Abstract
OBJECTIVE
Tamoxifen reduces the risk of developing breast cancer but also affects the risks of certain vascular and neoplastic events. Our purpose was to estimate the effects of tamoxifen on potentially life-threatening vascular and neoplastic outcomes.
DESIGN
Random effects meta-analysis of published randomized controlled trials.
PATIENTS
Participants in all trials in which a treatment arm that included tamoxifen was compared to a similar control arm. Breast cancer risk reduction and treatment trials were included.
INTERVENTIONS
Tamoxifen at variable dose and duration.
MEASUREMENTS AND MAIN RESULTS
Thirty-two trials (52,929 patients) reported one or more outcomes of interest. Tamoxifen was associated with significantly increased risks of endometrial cancer (relative risk [RR] 2.70; 95% CI, 1.94 to 3.75), gastrointestinal cancers (RR 1.31; 95% CI, 1.01 to 1.69), strokes (RR 1.49; 95% CI, 1.16 to 1.90), and pulmonary emboli (RR 1.88; 95% CI, 1.77 to 3.01). Tamoxifen had no effect on secondary malignancies other than endometrial and gastrointestinal cancers (RR 0.96; 95% CI, 0.81 to 1.13). In contrast, tamoxifen significantly decreased myocardial infarction deaths (RR 0.62; 95% CI, 0.41 to 0.93) and was associated with a statistically insignificant decrease in myocardial infarction incidence (RR 0.90; 95% CI, 0.66 to 1.23). Postmenopausal women had greater risk increases for neoplastic outcomes.
CONCLUSIONS
This meta-analysis of randomized trials found tamoxifen use to be significantly associated with several neoplastic and vascular outcomes. Consideration of tamoxifen use requires balance of potential benefits and risks.
Keywords: tamoxifen, adverse events, selective estrogen receptor modulators, chemoprevention
Tamoxifen, a selective estrogen receptor modulator with both estrogen agonist and antagonistic properties, is among the most widely prescribed breast cancer reduction therapies.1 It is the only agent currently approved by the U.S. Food and Drug Administration (FDA) for the reduction of breast cancer risk in women at increased risk for this disease, and may be considered for women with a 15-year projected breast cancer risk of ≥1.66%.2
As a mixed estrogen agonist and antagonist, tamoxifen affects a variety of clinical conditions in addition to breast cancer. Tamoxifen is generally well tolerated but is infrequently associated with life-threatening events including development of endometrial cancer and pulmonary emboli.3 Precise estimation of the magnitude of the risk of life-threatening conditions which may be associated with tamoxifen use are of special significance when considering its use for breast cancer risk reduction. The only published meta-analyses of adverse outcomes with tamoxifen were performed by the Early Breast Cancer Trialists' Collaborative Group.4 They did not examine the incidence of many outcomes of possible significance such as stroke and myocardial infarction, and their analyses only included patients receiving tamoxifen for breast cancer treatment. Furthermore, they have not conducted analyses of potentially higher risk groups such as postmenopausal women.
The purpose of the present study was to estimate the effects of tamoxifen on potentially life-threatening vascular and neoplastic outcomes by conducting a meta-analysis of data from all published randomized controlled trials involving its use. We analyze results separately for patients receiving tamoxifen for different indications and for different patient subgroups. We did not include results for raloxifene, another estrogen receptor, because the data supporting its efficacy for breast cancer risk reduction are still preliminary.3
METHODS
We performed a search of the medline and cancerlit computerized databases (1966 to November 2002) using the medical subject headings tamoxifenestrogen antagonists, and textwords tamoxifen, selective estrogen receptor modulator, and SERM. In addition, we performed a manual search using the authors' reference files, reference lists from original communications, and experts in the field. We retrieved citations for further evaluation if the drug therapy in the treatment arm differed from that in the control arm solely by the presence of tamoxifen (e.g., there were no other treatment variables besides tamoxifen, and tamoxifen was not used in both treatment and control arms). Retrieval occurred regardless of whether the study goal was breast cancer treatment, breast cancer risk reduction, or unrelated to breast cancer.
We restricted the search to randomized controlled trials that were published in English and conducted on human subjects. The 777 abstracts or full-text articles that were identified using this search strategy were screened in duplicate, the majority by either RB and SG or by RB and RH. Five hundred articles were excluded because they did not report on clinical outcomes of interest, 63 were excluded because the treatment arm did not differ from the control arm solely by the presence of tamoxifen, 12 were excluded because enrollees had previous exposure to tamoxifen, and 16 were excluded because the treatment and control groups were not randomized.
We abstracted information from the remaining 186 articles (86 trials) on patient demographics, study characteristics, and clinical outcomes. Demographic data included age, sex, race, and menopausal status. Study characteristics included treatment objective, utilization of chemotherapy and/or radiotherapy, duration and dose of tamoxifen, blinding, randomization method, and follow-up period. Clinical outcomes included incidence and deaths from stroke, pulmonary emboli, myocardial infarction, deep venous thromboses, endometrial cancer, colorectal cancers, all gastrointestinal cancers combined, all nonbreast cancers combined, and all cancers other than breast, endometrial, and gastrointestinal combined. Gastrointestinal cancers were defined to include cancers of the esophagus, stomach, pancreas, liver, biliary tract (including gallbladder), small intestine, colon, and rectum. Because many trials did not report tumors of the colon and rectum separately, we combined these into one category. Thirty-two trials reported summary data on one or more outcomes of interest, and these were used in our meta-analysis. Data abstraction was performed in duplicate by either RB and SG or RB and RH, and discrepancies were jointly reviewed until consensus was reached.
We only abstracted outcomes if they were labeled precisely (e.g., an event labeled cardiovascular disease would not be considered myocardial infarction). We grouped cancers in situ together with invasive cancers. We used age >50 as a proxy for postmenopausal status and median values as an approximation for mean values when the latter were not reported. Outcomes among breast cancer patients with tumor recurrence were not distinguished from outcomes among patients with no known recurrence. When more than one article was published from a single trial, we used the latest report with information on the outcome of interest.
