Abstract
BACKGROUND
Opportunistic disease screening is the routine, asymptomatic disease screening of patients at the time of a physician encounter for other reasons. While the prevalence of unrecognized diabetes in community populations is well known, the prevalence in clinical populations is unknown.
OBJECTIVE
To describe the prevalence, predictors, and clinical severity of unrecognized diabetes among outpatients at a major medical center.
DESIGN AND SETTING
A cross-sectional observational study at the Durham Veterans Affairs Medical Center.
SUBJECTS
Outpatients without recognized diabetes (N = 1,253).
METHODS
We screened patients for diabetes by using an initial random Hemoglobin A1c (HbA1c) measurement, and then obtaining follow-up fasting plasma glucose (FPG) for all subjects with HbA1c ≥6.0%. A case of unrecognized diabetes was defined as either HbA1c ≥7.0% or FPG ≥7 mmol/L (126 mg/dL). Height and weight were obtained for all subjects. We also obtained resting blood pressure, fasting lipids, and urine protein in subjects with HbA1c ≥6.0%.
RESULTS
The prevalence of unrecognized diabetes was 4.5% (95% confidence interval [CI], 3.4 to 5.7). Factors associated with unrecognized diabetes were the diagnosis of hypertension (adjusted odds ratio [OR], 2.5; P = .004), weight >120% of ideal (adjusted OR, 2.2; P = .02), and history of a parent or sibling with diabetes (adjusted OR, 1.7; P = .06). Having a primary care provider did not raise or lower the risk for unrecognized diabetes (P = .73). Based on the new diagnosis, most patients (61%) found to have diabetes required a change in treatment either of their blood sugar or comorbid hypertension or hyperlipidemia in order to achieve targets recommended in published treatment guidelines. Patients reporting a primary care provider were no less likely to require a change in treatment (P = .20).
CONCLUSIONS
If diabetes screening is an effective intervention, opportunistic screening for diabetes may be the preferred method for screening, because there is substantial potential for case-finding in a medical center outpatient setting. A majority of patients with diabetes diagnosed at opportunistic screening will require a change in treatment of blood sugar, blood pressure, or lipids to receive optimal care.
Keywords: diabetes, disease screening, metabolic syndrome
Systematic screening for diabetes is a potentially attractive intervention, because diabetes is a prevalent,1,2 costly,3 and highly morbid illness, and because there is a long asymptomatic phase to the illness.4 However, few studies support diabetes screening, and computer models suggest borderline cost-effectiveness for diabetes screening.5 For diabetes screening to be cost-effective, it will be important to utilize a screening strategy that maximizes the yield of screening. Additionally, the quality of follow-up care for patients diagnosed with diabetes at screening will be important to cost-effectiveness. One decision that affects the yield and follow-up is the choice of a location for screening.
There are 2 potential strategies for diabetes screening: community- or population-based screening, and opportunistic screening. Opportunistic screening strategies take advantage of the presentation of the patient to the doctor or medical center (scheduled visit, emergency room visit, inpatient stay) to deliver a public health screening intervention.6 Traditionally, opportunistic screening is thought to increase yield because patients accessing the health care system may have increased risk factors for chronic diseases.6 Additionally, opportunistic screening identifies patients in the context of a physician-patient relationship and presents fewer barriers to appropriate follow-up of a positive test.7 However, the yield of opportunistic diabetes screening could also be substantially lower than in community-based screening, because patients seeing doctors receive some measure of testing for chronic diseases8 and thus may have their diabetes diagnosed. For example, patients followed for hypertension (HTN) may receive ad hoc diabetes screening by virtue of chemistry panels ordered to monitor potassium or kidney function. The degree to which this occurs is unknown.
There have been many recent publications assessing the prevalence and severity of diabetes found at systematic community-based screening.6,9,10 None of these studies have examined the relationship between being enrolled in a health care system and case finding for diabetes. Our primary objective in this study was to estimate the prevalence and assess the severity of unrecognized diabetes in the setting of opportunistic screening, that is, among medical center outpatients.
