Abstract
The regulatory circuit for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 (KSHV/HHV-8) gene expression bears resemblance to that of Epstein-Barr virus (EBV), but with interesting differences. Based on protein sequence similarities and synteny to their EBV counterparts, two KSHV/HHV-8 viral regulatory factors, HHV-8 Rta and K-bZIP, encoded by open reading frame (ORF) 50 and ORF K8, respectively, have been identified. Rta is an immediate early transcriptional activator that activates lytic viral replication and mediates viral reactivation from latency, while ORF K8 is an early gene activated by Rta. Extensive splicing of ORF K8 mRNA leads to the production of K-bZIP, a protein of the basic domain-leucine zipper (bZIP) family. The role of K-bZIP in viral replication, however, remains unresolved. Here, we report that K-bZIP is a nuclear protein that binds Rta directly both in vivo and in vitro and represses Rta-mediated transactivation of the K-bZIP promoter. We further demonstrate that the leucine zipper domain of K-bZIP is required for Rta binding and a K-bZIP mutant lacking the leucine zipper does not repress Rta activity. Finally, the K-bZIP-mediated repression of Rta transactivation cannot be restored by overexpression of the transcriptional coactivator p300 or the p300-CBP-associated factor, P/CAF. Our results suggest that K-bZIP is involved in a feedback circuit to turn off its own expression and possibly the expression of other early genes activated by Rta.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is a newly identified human gammaherpesvirus that is strongly linked to the development of Kaposi's sarcoma and lymphoproliferative diseases, including body cavity-based B-cell lymphoma, otherwise known as primary effusion lymphoma (PEL), and Castleman's disease (1-4, 24, 26, 29). Like other herpesviruses, KSHV/HHV-8 follows an orderly program of gene expression during its replication cycle. Lytic viral replication is initiated by the expression of immediate-early genes whose gene products then activate the expression of early genes.
The mechanism of viral reactivation from latency has been well characterized for Epstein-Barr virus (EBV)—another member of the gammaherpesvirus family—where the immediately-early transactivator, Zta (also called EB1, Zebra, or BZLF1), of EBV induces the viral lytic cycle (16, 20) by augmenting transcription of both itself and EBV Rta after binding to cis-regulatory elements in their respective promoters (16, 28). Zta and Rta together activate expression of a third transactivator, Mta (BMLF1) (6, 9, 11, 15). The concerted action of these three viral regulatory factors then leads to the sequential activation of early gene expression followed by DNA replication and late gene expression (7, 8, 25, 28).
Based on protein sequence similarities and synteny to their EBV counterparts, two putative HHV-8 viral transcriptional regulators, HHV-8 Rta and K-bZIP, encoded by open reading frame (ORF) 50 and ORF K8, respectively, have been identified (10, 17). Like their EBV “equivalents,” the polypeptides for HHV-8 Rta and K-bZIP are derived from splicing of a complex set of RNA transcripts (10, 17, 32). While EBV Rta and Zta act synergistically and are both important for EBV reactivation in latently infected B cells, HHV-8 Rta alone appears to be sufficient for viral reactivation (18, 19, 30). DNA transfection of an expression construct of Rta into cells harboring a latent KSHV/HHV-8 genome triggers lytic viral replication (30). Furthermore, KSHV/HHV-8 Rta becomes expressed immediately after induction of latently infected PEL-derived B cells by chemicals such as tetradecanoyl phorbol ester acetate and sodium butyrate (31). Rta, in turn, transactivates the expression of multiple KSHV/HHV-8 early gene products that include the ORF 6 product (single-stranded-DNA-binding protein), the ORF 9 product (DNA polymerase), the ORF 21 product (thymidine kinase), the ORF 57 product (Mta), the ORF 59 product (PF8), nut-1/PAN, K9 (vIRF1), K12 (kaposin), and importantly, K8 (K-bZIP) (5, 18, 19, 27).