We report risks using the measure of relative risk (RR), a ratio of how the risk of an outcome in the presence of tamoxifen compares to the risk of that outcome in the absence of tamoxifen. Relative risks and 95% confidence intervals (CI) were calculated for each trial by comparing the incidence rate among tamoxifen users to nonusers. Both fixed-effects models (which weigh studies according to the inverse of their within-study variance) and the more conservative random-effects models (which also incorporate between-study variance) were used to combine the risk ratios across studies.5–7 No significant heterogeneity was found in any of the meta-analyses performed; thus the fixed-effects and random-effects calculations yielded similar results.
The study sponsors had no role in the study design, collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication. All authors were asked to disclose apparent or real conflicts of interest that may have influenced their interpretation of the results. One author (RTC) has acted as a consultant for Astra Zeneca, a pharmaceutical company that manufactures hormonal chemotherapy for breast cancer. None of the other authors reported any conflicts of interest.
RESULTS
Trial Characteristics
Thirty-two separate randomized controlled trials8–48 with data for 52,929 patients reported on at least one neoplastic or vascular outcome. Four trials8–12 (28,193 participants) investigated breast cancer risk reduction, 25 trials13–45 (24,373 participants) investigated breast cancer treatment, and 3 trials46–48 (363 participants) were unrelated to breast cancer (Table 1). On average, patients were 54.8 years old at randomization and received tamoxifen for 4.3 years. Among the 6 trials8,13,28,30,37,44 that reported racial composition, 91.9% of patients were white. Average follow-up time was longer for treatment trials (6.7 years) than for risk reduction trials (4.7 years). Compared to risk reduction trials, patients in tamoxifen treatment trials were older (59.9 vs 50.3 years) and more likely to be postmenopausal (79.8% vs 54.1%).
Table 1.
Characteristics of Tamoxifen Trials Included in Meta-analysis
| Trial | Number of Patients Analyzed | White, % | Postmenopause, % | Age | Dose (mg/day) | Duration of Exposure | Follow-up Interval |
|---|---|---|---|---|---|---|---|
| Risk reduction trials | |||||||
| NSABP P-18 | 13,175 | 96.5 | 60.7 | NR | 20 | 4.0 | 4.0 |
| Royal Mars9 | 2471 | NR | 33.6 | 47 | 20 | 5.8 | 5.8 |
| Italian10,11 | 5408 | NR | NR | 51 | 20 | 5.0 | 6.8 |
| IBIS-I12 | 7139 | NR | 49.1 | 51 | 20 | 5.0 | 4.2 |
| Subtotal | 28,193 | 96.5 | 54.1 | 50.3 | 20 | 4.6 | 4.7 |
| Breast cancer treatment trials | |||||||
| NSABP B1413–15 | 2,885 | 91 | 69.5 | 55.0 | 20 | 5.0 | 8.9 |
| Barner16 | 167 | NR | 79 | 57.5 | 20 | NR | 6.3 |
| Christie17 | 961 | NR | 61.2 | 54.1 | 20 | 10.0 | NR |
| South Swed18,19 | 475 | NR | 100 | NR | 30 | 1.0 | 9.0 |
| DBCG18,20 | 1710 | NR | 100 | NR | 30 | 1.0 | 9.0 |
| Stockholm18,21–23 | 2729 | NR | 100 | NR | 40 | 2.9 | 9.0 |
| ECOG 117824 | 168 | NR | 100 | 70.5 | 20 | 2.0 | 10.0 |
| Alwyn25 | 74 | NR | 100 | 77.5 | 20 | NR | 3.8 |
| Ingle26 | 234 | NR | 100 | 61 | 20 | 1.3 | 5.0 |
| NCCTG27 | 400 | NR | 0 | 43.8 | 20 | 1.3 | 5.3 |
| SWOG28 | 966 | 91 | 100 | 60.5 | 20 | 1.0 | 6.0 |
| Love29 | 140 | NR | 100 | NR | 20 | 5.0 | 5.0 |
| NSABP B2430 | 1798 | 86 | 65 | NR | 20 | 5.0 | 6.2 |
| GROCTA31 | 336 | NR | 53 | NR | 30 | 5.0 | 5.0 |
| Gunderson32 | 350 | NR | NR | NR | 20 | 2.0 | 6.3 |
| Scottish33–36 | 1322 | NR | 82 | 58.9 | 20 | 5.0 | 15 |
| ECOG 617737,38 | 142 | 92 | 100 | 57.5 | 20 | 1.0 | 3.0 |
| ECOG 517737 | 365 | NR | 0 | 57.5 | 20 | 1.0 | 3.6 |
| Cocconi39 | 133 | NR | 100 | 57.5 | 20 | NR | NR |
| Cocconi40 | 49 | NR | 89.7 | 67 | 20 | 0.7 | 6.0 |
| EORTC.E41 | 131 | NR | 100 | NR | 40 | NR | 5.4 |
| EORTC.b45 | 107 | NR | 0 | 43.0 | 40 | 3.0 | 7.3 |
| DBCG 82-B42 | 634 | NR | 0 | NR | 30 | 1 | 10 |
| ATAC43 | 6189 | NR | 100 | 64.2 | 20 | 5 | 2.8 |
| NSABP B-2344 | 1982 | 78 | 45 | NR | 20 | 5 | 5.4 |
| Subtotal | 24,373 | 86.5 | 79.8 | 59.9 | 23.8 | 4.1 | 6.7 |
| Trials unrelated to breast cancer | |||||||
| Rusthoven46 | 195 | NR | NR | 50.6 | 40 | 0.4 | NR |
| Agarwala47 | 56 | NR | 50 | 55 | 20 | NR | NR |
| Cocconi48 | 112 | NR | 72 | 59 | 40 | 0.9 | NR |
| Subtotal | 363 | NR | 69.0 | 53.5 | 37.1 | 0.6 | NR |
| All trials | 52,929 | 91.9 | 66.2 | 54.8 | 21.8 | 4.3 | 5.6 |
NSABP, National Surgical Adjuvant Breast and Bowel Project; IBIS, International Breast Cancer Intervention Study Group; DBCG, Danish Breast Cancer Cooperative Group; ECOG, Eastern Cooperative Oncology Group; NCCTG, North Central Cancer Treatment Group; SWOG, Southwest Oncology Group; GROCTA, Gruppo di Ricerca in Oncologia Clinica e Terapie Associate; EORTC, European Organization for the Research and Treatment of Cancer; ATAC, Arimidex, Tamoxifen Alone or in Combination.