METHODS
Patients
We identified all patients aged 45 to 64 who had kept an outpatient visit at the Durham Veterans Affairs Medical Center (DVAMC) between October 1996 and March 1999. We sent all these patients a 1-page questionnaire that asked if the patient had diabetes and if we could contact them by telephone for a research study. Respondents denying knowledge of diabetes or “high blood sugar” and agreeing to be telephoned were contacted for enrollment into the study.
To determine if volunteer bias affected the prevalence of diabetes in our sample, we reviewed the electronic medical records of 155 randomly selected patients who did not respond to our survey for the purposes of evaluating diabetes risk factors in these patients. Each of the 155 patients was assigned a “chart review date,” which was the same date as the enrollment date of a randomly selected patient from our study sample. We ascertained height, weight, and presence or absence of the diagnosis of hypertension at the visit closest to the chart review date.
Diabetes Screening Protocol
We obtained written informed consent from all subjects prior to enrollment. The study and enrollment strategy were approved by the Institutional Review Board of the DVAMC. At the initial visit, we excluded patients who said they had diabetes, had had a prescription filled at the DVAMC pharmacy for a hypoglycemic medication, had a short life expectancy (incurable cancer, heart disease, or lung disease requiring oxygen), or had no easy access to a telephone. We obtained hemoglobin A1c (HbA1c) measures on all subjects. All subjects with HbA1c ≥6.0% were invited back for follow-up fasting plasma glucose (FPG). Additionally, a convenience sample of 160 patients with HbA1c <6.0% also received FPG at the time of HbA1c measurement.
We defined a case of diabetes as HbA1c ≥7.0% or fasting plasma glucose ≥7 mmol/L (126 mg/dL). Although screening and diagnosis of diabetes by means of HbA1c is not standard, the approach was used for 2 reasons. First, the convenience of performing a nonfasting test allowed rapid enrollment of patients into the study. Second, the nonfasting test mimics a reasonable strategy that might be used by a medical center to perform mass screening of patients whether or not they are fasting. At the conservative diagnostic cut point that we chose for further evaluation (HbA1c = 6.0%, 2 standard deviations above mean and the upper limit of normal on standard machines) and in a screening population, the sensitivity of HbA1c for the diagnosis of diabetes is 75% to 93%.11,12
Outcome Measures and Covariates
At enrollment, we obtained multiple demographic and questionnaire measures. Having a primary care provider was assessed by patient self-report. Comorbidity and medical illnesses were assessed using the Kaplan-Feinstein comorbidity index for patients with diabetes.13 We modified this instrument for use in patient interview format. We also obtained height and weight on all subjects, and calculated the percentage of ideal body weight using Metropolitan Life weight tables.14
At the follow-up visit (after an HbA1c ≥6.0%), patients had their blood pressure (BP), fasting serum lipids, and urine albumin measured. Blood pressure was measured in the seated position by a nurse using a manual cuff.
Analysis
The target sample size of 1,255 was chosen in order to provide narrow confidence intervals (CIs) around the prevalence of unrecognized diabetes in the sample. We initially estimated the prevalence of unrecognized diabetes to be 7%; 1,255 patients were required to obtain 95% confidence intervals of ±1.4% around that estimate (i.e., 5.6% to 8.4%). We measured the prevalence of unrecognized diabetes in the sample by dividing actual cases identified by the total number in the sample. Descriptive statistics were computed for all variables of interest. Bivariate comparisons were performed using t tests for continuous variables, and χ2 for categorical variables. We performed multivariable modeling for categorical outcome variables using logistic regression. All analyses were performed using the SAS analysis system (SAS Institute, Cary, NC).