K-bZIP is a homodimeric phosphoprotein of 237 amino acid residues that contains a prototypic basic region-leucine zipper (bZIP) domain at the carboxyl terminus (17). K-bZIP derives its sequence from three coding exons and one untranslated exon via splicing of the K8 ORF mRNA (17). While K8 ORF mRNA can yield multiple gene products via alternative splicing, K-bZIP appears to be the predominant isoform (14, 17, 23). Although the amino acid sequence of the bZIP domain of K-bZIP is reminiscent of that of EBV Zta (17), K-bZIP differs from EBV Zta functionally (10, 23). Unlike EBV Zta, K-bZIP cannot activate lytic viral replication when expressed in latently infected B cells (23). Recently, K-bZIP has been shown to interact with p53 and repress the transcriptional activity of p53 (22). This activity of K-bZIP presumably inhibits p53-mediated apoptosis and can facilitate viral replication (22). K-bZIP also appears to play a role in viral replication when cotransfected with genes encoding the core HHV-8 replication proteins (ORF40/41 primase-associated factors, ORF6 single-stranded DNA-binding proteins, ORF59 polymerase processivity factor, ORF9 polymerase, ORF44 helicase, and ORF56 primase), which form large globular “pseudo-replication compartments” that exclude cellular DNA, K-bZIP was found to localize to pseudo-replication compartments (33). Association of K-bZIP with the punctate cellular promyelocytic leukemia protein (PML)-associated nuclear bodies and viral replication complex has also been reported (33).
The fact that KSHV/HHV-8 Rta is functionally equivalent to EBV Zta in viral reactivation raises interesting questions regarding the role of K-bZIP in KSHV/HHV-8 viral gene expression. Here, we show that K-bZIP is a nuclear protein that binds directly to KSHV/HHV-8 Rta and represses transactivation of its own promoter by Rta. The leucine zipper domain of K-bZIP is required for Rta binding, and a K-bZIP mutant lacking the leucine zipper domain failed to repress Rta-mediated transactivation. Finally, the repression of Rta transactivation by K-bZIP cannot be reversed by overexpression of transcription coactivators p300 and/or P/CAF.
K-bZIP represses Rta-mediated transactivation.
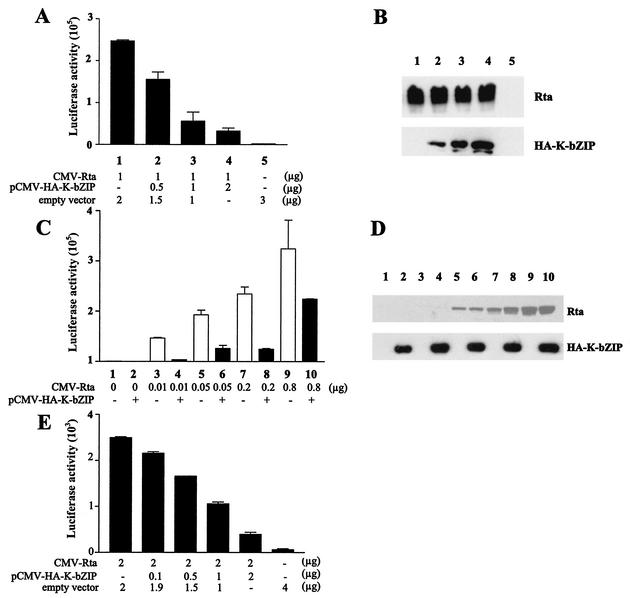
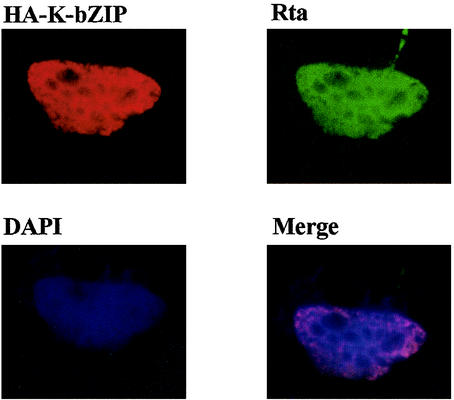
Because EBV Zta and Rta act synergistically to promote viral gene expression, we sought to investigate whether K-bZIP may also collaborate with KSHV/HHV-8 Rta to activate viral transcription. KSHV/HHV-8 Rta has been shown to transactivate many early genes, including ORF K8, which encodes K-bZIP (19). We previously constructed a reporter, RtaRE220-Luc, in which a DNA fragment containing a 220-bp sequence upstream of the ORF K8 translational start site was inserted upstream of a promoterless firefly luciferase gene in a reporter plasmid, pA3Pluc. To determine whether K-bZIP can affect Rta function, human embryonic kidney (HEK) 293 cells were transiently cotransfected with CMV-Rta, RtaRE220-Luc, and increasing amounts of pCMV-HA-K-bZIP. As anticipated, Rta greatly augmented luciferase expression driven by the ORF K8 promoter in RtaRE220-Luc (Fig. 1A, compare lanes 1 and 5). Coexpression of K-bZIP, however, resulted in repression of the transactivating activity of Rta in a dose-dependent manner (Fig. 1A and B, bottom) and over a wide range of Rta concentrations (Fig. 1C and D). The diminution of Rta activity is not due to a reduction in Rta expression caused by K-bZIP, as indicated by immunoblotting of cell extracts prepared from HEK 293 cells cotransfected with both Rta and K-bZIP expression constructs driven by the cytomegalovirus (CMV) immediate-early enhancer-promoter (Fig. 1B and D). The fact that K-bZIP repressed Rta-mediated transactivation in a dose-dependent manner and yet had no effect on Rta expression driven by the CMV immediate-early enhancer-promoter suggests that the effect of K-bZIP on Rta is specific. The suppressive effect of K-bZIP on Rta was also seen in an EBV-positive human B-lymphoblast line, NC37, after DNA transfection using the GENEporter 2 transfection reagent (Gene Therapy