Because many trials exclusively enrolled postmenopausal women and few of the other trials stratified results by menopausal status, there was little information on outcomes among premenopausal women. Because few deaths were attributed to any particular outcome, with the exception of myocardial infarctions (MI), we do not report combined mortality estimates for outcomes other than MI.
Risk Estimates
Tamoxifen was associated with increased risks of several adverse events including stroke, venous thromboembolism, and cancers of the gastrointestinal tract and endometrium. Tamoxifen appeared to be associated with a decreased risk of death from myocardial infarction, though no impact on the incidence of myocardial infarction was observed.
Strokes
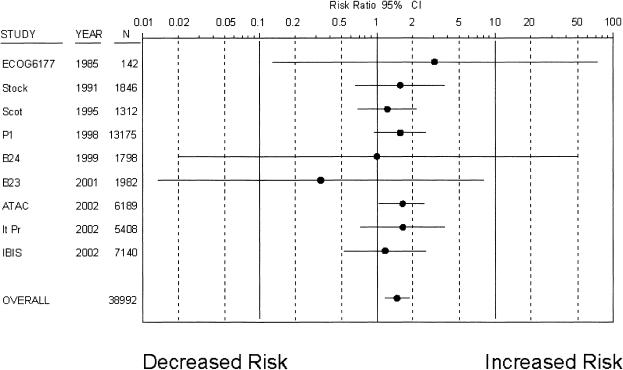
Nine trials8,9,11,12,30,34,37,38,43,44 with 38,992 patients reported a total of 260 strokes over a mean follow-up of 5.1 years (Fig. 1), with an average incidence of 10.4 strokes per 10,000 patient years in control groups. Tamoxifen was associated with a statistically significant increased risk of stroke (RR 1.49; 95% CI, 1.16 to 1.90). When women in risk reduction trials, breast cancer treatment trials, or postmenopausal were considered separately, risk increases with tamoxifen were similar and remained significantly elevated (Table 2). There was no evidence of treatment interactions, as the combined risk estimate was nearly identical among trials in which tamoxifen was the only treatment.8,9,11,12,30,34
Figure 1.
Risk ratio of stroke associated with tamoxifen use (• depicts the risk ratio of stroke; solid lines depict the 95% CI; * indicates studies with placebo-control groups). Trials are listed in chronological order.
Table 2.
Relative Risks (95% CI) Associated with Tamoxifen Use for Selected Vascular and Neoplastic Outcomes*
| All Trials | Trials of Post-menopausal Women | Breast Cancer Treatment Trials | Breast Cancer Risk Reduction Trials | Trials with Tamoxifen as Only Treatment | |
|---|---|---|---|---|---|
| Strokes | 1.49 (1.16 to 1.90) | 1.68 (1.22 to 2.32) | 1.48 (1.07 to 2.04) | 1.50 (1.03 to 2.20) | 1.41 (1.03 to 1.94) |
| Number of events/patients in treatment groups | 156/19,498 | 100/8,093 | 90/6,650 | 66/12,850 | 91/14,410 |
| Number of events/patients in control groups | 104/19,494 | 59/8,082 | 60/6,621 | 44/12,873 | 64/14,423 |
| Myocardial infarctions (incidence) | 0.90 (0.66 to 1.23) | 0.89 (0.48 to 1.64) | 0.74 (0.47 to 1.16) | 1.08 (0.70 to 1.68) | 0.90 (0.62 to 1.30) |
| Number of events/patients in treatment groups | 74/14,769 | 19/1,258 | 33/1,919 | 41/12,850 | 55/13,511 |
| Number of events/patients in control groups | 82/14,773 | 21/1,249 | 44/1,900 | 38/12,873 | 61/13,524 |
| Myocardial infarctions (death) | 0.62 (0.41 to 0.93) | 0.53 (0.32 to 0.87) | 0.55 (0.36 to 0.87) | 1.13 (0.34 to 3.78) | 0.54 (0.34 to 0.88) |
| Number of events/patients in treatment groups | 37/13,891 | 23/3,778 | 28/3,741 | 9/10,150 | 26/12,295 |
| Number of events/patients in control groups | 61/13,899 | 45/3,734 | 53/3,734 | 8/10,165 | 48/12,310 |
| Pulmonary emboli | 1.88 (1.17 to 3.01) | 2.46 (1.24 to 4.91) | 2.02 (0.84 to 4.87) | 1.85 (1.05 to 3.25) | 1.77 (1.04 to 2.99) |
| Number of events/patients in treatment groups | 53/18,295 | 30/6,566 | 17/4,106 | 36/14,088 | 44/16,691 |
| Number of events/patients in control groups | 26/18,309 | 11/6,588 | 7/4,105 | 19/14,106 | 21/15,892 |
| Gastrointestinal cancers | 1.31 (1.01 to 1.69) | 1.77 (1.20 to 2.62) | 1.50 (1.11 to 2.04) | 0.95 (0.59 to 1.51) | 1.18 (0.90 to 1.56) |
| Number of events/patients in treatment groups | 140/16,937 | 73/3,324 | 106/5,549 | 34/11,388 | 110/14,650 |
| Number of events/patients in control groups | 104/16,916 | 39/3,310 | 68/5,518 | 36/11,398 | 92/14,676 |
| Endometrial cancers | 2.70 (1.94 to 3.75) | 3.18 (2.09 to 4.85) | 3.28 (2.09 to 5.14) | 2.16 (1.33 to 3.50) | 2.56 (1.77 to 3.71) |
| Number of events/patients in treatment groups | 140/22,992 | 98/15,070 | 88/11,604 | 52/11,388 | 107/16,214 |
| Number of events/patients in control groups | 45/22,944 | 27/15,047 | 21/11,546 | 24/11,398 | 39/16,231 |
Risks refer to incidences except where indicated otherwise.CI, confidence interval.