RESULTS
Subjects
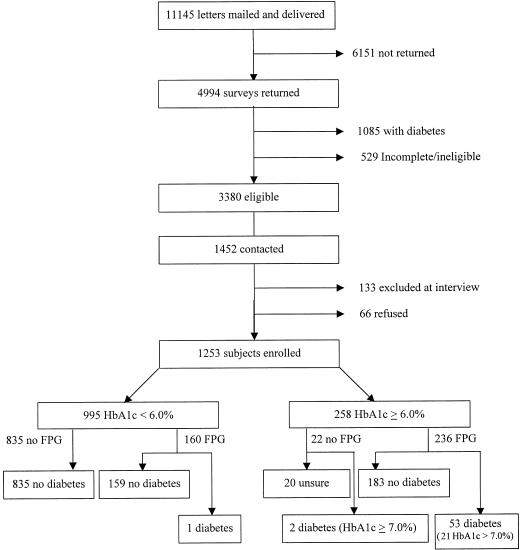
The numbers of patients passing through each phase of the enrollment protocol is shown in Figure 1. Of the 11,145 letters that were mailed and delivered, 4,994 (45%) subjects responded. Of these, 1,085 subjects (22%) reported having diabetes, and 529 surveys (11%) were incomplete or otherwise ineligible, leaving 3,380 eligible for the study. In order to enroll 1,255 patients into the study, we contacted 1,452 (43% of eligible respondents) patients. The remaining 1,928 patients were either not successfully contacted after at least 3 phone calls were attempted and a follow-up letter was sent, or no attempt was made to contact them because the target sample size had been achieved. Two subjects were later found to have known they had diabetes and were excluded, leaving 1,253 patients.
FIGURE 1.
Enrollment of patients in the study.
The baseline demographic and clinical characteristics of the 1,253 enrolled patients are described in Table 1. Our sample had rates of diabetes risk factors similar to those of the general population of the American South. Approximately 29% of subjects described their race as African-American, and 60% were over 120% of their ideal body weight. Self-reported HTN was present in 53%, and 34% had moderate or severe comorbidities as measured by the Kaplan-Feinstein comorbidity index.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Patients (N = 1,253)
| Demographic characteristics | |
| Mean age, y (SD) | 55 (6) |
| Gender, % male | 94 |
| Enrolled in primary care at DVAMC (self-report), % | 72 |
| Diabetes risk factors, % | |
| Race | |
| White | 69 |
| African American | 29 |
| Other | 2 |
| Family history of diabetes | 38 |
| Body weight >120% of ideal | 60 |
| Diagnosis of hypertension | 53 |
| Clinical characteristics | |
| Comorbidity (Kaplan-Feinstein scale), % | |
| None | 5 |
| Mild | 61 |
| Moderate | 21 |
| Severe | 13 |
| SF-36 Physical Component Score (mean) | 36 (12) |
| SF-36 Mental Component Score (mean) | 50 (14) |
| HbA1c (%) | 5.6 (0.7) |
DVAMC, Durham Veterans Affairs Medical Center.
Prevalence of Unrecognized Diabetes
Of the 1,253 subjects enrolled, 258 (21%) had HbA1c ≥6.0%. Of these, we obtained data adequate to make or dismiss a diagnosis of diabetes for 238 (92%) (see Fig. 1). Thirty-two subjects had FPG ≥7.0 mmol/L and 6.0%≤ HbA1c ≤6.9%; 19 subjects had HbA1c ≥7.0% and FPG ≥7.0 mmol/L; and 2 patients had HbA1c ≥7.0% and FPG <7.0 mmol/L. Two subjects had HbA1c ≥7.0% and no FPG. These 55 patients were identified as cases of unrecognized diabetes. In addition, of the 160 patients with HbA1c <6.0% selected to have FPG, 1 (0.6%) had an FPG = 7.0 mmol/L and was defined as a case of unrecognized diabetes. Thus, the demonstrated prevalence of unrecognized diabetes in the sample was 4.5% (95% CI, 3.4% to 5.7%).
Factors Associated with Unrecognized Diabetes
Three variables were associated with unrecognized diabetes in bivariate analyses (Table 2). Two of these remained significant in multivariable logistic models, while the third was close to statistical significance. These were: self-reported hypertension (adjusted odds ratio [OR], 2.5; P = .004); weight >120% of ideal body weight (adjusted OR, 2.2; P = .02); and self-reported history of a parent or sibling with diabetes (adjusted OR, 1.7; P = .06). All other measured variables, including race, were not associated with unrecognized diabetes in our sample. The prevalence of unrecognized diabetes was very low (0.5%) for patients with none of the 3 risk factors, and increased to as high as approximately 10% for patients with all 3 risk factors.