Systems, San Diego, Calif.). Interestingly and in contrast to a previous report that K-bZIP is localized to the PML bodies in PEL cells and in 293T cells that overexpress the PML protein (13, 33), immunofluorescence showed that K-bZIP localizes in the nuclei of transfected HeLa cells in a diffuse staining pattern (Fig. 2A), analogous to Rta (Fig. 2B). The overall staining patterns of Rta and Zta appear to overlap (Fig. 2D), suggesting that they may be colocalized. The difference in K-bZIP localization in HeLa versus PEL and 293T cells may be a consequence of overexpression of PML protein in the latter cell types.
FIG. 1.
K-bZIP represses Rta-mediated transactivation. (A) Transactivation of RtaRE220-Luc by Rta is repressed by K-bZIP. A DNA fragment containing a 220-bp nucleotide sequence upstream of the ORF K8 translational start site was inserted upstream of the promoterless firefly luciferase gene in a reporter plasmid, pA3Pluc, to generate RtaRE220-Luc. RtaRE220-Luc was then used as a reporter for Rta-mediated transactivation. The cDNA for KSHV/HHV-8 Rta was amplified from a lambda phage-based cDNA library of a PEL cell line, BCBL1, using the primers 5′ AGATCT(BglII)CCATGG CGC AAG ATG ACAAG and 5′ GAATTC(EcoRI)TCA GTC TCG GAA GTA ATT ACG. The PCR product was cloned into the PCR2.1 TA-cloning vector, digested with BglII and EcoRI, and ligated into the BamHI and EcoRI sites of pcDNA3.1(+) vector to obtain CMV-Rta. The K-bZIP expression vector, pCMV-HA-K-bZIP, has been described (17). Approximately 106 HEK 293 cells/well in a six-well plate were cotransfected with the RtaRE220 (0.5 μg) in the presence (lanes 1 to 4) or absence (lane 5) of the Rta expression plasmid, CMV-Rta (1 μg), together with 0, 0.5, 1 and 2 μg of pCMV-HA-K-bZIP (lanes 1-4, respectively) using Lipofectamine (Invitrogen Corp.) as prescribed by the manufacturer. The total amount of transfected DNA (3 μg) in each well was kept constant by the addition of the empty vector plasmid pcDNA3.1(+). Cell lysates were prepared 48 h after transfection by dissolving the DNA-transfected cells from each well in 200 μl of the reporter lysis buffer (Promega Corp.). Twenty microliters of the lysate was then placed in each well of a 96-well plate. After injection of 100 μl of luciferase substrate buffer, the luciferase activity was measured immediately in an MLX microtiter plate luminometer (Dynex Technologies). Transfections and luciferase assays for each experiment were performed in triplicate with the averages and standard deviations of luciferase activities shown. (B) Expression of Rta driven by the CMV immediate early enhancer-promoter is not affected by K-bZIP. HEK 293 cells were transfected as described for panel A. Twenty micrograms of total cell proteins from each transfection was resolved in an SDS-12% polyacrylamide gel and immunoblotted with a rabbit polyclonal antibody against a peptide containing amino acid residues 527 to 539 (KKRKALTVPEADT) of Rta and an anti-HA mouse monoclonal antibody (Santa Cruz Biotechnology, Inc.). (C) Repression of Rta-mediated transactivation by K-bZIP at different Rta-to-K-bZIP ratios. DNA transfection was carried out as described for panel A, with increasing amounts of CMV-Rta and a fixed amount of K-bZIP. (D) Detection of Rta and K-bZIP in cell lysates from panel C. Immunoblots were carried out as for panel B using antibodies against Rta and HA epitopes. Each lane contains 10 μl of cell lysates (∼20 μg of total protein). (E) Repression of Rta transactivation by K-bZIP in NC37 cells. Two million NC37 B-cells in each well of a six-well plate were cotransfected with 2 μg of CMV-Rta and 0.1, 0.5, 1, or 2 μg of pCMV-HA-K-bZIP, using the GENEporter 2 transfection reagent (Gene Therapy System) as per the manufacturer's protocol. Forty-eight hours after transfection, cells were collected and luciferase assays were performed as described for panel A. A TK-promoter driven Renilla luciferase vector, pTK-RL (Promega Corp.), was used to normalize the transfection efficiency. The total amount of plasmid DNA in each well was kept constant by adding pcDNA3.1(+) empty vector.