Myocardial Infarctions
Only 6 trials8,11,12,22,34,37 (29,542 participants) reported on the incidence of myocardial infarction, whereas 12 trials8,12,15,16,23,24,26,32,35,38–40 (totaling 27,790 participants) reported on myocardial infarction deaths. A total of 74 myocardial infarctions occurred in treatment groups and 82 occurred in control groups over a mean follow-up of 5.5 years. Tamoxifen did not affect the risk of incident myocardial infarction in the aggregate or in any subgroup analyses (Table 2). There was no evidence of treatment interactions, as the combined risk estimate was similar among trials in which tamoxifen was the only treatment.8,12,34
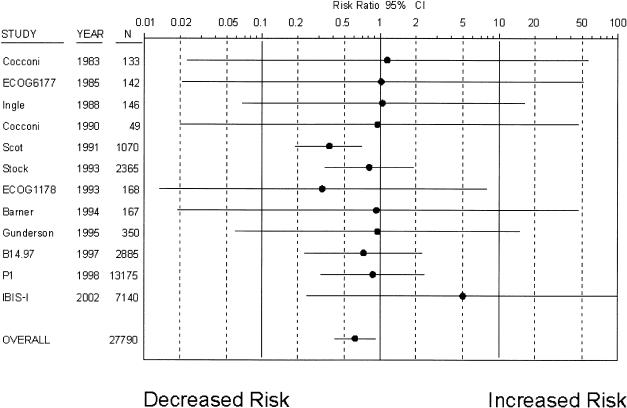
Tamoxifen was, however, associated with a significant reduction in the risk of myocardial infarction death (RR 0.62; 95% CI 0.41 to 0.93), based on 37 deaths occurring in treatment groups and 61 deaths occurring in control groups over 5.6 years of follow-up (Fig. 2). The rate of deaths from myocardial infarction in control groups was 7.8 per 10,000 patient years. Although there was no statistical suggestion of heterogeneity, review of the data suggested that one of the 12 trials35 disproportionately contributed to this treatment effect. Excluding this trial from the analysis modified the risk estimate considerably, leading to a loss of statistical significance (RR 0.81; 95% CI 0.48 to 1.37). There was no evidence of treatment interactions, as the combined risk estimate was nearly identical among the 6 trials in which tamoxifen was the only treatment.8,12,15,16,24,35 When women who were postmenopausal or in treatment trials were considered separately, decreases in myocardial infarction deaths were similar and remained statistically significant. However, the 2 risk reduction trials reporting on this outcome8,12 found no significant association between tamoxifen use and myocardial infarction death.
Figure 2.
Risk ratio of myocardial infarction death associated with tamoxifen use (•, risk ratio; solid lines, 95% CI; *, studies with placebo-control). Trials are listed in chronological order.
Other Vascular Outcomes
Eleven trials8–13,17,23,28,30,37,42 with 36,604 patients reported on the incidence of pulmonary emboli. Seventy-nine pulmonary emboli occurred over a mean follow-up of 5.5 years, with an incidence of 2.6 per 10,000 patient years in control groups. Tamoxifen was associated with a significantly increased risk of pulmonary emboli (RR 1.88; 95% CI, 1.17 to 3.01). Fifteen trials8–13,17,20,27,28,30,37,38,42–44 with 35,817 patients reported the incidence of deep venous thromboses, and tamoxifen was associated with a nearly identical increase (RR 1.87; 95% CI, 1.33 to 2.64). When women who were postmenopausal, in treatment trials, and in risk reduction trials were analyzed separately, risk increases for pulmonary emboli were similar.
Gastrointestinal Cancers
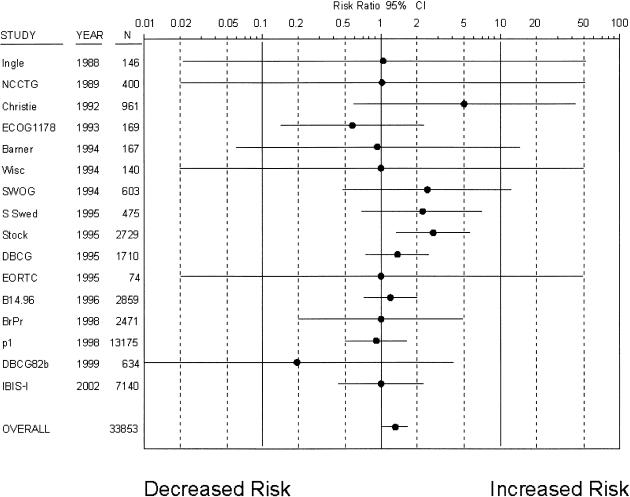
Sixteen trials8,10,12,13,16–18,24–29,42 with 33,853 patients reported the incidence of gastrointestinal cancers (Fig. 3). A total of 140 gastrointestinal cancers occurred in treatment groups and 104 occurred in control groups over a mean follow-up of 5.6 years, and tamoxifen was associated with a modest but statistically significant risk increase (RR 1.31; 95% CI, 1.01 to 1.69). The incidence of gastrointestinal cancers in control groups was 10.9 per 10,000 patient years. Eighteen trials8,11–13,16–18,20,24–29,36,42,43 reported on colorectal cancers specifically (229 cancers), and tamoxifen was not associated with a statistically significant risk increase (RR 1.16; 95% CI, 0.86 to 1.57).
Figure 3.
Risk ratio of gastrointestinal cancers associated with tamoxifen (•, risk ratio; solid lines, 95% CI; *, studies with placebo-control). Trials are listed in chronological order.
When subgroups of patients were considered separately (Table 2), postmenopausal women had higher risk elevations than were observed in all trials combined. While women in treatment trials had somewhat higher risk elevations, women in risk reduction trials appeared to have no increase in gastrointestinal cancer risk. There was no evidence of treatment interactions, as subgroup analyses of trials in which tamoxifen was the only treatment8,10,12,13,16–18,24,29 produced similar risk estimates.
Although there was no statistical suggestion of heterogeneity, review of the data suggested that the three trials conducted in Scandinavian countries18 contributed disproportionately to the treatment effect, reporting similar increases in risk with tamoxifen (RR 1.9; 95% CI, 1.2 to 2.9). In contrast, the majority of trials and the single largest trial (NASBP P-1) did not report significant risk increases.
