] [PubMed] [Google Scholar]
- 125.Johnson M, Lin M, Mangalik S, Murphy D, Kramer A. Patients’ perceptions of physicians’ recommendations for comfort care differ by patient age and gender. J Gen Intern Med. 2000;15:248–55. doi: 10.1111/j.1525-1497.2000.07004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Majeed Z, Moser K, Maxwell R. Age, sex, and practice variations in the use of statins in general practice in England and Wales. J Public Health Med. 2000;22:275–9. doi: 10.1093/pubmed/22.3.275. [DOI] [PubMed] [Google Scholar]
- 127.Savoie I, Kazanjian A. Utilization of lipid-lowering drugs in men and women: a reflection of the research evidence? J Clin Epidemiol. 2002;55:95–101. doi: 10.1016/s0895-4356(01)00436-x. [DOI] [PubMed] [Google Scholar]
- 128.Hippisley-Cox J, Pringle M, Crown N, Meal A, Wynn A. Sex inequalities in ischaemic heart disease in general practice: cross-sectional survey. BMJ. 2001;322:832. doi: 10.1136/bmj.322.7290.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bowker T, Clayton T, Ingham J, et al. British Cardiac Society survey of the potential for the secondary prevention of coronary disease: ASPIRE (Action on Secondary Prevention through Intervention to Reduce Events) Heart. 1996;75:334–42. doi: 10.1136/hrt.75.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bannerman A, Hamilton K, Isles C, et al. Myocardial infarction in men and women under 65 years of age: no evidence of gender bias. Scott Med J. 2001;46:73–8. doi: 10.1177/003693300104600304. [DOI] [PubMed] [Google Scholar]
- 131.Wei L, Wang J, Thompson P, Wong S, Struthers A, MacDonald T. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow-up study. Heart. 2002;88:229–33. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sgadari A, Incalzi R, Onder G, Pedone C, Gambassi G. Lipid-lowering therapy in patients with coronary artery disease: sex or age bias? Arch Intern Med. 2000;160:2684–5. doi: 10.1001/archinte.160.17.2684. [DOI] [PubMed] [Google Scholar]
- 133.Pilote L, Beck C, Richard H, Eisenberg M. The effects of cost-sharing on essential drug prescriptions, utilization of medical care and outcomes after acute myocardial infarction in elderly patients. CMAJ. 2002;167:246–52. [PMC free article] [PubMed] [Google Scholar]
- 134.Evenson K, Fleury J. Barriers to outpatient cardiac rehabilitation participation and adherence. J Cardiopulm Rehabil. 2000;20:241–6. doi: 10.1097/00008483-200007000-00005. [DOI] [PubMed] [Google Scholar]