FIG. 2.
Nuclear localization of K-bZIP and Rta. Approximately 105 HeLa cells were seeded on each of the coverslips in a six-well plate and cotransfected with pCMV-HA-K-bZIP and CMV-Rta by Lipofectamine (Invitrogen Corp.). Forty-eight hours after DNA transfection, cells on coverslips were fixed with paraformaldehyde and incubated with both rabbit anti-Rta and mouse anti-HA antibodies (1:500 dilution in phosphate-buffered saline-3% bovine serum albumin). After three washes with 2 ml of phosphate-buffered saline each, fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (1:400; Vector Laboratories Inc.) and Cy3-conjugated anti-mouse immunoglobulin G (1:10,000; Sigma) were applied for 1 h at room temperature. Cells were washed again with 2 ml of phosphate-buffered saline five times. Cells were then mounted with one drop of Fluoromount-G (Southern Biotechnology Associates, Inc.) which contained 0.5 μg of 4′,6′-diamidino-2-phenylindole (DAPI) per ml and visualized with a fluorescence microscope. The fluorescence images from Cy3 (HA-K-bZIP), fluorescein isothiocyanate (Rta), and DAPI were collected separately and overlaid by a computer to create the three-color images (merge).
K-bZIP binds KSHV/HHV-8 Rta in vivo and in vitro.
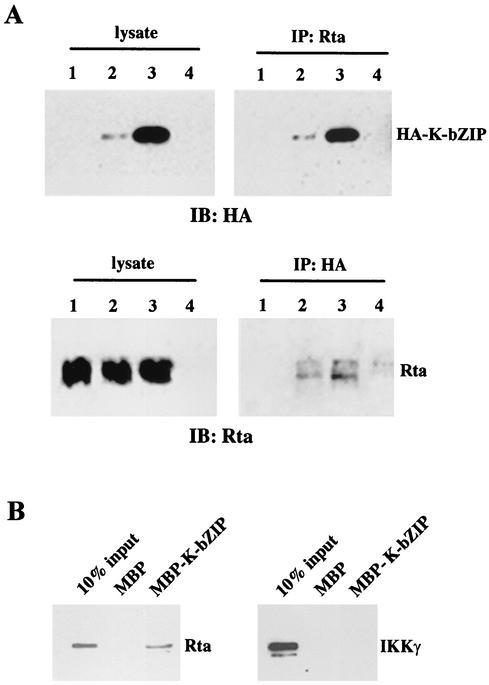
We next investigated whether K-bZIP interacts with Rta directly. HA-tagged K-bZIP and Rta expression plasmids (pCMV-HA-K-bZIP and CMV-Rta, respectively) were cotransfected into HEK 293 cells by lipofection. Forty-eight hours after transfection, cells were harvested, lysed, and sonicated. After centrifugation to remove cell debris, Rta was immunoprecipitated with a rabbit antiserum generated against a peptide (KKRKALTVPEADT) containing amino acid residues 527 to 539 of Rta (a generous gift from Gary Hayward), resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and probed for the presence of Rta-bound HA-K-bZIP using a monoclonal hemagglutinin (HA) antibody. As anticipated, HA-K-bZIP coimmunoprecipitated with Rta (Fig. 3A, top, lanes 2 and 3), while immunoprecipitates of HEK 293 cells transfected with CMV-Rta alone (lane 1) or mock transfected with pcDNA3.1(+) vector (lane 4) did not contain HA-K-bZIP. In the converse experiment, anti-HA antibody coimmunoprecipitated Rta only in the presence of HA-K-bZIP (Fig. 3A, bottom, lanes 2 and 3). We also noticed that the K-bZIP expressed in HEK 293 cells migrated at a position that corresponds to a molecular mass of 37 kDa (data not shown), greater than the predicted molecular mass of 27 kDa based on the amino acid sequence, apparently as a result of posttranslational modifications by cyclin-dependent kinases as previously reported (23). To determine if K-bZIP and Rta interact directly, we derived a maltose-binding protein (MBP)-K-bZIP fusion construct by joining the coding sequence of K-bZIP with that of MBP. MBP-K-bZIP was then expressed, purified by using amylose resin, and used in a pull-down assay together with purified Rta protein derived from an Escherichia coli expression system. As shown in Fig. 3B, left panel, Rta was bound by MBP-K-bZIP but not by the MBP control. No detectable binding to MBP-K-bZIP or MBP was observed for the control I-κB kinase γ-regulatory subunit (IKKγ) (Fig. 3B, right panel).