Other Neoplastic Outcomes
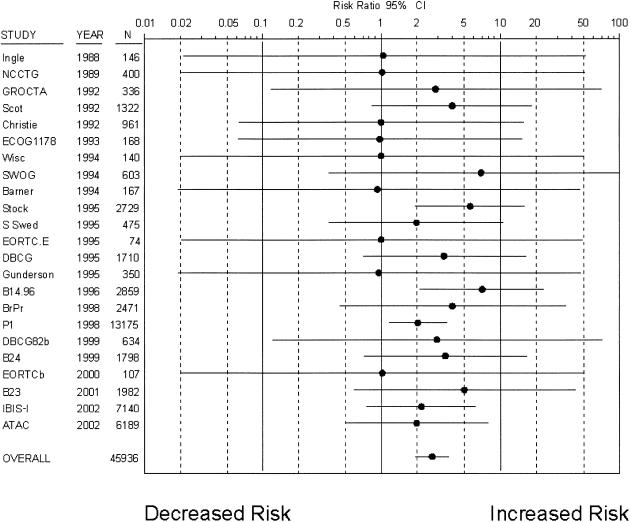
Twenty-three trials8,10,12,14,16–18,24–33,36,42–45 with 45,936 patients reported on endometrial cancers as a clinical outcome. A total of 185 endometrial cancers occurred over a mean follow-up of 5.4 years (Fig. 4), with an incidence of 3.6 per 10,000 patient years in control groups. Tamoxifen was associated with a statistically significant risk increase (RR 2.70; 95% CI, 1.94 to 3.75). When women who were postmenopausal or in treatment trials were considered separately (Table 2), risk increases were greater. However, when women in risk reduction trials were considered separately, their risk increase was more modest but still statistically significant (RR 2.16; 95% CI, 1.33 to 3.50). The risk estimate was similar among the 13 placebo-controlled trials.8,10,12,14,16–18,21,24,29,30,33,36
Figure 4.
Relative risk of endometrial cancers associated with tamoxifen use (•, relative risk; solid lines, 95% CI; *, studies with placebo-control). Trials are listed in chronological order.
To further investigate whether tamoxifen was associated with an increased risk of cancers not of breast or endometrial origin, these tumors were grouped together as a single outcome measure. Twenty trials8,9,11–13,17,18,20,24,26–31,36,42–44 with 50,605 patients reported a thorough inventory of malignancies, with 646 cancers other than breast or endometrial origin occurring in treatment groups and 625 occurring in control groups over a mean follow-up of 5.6 years. Tamoxifen had no statistically significant effect on their incidence (RR 1.04; 95% CI, 0.92 to 1.17), although the 13 placebo-controlled trials8,9,11–13,17,18,20,21,24,29,30,36 showed a trend toward an increase (RR 1.11; 95% CI, 0.98 to 1.27). When gastrointestinal tumors were subtracted from this group, the risk estimates were slightly lower (all trials: RR 0.96; 95% CI, 0.81 to 1.13; placebo-controlled trials, RR 1.04; 95% CI, 0.86 to 1.25). Risk estimates were similar for postmenopausal women.
DISCUSSION
In this meta-analysis of randomized controlled trials focused on adverse outcomes, tamoxifen use was significantly associated with a higher risk of developing endometrial cancer, deep venous thrombosis, strokes, pulmonary emboli, and all gastrointestinal cancers combined. In the most recent Early Breast Cancer Trialists' Collaborative Group (EBCTCG) report,4 tamoxifen use was associated with a nonsignificant trend suggesting increased risk of deaths from strokes and pulmonary emboli. In the NSABP P-1 trial (National Surgical Adjuvant Breast and Bowel Project),8 tamoxifen use was associated with a nonsignificant increase in stroke. Thus, the current analysis of all published randomized trials identified several statistically significant associations in areas where nonsignificant trends had been previously reported. Kinsinger et al.49 recently published a summary of the evidence of breast cancer chemoprevention. Our analysis augments these results by 1) using statistical methodology to produce summary risk estimates; 2) incorporating the recently published results of the fourth tamoxifen breast cancer prevention trial (International Breast Cancer Intervention Study Group; IBIS); and 3) including analyses for patients using tamoxifen for reasons other than breast cancer prevention.
For most adverse outcomes analyzed, our risk estimates were similar to the largest meta-analysis describing tamoxifen outcomes (EBCTCG),4 as well as the largest chemoprevention trial (NSABP P-1).8 Our estimate for endometrial cancer risk (RR 2.70) was similar to both the EBCTCG (RR 2.58) and the NSABP P-1 (RR 2.53) results. Our estimate for colorectal cancer risk (RR 1.16) was also similar to these results (RR 1.11 and 1.25, respectively). Additionally, the NSABP P-1 trial reported risk increases similar to the present analysis for stroke (RR 1.59 vs 1.49). However, our finding that tamoxifen may decrease myocardial infarction deaths is in contrast to these reports. The EBCTCG review did not report on myocardial infarction deaths specifically but noted no statistically significant effect on all causes of cardiac mortality. The NSABP P-1 trial did not find a significant effect of tamoxifen on fatal or nonfatal myocardial infarctions, but the low number of events (15) in this younger group of women limited the statistical power of this analysis. Our estimate for increased gastrointestinal cancer risk (RR 1.31) was also in contrast to the NSABP P-1 result (RR 0.91); the EBCTCG did not analyze this outcome. Notably, we found a particularly high risk for gastrointestinal cancers in postmenopausal women (RR 1.77), and therefore heterogeneity among patients with different menopausal status may explain some of this variation. Observational data also suggest an increased risk for gastrointestinal cancers with tamoxifen. In a retrospectively analyzed U.S. cohort of breast cancer patients,50 use of tamoxifen was associated with a trend toward increased incidence of colorectal cancer (RR 1.46; 95% CI, 0.92 to 2.31). In another retrospective cohort study of breast cancer survivors in Japan,51 women exposed to tamoxifen had a trend toward increased risk for stomach cancer (RR 1.37; 95% CI, 0.76 to 2.38).
Tamoxifen mimics the effect of estrogen on certain organs. Indeed, data from the recently published randomized controlled trial of hormone replacement therapy, the Women's Health Initiative,52 was strikingly similar to some of the risk estimates from the current analyses (stroke, RR 1.41 vs 1.49; pulmonary embolism, RR 2.13 vs 1.88). Tamoxifen may act as an estrogen antagonist on other organs, and this may explain why the Women's Health Initiative found effects of similar magnitude but opposite directions to risk estimates in the present analysis for myocardial infarctions (RR 1.32; 95% CI, 1.02 to 1.72) and colorectal cancers (RR 0.63; 95% CI, 0.43 to 0.92).
Tamoxifen may have opposing effects on thrombosis and atherogenesis, two important determinates of vascular pathology. Tamoxifen has been shown to decrease coronary plaques in vivo,53,54 improve lipid profiles,55,56 reduce C-reactive protein,57 and modulate nitric oxide production.58 In contrast, it may alter the balance of clotting proteins in a manner that promotes thrombosis.59 It is possible that the discrepancy between increases in pulmonary emboli and stroke and decreases in myocardial infarction deaths arises from differences in the relative contributions of atherogenesis and thrombosis to these outcomes. Furthermore, the relative importance of these effects may be different for populations with different coronary artery disease risks. The greatest reduction in myocardial infarction deaths with tamoxifen use was reported among a Scottish population, a group with a particularly high prevalence of hyperlipidemia and coronary artery disease.34,35 A posthoc analysis of NSABP P-1 trial coronary outcomes60 reported that patients with active coronary disease receiving tamoxifen had fewer and less lethal myocardial infarctions (6 vs 9 events and 0 vs 4 deaths; not statistically significant). Furthermore, in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial,61 raloxifene, another selective estrogen receptor modulator, was associated with a similar risk reduction in cardiovascular events of 0.60 (95% CI, 0.38 to 0.95) among women at increased cardiovascular risk but not among all enrolled women.
The meta-analyses published by the Early Breast Cancer Trialists' Collaborative Group have become a landmark source on breast cancer interventions such as tamoxifen. Their analytic process has many strengths, including reliance on updated primary data, face-to-face trialist interaction, and publication of results only with the concurrence of all trialists. The considerable time and effort requested to complete this process is reflected in the 32-month interval between the 1995 Early Trialist meetings (when preliminary results were presented) to eventual publication in the literature.4 Albeit by different methodology, the approach utilized in the current analysis also identified several statistically significant associations in a timely fashion. Such a literature-based meta-analysis of comorbidities might be applicable in other cancer therapy settings where the EBCTCG approach has been somewhat less successful, such as for trials involving colorectal cancer.
Although some of the relative risk increases for adverse outcomes may seem large, the absolute risk increases are not as dramatic. If all adverse outcomes with statistically significant risk increases in the present analysis are considered together (pulmonary emboli, stroke, gastrointestinal cancers, endometrial cancers), the absolute risk for any event after 5 years of tamoxifen treatment is 0.84%, corresponding to one adverse outcome for every 118 patients treated. In comparison, the number needed to treat to prevent one breast cancer in a woman with the minimum risk for which tamoxifen is indicated (1.66% after 5 years) is 159, assuming a risk reduction of 38%.62 For a higher risk woman (5% 5-year risk), the number needed to treat would be 53.
Limitations
Reporting bias, a reduced likelihood to include nonsignificant findings in a published report, is a type of publication bias that may have been an important limitation of this meta-analysis. Secondary outcomes are often recorded retrospectively and not as systematically as primary outcomes. Reporting bias may result in underestimation of absolute risk when surveillance is limited because an adverse event has not yet been recognized, and may result in overestimation of both absolute and relative risks when only studies with unusually high incidences of secondary outcomes report those results.
The potential impact of reporting bias can be seen when tamoxifen and endometrial cancer risk is considered. Endometrial cancer risk was not reported as a risk associated with tamoxifen until 1994.28 Trials conducted before this time may have reported no increased risk simply because the data needed to determine this risk were not systematically collected or assessed, only reporting this endpoint if they noticed a large difference in rates between treatment and control groups.
Although reporting bias is an important limitation of any meta-analysis that is not based on primary data collection, there is some evidence that our results were not critically undermined by this factor: 1) our estimates for many risks were similar to those reported by the largest study in which they were prespecified endpoints (NASBP P1);8 2) the incidences of malignancies other than breast, endometrial, and gastrointestinal cancers were nearly identical in the treatment and control groups; and 3) funnel plots of the outcome measures did not demonstrate the asymmetry that sometimes characterizes publication bias (data not shown). The present analyses should be viewed as exploratory and used to guide the collecting and reporting of adverse events in future trials, because systematically combing events that may not have been systematically collected or assessed can be challenging.
Several other methodological limitations should be considered when interpreting these results. Because this study was based on published reports rather than primary data analysis, the ability to identify patient characteristics associated with greater risks was limited. Different groups of studies reported on each adverse outcome, and each risk estimate may have reflected differences in true effects or biases particular to the studies from which the risk estimate was derived. In many breast cancer treatment trials, only initial secondary tumors are reported and not subsequent ones, so all tumors may not have been captured in our analysis. Reporting of causes of deaths in cancer patients is often imprecise because of difficulty establishing whether death is due to the tumor or to a specific event that the tumor may have influenced. Lastly, the majority of studies were performed on women of European ancestry, and the results of this meta-analysis may not be generalizable to women of other races or ethnicities.
CONCLUSIONS
In summary, this meta-analysis found tamoxifen use to be significantly associated with several neoplastic and vascular outcomes. These results highlight the importance of prospectively identifying and investigating important secondary health outcomes when evaluating risk reduction therapies. Predicting whether tamoxifen will cause more harm than benefit for an individual woman, particularly when used for breast cancer risk reduction, requires the consideration of her individual risks for developing these outcomes (e.g., her absolute risk) as well as her risk for developing breast cancer. This quantitative summary of relative risks adds to the literature and may assist health care providers and patients who are weighing these complex issues.
Acknowledgments
This research was supported by National Library of Medicine grant # T15-LM07092-09, the Pharmaceutical Research and Manufacturers' Association, the Robert Wood Johnson Foundation, and AHRQ grant #R25-HS09796.