FIG. 3.
K-bZIP interacts with Rta in vivo and in vitro. (A) K-bZIP and Rta coimmunoprecipitate. Approximately 106 HEK 293 cells in each well of a six-well plate were transiently transfected with 1 μg of CMV-Rta alone (lanes 1) or together with 1 (lanes 2) or (lanes 3) 2 μg of pCMV-HA-K-bZIP or 1 μg of the empty vector pcDNA3.1(+) (lanes 4). Forty-eight hours after transfection, 500 μg of whole-cell lysates prepared in 250 μl of radioimmunoprecipitation assay buffer (9.1 mM dibasic sodium phosphate, 1.7 mM monobasic sodium phosphate, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS) containing 1× protease inhibitor cocktail (Roche Diagnostics) was immunoprecipitated with 10 μl of a rabbit polyclonal Rta antibody or 10 μl of an HA monoclonal antibody together with 20 μl of protein A-Sepharose beads (Invitrogen) overnight at 4°C. After the beads had been washed with radioimmunoprecipitation assay buffer four times, the bead-bound proteins were heated in the loading buffer and resolved on an SDS-12% polyacrylamide gel, transferred to a nitrocellulose membrane, and immunoblotted with anti-HA antibody (IP: Rta) or anti-Rta antibody (IP: HA). Panels labeled “lysate” show HA-K-bZIP (top) and Rta (bottom) expression in total cell lysates. (B) K-bZIP directly binds Rta. The complete coding sequence of K-bZIP was PCR amplified from pCMV-HA-K-bZIP by using the primers 5′-GCCGAATTC(EcoRI)ATGCCCAGAATGA-3′ and 5′-CGGGATCC(BamHI)TCAACATGGTGGGA-3′, digested with EcoRI and BamHI, and inserted in frame into the EcoRI and BamHI site of pMAL Cx2 (New England Biolabs, Inc.) to produce pMBP-K-bZIP. The MBP-K-bZIP fusion protein was expressed and purified according to the manufacturer's specifications. To derive Rta protein, the complete coding sequence of Rta was amplified by PCR from CMV-Rta and inserted into a bacterial expression vector, pTrc2His2-TOPO (Invitrogen Corp.), to produce pTrc2His2-TOPO-Rta, where Rta was tagged with a c-Myc epitope and a hexahistidine extension at the COOH terminus. Expression and purification of hexahistidine-tagged Rta protein by using Ni2+-nitrilotriacetic acid-Sepharose (Qiagen Inc.) were as described previously (34). Five micrograms of MBP or MBP-K-bZIP was incubated with 2 μg of Rta or glutathione S-transferase-IKKγ together with 30 μl of amylose resin (New England Biolabs, Inc.). The resin was then washed with 1 ml of a buffer containing 20 mM Tris-HCl (pH 7.5) and 100 mM NaCl four times. Protein-bound resin was boiled in SDS-PAGE loading buffer and the proteins were resolved in a SDS-12% polyacrylamide gel, transferred to a nitrocellulose membrane, and immunoblotted with Rta or IKKγ antibody.
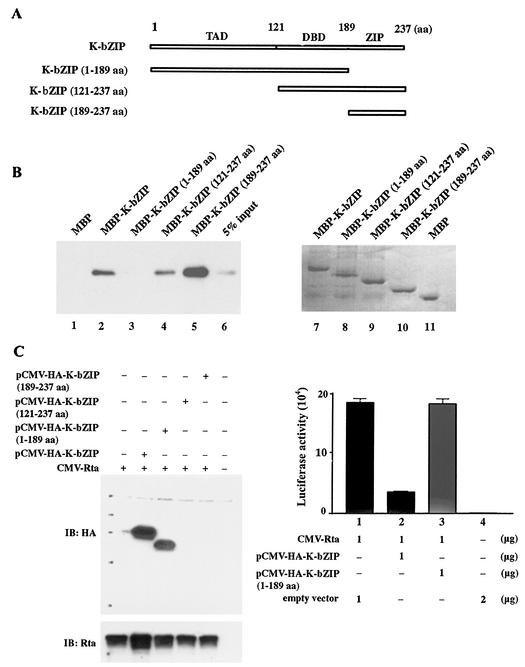
K-bZIP/Rta interaction requires the bZIP domain of K-bZIP.