REFERENCES
- 1.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT. Reducing the risk of breast cancer. N Engl J Med. 1998;343:191–8. doi: 10.1056/NEJM200007203430307. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Col NF, Winer EP, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase reduction. J Clin Oncol. 2002;20:3328–43. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 5.Hasselblad V, McCrory DC. Meta-analytic tools for medical decision making: a practical guide. Med Decis Making. 1995;15:81–96. doi: 10.1177/0272989X9501500112. [DOI] [PubMed] [Google Scholar]
- 6.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Int Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Berlin JA, Laird NM, Sacks HS, et al. A comparison of statistical methods for combining event rates from clinical trials. Stat Med. 1989;8:141–51. doi: 10.1002/sim.4780080202. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Constantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Powles T, Eles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;252:98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Lancet. 1998;352:93–7. doi: 10.1016/s0140-6736(98)85011-3. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Maisonneuve P, Sacchini V, et al. Tamoxifen for breast cancer among hysterectomized women. Lancet. 2002;359:1122–4. doi: 10.1016/S0140-6736(02)08159-X. [DOI] [PubMed] [Google Scholar]
- 12.IBIS Investigators. First results from the International Breast Cancer Intervention Study (IBIS-1): a randomised prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–42. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Constantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86:527–37. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 15.Constantino JP, Kuller LH, Ives DG, et al. Coronary heart disease mortality and adjuvant tamoxifen therapy. J Natl Cancer Inst. 1997;89:776–82. doi: 10.1093/jnci/89.11.776. [DOI] [PubMed] [Google Scholar]
- 16.Barner M, Bacchi M, Goldhirsch A, et al. First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase 3 multicenter study comparing systemic treatment with observation after excision and radiation. J Clin Oncol. 1994;12:2071–7. doi: 10.1200/JCO.1994.12.10.2071. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro G, Swindell R. The Christie Hospital Adjuvant Tamoxifen Trial. J Natl Cancer Inst Monogr. 1992;11:121–5. [PubMed] [Google Scholar]
- 18.Rutqvist LE, Signomklao H Johansson, et al. Adjuvant tamoxifen therapy for early stage breast cancer and secondary primary malignancies. J Natl Cancer Inst. 1995;87:645–51. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- 19.Ryden S, Ferno M, Moller T, et al. Long term effects of adjuvant tamoxifen and/or radiotherapy. Acta Oncol. 1992;31:271–4. doi: 10.3109/02841869209088914. [DOI] [PubMed] [Google Scholar]
- 20.Andersson M, Storm HH, Mouridsen HT. Incidence of new primary cancers after adjuvant tamoxifen therapy and radiotherapy for early breast cancer. J Natl Cancer Inst. 1991;83:1013–7. doi: 10.1093/jnci/83.14.1013. [DOI] [PubMed] [Google Scholar]
- 21.Dalberg K, Johansson H, Johansson U. A randomized trial of long term adjuvant tamoxifen plus postoperative radiation therapy versus radiation therapy alone for patients with early stage breast carcinoma treated with breast-conserving surgery. Cancer. 1998;82:2204–11. [PubMed] [Google Scholar]
- 22.Rutqvist LE, Mattson A. Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. J Natl Cancer Inst. 1993;85:1398–406. doi: 10.1093/jnci/85.17.1398. [DOI] [PubMed] [Google Scholar]
- 23.Fornander T, Rutqvist LE, Cedermark B, et al. Adjuvant tamoxifen in early-stage breast cancer: effects on intercurrent morbidity and mortality. J Clin Oncol. 1991;9:1740–8. doi: 10.1200/JCO.1991.9.10.1740. [DOI] [PubMed] [Google Scholar]
- 24.Cummings FJ, Gray R, Tormey DC, et al. Adjuvant tamoxifen versus placebo in elderly women with node-positive breast cancer: long term follow-up and causes of death. J Clin Oncol. 1993;11:29–35. doi: 10.1200/JCO.1993.11.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Alwyn van Zyl J, Muller AG. Tumor excision plus continuous tamoxifen compared with modified radical mastectomy in patients over 70 years of age with operable breast cancer. J Surg Oncol. 1995;59:151–4. doi: 10.1002/jso.2930590304. [DOI] [PubMed] [Google Scholar]
- 26.Ingle JN, Everson LK, Wieland HS, et al. Randomized trial of observation versus adjuvant therapy with cyclophosphamide, fluorouracil, prednisone with or without tamoxifen following mastectomy in postmenopausal women with node-positive breast cancer. J Clin Oncol. 1988;6:1388–96. doi: 10.1200/JCO.1988.6.9.1388. [DOI] [PubMed] [Google Scholar]
- 27.Ingle JN, Everson LK, Weiland HS, et al. Randomized trial to evaluate the addition of tamoxifen to cyclophosphamide, 5-fluorouracil, prednisone adjuvant therapy in premenopausal women with node-positive breast cancer. Cancer. 1989;63:1257–64. doi: 10.1002/1097-0142(19890401)63:7<1257::aid-cncr2820630705>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Rivkin SE, Green S, Metch B, et al. Adjuvant CMFVP versus tamoxifen versus concurrent CMFVP and tamoxifen for postmenopausal, node-positive, and estrogen receptor-positive breast cancer patients: a Southwest Oncology Group Study. J Clin Oncol. 1994;12:2078–85. doi: 10.1200/JCO.1994.12.10.2078. [DOI] [PubMed] [Google Scholar]
- 29.Love RR, Wiebe DA, Feyzi JM, et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women after 5 years of treatment. J Natl Cancer Inst. 1994;86:1534–9. doi: 10.1093/jnci/86.20.1534. [DOI] [PubMed] [Google Scholar]
- 30.Fisher B, Digham J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomized controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 31.Boccardo F, Rubagotti A, Amoroso D, et al. Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen-receptor positive breast cancer patients. An update at 7 years of the 1st GROCTA (Breast Cancer Adjuvant Chemo-hormone Therapy Cooperative Group) trial. Eur J Cancer. 1992;28:673–80. doi: 10.1016/s0959-8049(05)80123-6. [DOI] [PubMed] [Google Scholar]
- 32.Gundersen S, Hannisdal E, Soreide JA, et al. Adjuvant tamoxifen for pre- and postmenopausal women with estrogen receptor positive, node positive breast cancer: a randomized study. Breast Cancer Res Treat. 1995;36:49–53. doi: 10.1007/BF00690184. [DOI] [PubMed] [Google Scholar]
- 33.Stewart HJ. The Scottish trial of adjuvant tamoxifen in node-negative breast cancer. J Natl Cancer Inst Monogr. 1992;11:117–20. [PubMed] [Google Scholar]
- 34.McDonald CC, Alexander FE, Whyte BW, et al. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomized trial. BMJ. 1995;311:977–80. doi: 10.1136/bmj.311.7011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald CC, Stewart HJ. Fatal myocardial infarction in the Scottish adjuvant tamoxifen trial. BMJ. 1991;303:435–7. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart HJ, Prescott RJ, Forrest PM. Scottish Adjuvant Tamoxifen Trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93:456–62. doi: 10.1093/jnci/93.6.456. [DOI] [PubMed] [Google Scholar]
- 37.Taylor SG, Kalish LA, Olson JE, et al. Adjuvant CMFP versus CMFP plus tamoxifen versus observation alone in postmenopausal, node-positive breast cancer patients: three year results of an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1985;3:144–54. doi: 10.1200/JCO.1985.3.2.144. [DOI] [PubMed] [Google Scholar]
- 38.Tormey DC, Gray R, Taylor SG, et al. Postoperative chemotherapy and chemohormonal therapy in women with node-positive breast cancer. J Natl Cancer Inst Monogr. 1986;1:75–80. [PubMed] [Google Scholar]
- 39.Cocconi G, De Lisi V, Boni C, et al. Chemotherapy versus combination of chemotherapy and endocrine therapy in advanced breast cancer. Cancer. 1983;51:581–8. doi: 10.1002/1097-0142(19830215)51:4<581::aid-cncr2820510404>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.Cocconi G, Di Blasio B, Bisagni G, et al. Neoadjuvant chemotherapy or chemotherapy and endocrine therapy in locally advanced breast carcinoma. Am J Clin Oncol. 1990;13:226–32. doi: 10.1097/00000421-199006000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Robertson JR, Ellis IO, Elston CW, et al. Mastectomy of tamoxifen as initial therapy for operable breast cancer in elderly patients: 5 year follow-up. Eur J Cancer. 1992;28A:908–10. doi: 10.1016/0959-8049(92)90148-u. [DOI] [PubMed] [Google Scholar]
- 42.Andersson M, Kamby C, Jensen MB, et al. Tamoxifen in high-risk premenopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish Breast Cancer Co-operative Group DBCG 82B Trial. Eur J Cancer. 1999;35:1659–66. doi: 10.1016/s0959-8049(99)00141-0. [DOI] [PubMed] [Google Scholar]
- 43.The ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists' Group. Anastrazol alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359:2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 44.Fisher B, Anderson S, Tan- Chiu E, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B23. J Clin Onc. 2001;19:931–42. doi: 10.1200/JCO.2001.19.4.931. [DOI] [PubMed] [Google Scholar]
- 45.Klijn JG, Beex LV, Mauriac L, et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: a randomized study. J Natl Cancer Inst. 2000;92:903–11. doi: 10.1093/jnci/92.11.903. [DOI] [PubMed] [Google Scholar]
- 46.Rusthoven JJ, Quirt IC, Iscoe NA, et al. Randomized, double-blind, placebo-controlled trial comparing the response rates of carmustine, dacarbazine, and cisplatin with and without tamoxifen in patients with metastatic melanoma. J Clin Oncol. 1996;14:2083–90. doi: 10.1200/JCO.1996.14.7.2083. [DOI] [PubMed] [Google Scholar]
- 47.Agarwala SS, Ferri W, Gooding W, et al. A phase 3 randomized trial of dacarbazine and carboplatin with and without tamoxifen in the treatment of patients with metastatic melanoma. Cancer. 1999;85:1979–84. [PubMed] [Google Scholar]
- 48.Cocconi G, Bella M, Calabresi F, et al. Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. N Engl J Med. 1992;327:516–23. doi: 10.1056/NEJM199208203270803. [DOI] [PubMed] [Google Scholar]
- 49.Kinsinger LS, Harris R, Woolf SH, et al. Chemoprevention of breast cancer: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:59–67. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- 50.Newcomb P, Solomon C, White E. Tamoxifen and risk of large bowel cancer in women with breast cancer. Breast Cancer Res Treat. 1999;53:271–7. doi: 10.1023/a:1006117220284. [DOI] [PubMed] [Google Scholar]
- 51.Matsuyama Y, Tominaga T, Nomura Y, et al. Second cancers after adjuvant tamoxifen therapy for breast cancer in Japan. Ann Oncol. 2000;11:1337–43. doi: 10.1093/oxfordjournals.annonc.a010406. [DOI] [PubMed] [Google Scholar]
- 52.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 53.Williams KJ, Wagner JD, Zhang L, et al. Tamoxifen inhibits arterial accumulation of LDL degradation products and progression of coronary artery atherosclerosis in monkeys. Arterioscler Thromb Vasc Biol. 1997;17:403–8. doi: 10.1161/01.atv.17.2.403. [DOI] [PubMed] [Google Scholar]
- 54.Williams KJ, Honore EK, Adams MR. Contrasting effects of conjugated estrogens and tamoxifen on dilator responses of atherosclerotic epicardial coronary arteries nonhuman primates. Circulation. 1997;96:1970–75. doi: 10.1161/01.cir.96.6.1970. [DOI] [PubMed] [Google Scholar]
- 55.Clarke SC, Schofield PM, Grace AA, et al. Tamoxifen effects on endothelial function and cardiovascular risk factors in men with advanced atherosclerosis. Circulation. 2001;103:1497–502. doi: 10.1161/01.cir.103.11.1497. [DOI] [PubMed] [Google Scholar]
- 56.Love RR, Wiebe DA, Newcomb PA, et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991;115:860–4. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- 57.Cushman M, Tracy JP, Song RP, et al. Tamoxifen and cardiac risk factors in healthy women: suggestion of an anti-inflammatory effect. Arterioscler Thromb Vasc Biol. 2001;21:255–61. doi: 10.1161/01.atv.21.2.255. [DOI] [PubMed] [Google Scholar]
- 58.Figtree GA, Webb CM, Collins P. Tamoxifen acutely relaxes coronary arteries by an endothelium-, nitric oxide-, and estrogen receptor-dependent mechanism. J Pharmacol Exp Ther. 2000;295:519–23. [PubMed] [Google Scholar]
- 59.Love RR, Surawicz TS, Williams EC. Antithrombin III level, fibrinogen level, and platelet count changes with adjuvant tamoxifen therapy. Arch Intern Med. 1992;152:317–20. [PubMed] [Google Scholar]
- 60.Reis SE, Constantino JP, Wickerham DL, et al. Cardiovascular effects of tamoxifen in women with and without heart disease: breast cancer prevention trial. J Natl Cancer Inst. 2001;93:16–21. doi: 10.1093/jnci/93.1.16. [DOI] [PubMed] [Google Scholar]
- 61.Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women. JAMA. 2002;287:847–57. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- 62.Kuzick J. Update on new studies in Europe. Eur J Cancer. 2002;38:S20. [Google Scholar]