As reported previously and depicted in Fig. 4A, K-bZIP contains three domains: the transactivation domain (TAD; amino acids 1 to 121), the DNA-binding domain (DBD), or basic domain (BD) (amino acids 121 to 189), and the ZIP domain (amino acids 190 to 237). To map the region in K-bZIP that binds Rta, we derived three deletion mutants of K-bZIP containing amino acid residues 1 to 189 (TAD-DBD), 121 to 238 (DBD-ZIP), and 189 to 238 (ZIP). These three deletions were fused to the MBP coding sequence individually, and fusion proteins were expressed, purified (Fig. 4B, lanes 7 to 11), and used in pull-down experiments together with purified Rta protein. As shown in Fig. 4B, the region containing the TAD and the BD (residues 1 to 189) did not show detectable interaction with Rta (lane 3). By contrast, the bZIP domain bound Rta (lane 4), and, importantly, the ZIP region of K-bZIP alone (lane 5) is sufficient for interaction with Rta. We next tested the individual K-bZIP deletions listed above for their abilities to repress Rta-mediated transactivation. K-bZIP(1-189) could be readily expressed and detected by immunoblotting (Fig. 4C, left panel). As expected, it had no effect on Rta function (Fig. 4C, right panel, lane 2). We could not detect expression of bZIP(121-238) and ZIP(189-238) deletions by immunoblotting (Fig. 4C, left panel), possibly due to the instability of these two deletions in mammalian cells. These results, nevertheless, are consistent with the notion that the region in K-bZIP that engages Rta resides in the ZIP domain.
FIG. 4.
K-bZIP-Rta interaction requires the bZIP domain of K-bZIP. (A) Domain organization of K-bZIP. Regions of K-bZIP included in the deletion mutants are indicated. aa, amino acids. (B) The ZIP domain of K-bZIP binds Rta. The coding sequences of K-bZIP(1-189), K-bZIP(121-237), and K-bZIP(189-237) were derived by PCR using the respective forward primers 5′-GCCGAATTC(EcoRI)ATGCCCAGAATGA-3′, 5′-GCCGAATTC (EcoRI)ATGCAGCTTCCAACT-3′, and 5′-GCCGAATTC(EcoRI) ATGCAGGCATTAGA-3′ and the reverse primers 5′-CGGGATCC(BamHI)TCAACATGGTGGGA-3′ [K-bZIP(121-237) and K-bZIP(189-237)] and 5′-CGGGATCC(BamHI)TCACTGCTGCAGCT-3′ [K-bZIP(1-189)] and fused to the MBP sequence as described for Fig. 3. MBP and MBP fusion proteins containing full-length K-bZIP and the three deletions were expressed and purified (lanes 7 to 11, stained with Coomassie brilliant blue), and 5 μg of each was used together with 2 μg of purified Rta protein in pull-down experiments (lanes 1 to 6, Rta immunoblot) as described for Fig. 3B. (C) K-bZIP lacking the ZIP domain failed to suppress Rta transactivation. Transient transfection of HEK 293 cells and luciferase assays were performed as described for Fig. 1 except that 1 μg each of pCMV-HA-K-bZIP, pCMV-HA-K-bZIP (encoding residues 1 to 189), pCMV-HA-K-bZIP (residues 121 to 237), and pCMV-HA-K-bZIP (residues 189 to 237) were used, respectively, together with 1 μg of CMV-Rta. The expression plasmids for the deletion mutants of HA-K-bZIP were constructed by fusing their respective coding sequences (PCR derived) with the coding region of the HA epitope in pCMV-HA (Invitrogen Corp.) via EcoRI and BamHI site. The forward primers used here were 5′-GCCGAATTC(EcoRI)TGCCCAGAATGA-3′ [K-bZIP(1-189)], 5′-GCCGAATTC(EcoRI)TGCAGCTTCCAACT-3′ [K-bZIP(121-237)], 5′-GCCGAATTC(EcoRI)TGCAGGCATTAGA-3′ [K-bZIP(189-237)]. The reverse primers were as described for the MBP fusion constructs in Fig. 3. Twenty micrograms of total cell proteins from each transfection was resolved in an SDS-12% polyacrylamide gel and immunoblotted (IB) with an anti-HA mouse monoclonal antibody and a rabbit polyclonal antibody against Rta. Luciferase assays were carried out as described for Fig. 1A.
Overexpression of CBP, p300, or P/CAF cannot reverse the repression of Rta-mediated transactivation by K-bZIP.
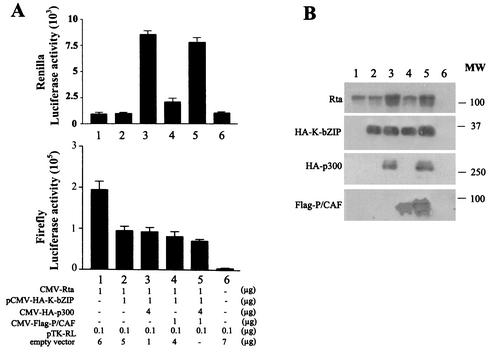
CREB-binding protein (CBP), p300, and their associated factor, P/CAF, are transcriptional coactivators that serve as general integrators of signal-dependent transcription. They possess intrinsic histone acetyltransferase activity (21) and are involved in interacting with sequence-specific DNA-binding transcription factors and basal transcriptional machinery to promote chromatin remodeling for transcription. CBP, p300, and P/CAF are often recruited by viral transactivators to augment viral gene expression. Previous studies have shown that the BD of K-bZIP interacts with CBP and represses CBP-mediated transcription (12). To investigate whether K-bZIP inhibition of Rta-mediated transactivation occurs due to K-bZIP inactivation of a limiting pool of p300 or P/CAF, we attempted to reverse the inhibitory effect of K-bZIP by increasing the levels of p300 and P/CAF exogenously. An inhibitory level of pCMV-K-bZIP was cotransfected with CMV-Rta and RtaRE220-Luc, together with a saturating amount of a CMV-p300 or a FLAG-epitope-tagged P/CAF expression construct, CMV-(f)P/CAF, and a plasmid containing a herpes simplex virus type 1 thymidine kinase (TK) promoter-driven Renilla luciferase gene as an internal control. As shown in Fig. 5, despite the fact that p300 can increase the expression of TK-Renilla luciferase (Fig. 5A, top, and B, lanes 3 and 5) and the CMV promoter-driven Rta and K-bZIP (Fig. 5B, lanes 3 and 5), the inhibitory effect of K-bZIP on Rta-mediated transactivation was unaffected by overexpression of p300 or P/CAF (Fig. 5A, bottom, and B, compare lanes 2 and 3 and lanes 4 and 5).
FIG. 5.
Overexpression of p300 or P/CAF cannot relieve the repression by K-bZIP. (A) Activities of luciferase reporters. Approximately 107 HEK 293 cells in 100-mm dishes were cotransfected with reporter plasmids RtaRE220 (0.5 μg), pTK-RL (0.1 μg), and the indicated amounts of CMV-Rta and pCMV-HA-K-bZIP together with CMV-HA-p300, CMV-P/CAF, or CMV-p300 plus CMV-P/CAF as for Fig. 4. The total amount of transfected DNA (7.6 μg) in each dish was kept constant by the addition of the empty vector plasmid pcDNA3.1(+). Preparation of cell lysates and luciferase assays were performed as described for Fig. 1A. (B) Immunoblot analyses of cell lysates from panel A. Immunoblots were carried out as for Fig. 1B by using antibodies against Rta, HA, and FLAG epitopes. Each lane contains 10 μl of cell lysates (∼20 μg of total protein). MW, molecular weight (in thousands).
In summary, our results suggest that HHV-8 Rta and K-bZIP are involved in an autoregulatory feedback loop whereby transactivation of K-bZIP expression by Rta leads to an increase in the level of K-bZIP, which in turn interacts directly with Rta to down-modulate Rta-mediated transactivation of K-bZIP expression and possibly the expression of other Rta-activated genes. The interaction between K-bZIP and Rta requires the ZIP region of K-bZIP. While the mechanism of Rta repression by K-bZIP is not clear at present, it does not appear to involve disruption of Rta binding to the Rta response element, as Rta binding to DNA is unaffected by the addition of K-bZIP (unpublished data). Finally, though speculative at this point, our inability to reverse K-bZIP repression by overexpressing p300 and/or P/CAF suggests that K-bZIP may disrupt the interaction between Rta and other transcriptional coactivators or basal factors. These data provide an interesting contrast to the well-established paradigm reported for the interaction between EBV Rta and Zta.
Acknowledgments
We thank Gary Hayward for the HHV-8 Rta antibody, Bala Chandran for the BCBL-1 cDNA library, Aviva Symes for the pA3Pluc plasmid, and Xin Xiang for assistance with fluorescence microscopy.
This work was supported by in-house research grant RO73FH from the USUHS.
REFERENCES
- 1.Boshoff, C., and R. A. Weiss. 1998. Kaposi's sarcoma-associated herpesvirus. Adv. Cancer Res. 75:57-86. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman, E., R. G. Nador, K. Aozasa, G. Delsol, J. W. Said, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am. J. Pathol. 149:53-57. [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J., K. Ueda, S. Sakakibara, T. Okuno, and K. Yamanishi. 2000. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor gene. J. Virol. 74:8623-8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook, I. D., F. Shanahan, and P. J. Farrell. 1994. Epstein-Barr virus SM protein. Virology 205:217-227. [DOI] [PubMed] [Google Scholar]
- 7.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 69:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flemington, E. K., A. M. Borras, J. P. Lytle, and S. H. Speck. 1992. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J. Virol. 66:922-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 18:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruffat, H., S. Portes-Sentis, A. Sergeant, and E. Manet. 1999. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) encodes a homologue of the Epstein-Barr virus bZip protein EB1. J. Gen. Virol. 80(Pt. 3):557-561. [DOI] [PubMed] [Google Scholar]
- 11.Holley-Guthrie, E. A., E. B. Quinlivan, E. C. Mar, and S. Kenney. 1990. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J. Virol. 64:3753-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang, S., Y. Gwack, H. Byun, C. Lim, and J. Choe. 2001. The Kaposi's sarcoma-associated herpesvirus K8 protein interacts with CREB-binding protein (CBP) and represses CBP-mediated transcription. J. Virol. 75:9509-9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katano, H., K. Ogawa-Goto, H. Hasegawa, T. Kurata, and T. Sata. 2001. Human-herpesvirus-8-encoded K8 protein colocalizes with the promyelocytic leukemia protein (PML) bodies and recruits p53 to the PML bodies. Virology 286:446-455. [DOI] [PubMed] [Google Scholar]
- 14.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 2000. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 269:335-344. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman, P. M., and A. J. Berk. 1994. A mechanism for TAFs in transcriptional activation: activation domain enhancement of TFIID-TFIIA-promoter DNA complex formation. Genes Dev. 8:995-1006. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman, P. M., P. O'Hare, G. S. Hayward, and S. D. Hayward. 1986. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J. Virol. 60:140-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, S. F., D. R. Robinson, G. Miller, and H. J. Kung. 1999. Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus. J. Virol. 73:1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 20.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 22.Park, J., T. Seo, S. Hwang, D. Lee, Y. Gwack, and J. Choe. 2000. The K-bZIP protein from Kaposi's sarcoma-associated herpesvirus interacts with p53 and represses its transcriptional activity. J. Virol. 74:11977-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polson, A. G., L. Huang, D. M. Lukac, J. D. Blethrow, D. O. Morgan, A. L. Burlingame, and D. Ganem. 2001. Kaposi's sarcoma-associated herpesvirus K-bZIP protein is phosphorylated by cyclin-dependent kinases. J. Virol. 75:3175-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepers, A., D. Pich, and W. Hammerschmidt. 1993. A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of Epstein-Barr virus. EMBO J. 12:3921-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz, T. F. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8): epidemiology and pathogenesis. J. Antimicrob. Chemother. 45(Suppl. T3):15-27. [DOI] [PubMed] [Google Scholar]
- 27.Seaman, W. T., D. Ye, R. X. Wang, E. E. Hale, M. Weisse, and E. B. Quinlivan. 1999. Gene expression from the ORF50/K8 region of Kaposi's sarcoma-associated herpesvirus. Virology 263:436-449. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 65:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 30.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, S., and Z. M. Zheng. 2002. Kaposi's sarcoma-associated herpesvirus K8 exon 3 contains three 5′-splice sites and harbors a K8.1 transcription start site. J. Biol. Chem. 277:14547-14556. [DOI] [PubMed] [Google Scholar]
- 33.Wu, F. Y., J. H. Ahn, D. J. Alcendor, W. J. Jang, J. Xiao, S. D. Hayward, and G. S. Hayward. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao, L. J., and C. Z. Giam. 1991. Interaction of the human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc. Natl. Acad. Sci. USA 88:11445-11449. [DOI] [PMC free article] [PubMed] [Google Scholar]