Table 2.
Independent Risk Factors for Diabetes in Screened Patients
| Risk Factor | Prevalence of Diabetes in Subjects With Factor, % | Prevalence of Diabetes in Subjects Without Factor, % | Adjusted Odds Ratio (95% CI)* |
|---|---|---|---|
| Hypertension | 6.3 | 2.6 | 2.5 (1.3 to 4.6) |
| Weight >120% of ideal body weight | 6.0 | 2.4 | 2.2 (1.1 to 4.2) |
| Family history of diabetes | 6.1 | 3.6 | 1.7 (0.97 to 2.9) |
| Primary care enrollment | 4.6 | 4.1 | 1.0 (0.54 to 2.0) |
Adjusted odds ratios are from a logistic regression model with all 4 variables.
CI, confidence interval.
Having a primary care doctor, either at the DVAMC or anywhere, did not raise or lower the risk for unrecognized diabetes in bivariate analyses. However, it is possible that this lack of association was confounded by a higher prevalence of diabetes risk factors (hypertension, obesity) in the population reporting primary care, so that effective screening for diabetes in primary care would be balanced by higher risk. To assess this confounding, we entered primary care status into the logistic regression model along with the 3 known risk factors. Primary care status was not associated with unrecognized diabetes even after controlling for other risk factors (P = .92; Table 2).
To assess the potential effect of volunteer bias, we reviewed the medical records of a random sample of 155 patients who did not respond to our survey. Of these, 21(14%) had diabetes. Because responders who acknowledged diabetes were excluded from our study, we excluded these patients from the analysis of nonresponders. Of the remaining 134, we obtained blood pressure measurements for 130, of whom 71 (55%; P = .72 for the difference compared to our sample) had hypertension recorded in their medical records. Of the 134, 89 had a height and weight on their medical record for the time frame of enrollment for our study. Of these 89, 41(46%) had weight greater than 120% of their ideal body weight (P = .01 for the difference compared to our sample). Thus, patients in our study sample were slightly more likely to be overweight than were nonresponder patients.
Clinical Status of Patients with Unrecognized Diabetes
The clinical status of patients diagnosed with diabetes at screening is shown in Table 3. Of the 56 subjects diagnosed with diabetes, 23 (41%) had HbA1c ≥7.0%, implying that their hyperglycemia was at a level warranting treatment. We obtained blood pressure measurements on 51/56 patients diagnosed with diabetes at screening. Of these 51, 25 (49%) had BP greater than 140/90, and 13 (25%) had BP between 140/90 and 130/80. Thus, three quarters of patients warranted tighter BP control. We also obtained fasting serum lipid fractions on 39/56 patients diagnosed with diabetes at screening. Of these, 7/39 (18%) had low-density lipoprotein (LDL) cholesterol >130 mg/dL, including 1 subject with LDL >160.
Table 3.
Clinical Characteristics of Patients with Unrecognized Diabetes
| Clinical Characteristic | Patients with New Diabetes, % | Patients with New Diabetes In Primary Care, % |
|---|---|---|
| HbA1c ≥7.0% | 41 | 33* |
| BP between 130/80 and 140/90 | 25 | 29 |
| LDL >3.37 mmol/L | 18 | 7* |
| Any of the above | 61 | 55 |
P < .05 between primary care and no primary care.
To illustrate the immediate impact of diabetes screening on disease treatment, we defined a subject with newly diagnosed diabetes as having a “treatment-altering diagnosis of diabetes” if the subject met 1 of 3 clinical criteria. Each of these clinical criteria represents a threshold at which evidence suggests that the diagnosis of diabetes will either initiate the treatment of blood sugar,15,16 or change the treatment of comorbid illness, i.e., hypertension and hyperlipidemia.17–21 If the comorbid illness was severe enough to require treatment in the absence of diabetes, this was not considered a “treatment-altering diagnosis.” The criteria were: HbA1c >7.0%; systolic blood pressure between 130 and 140 mmHg (without diastolic >90) or diastolic blood pressure between 80 and 90 mmHg (without systolic >140); and LDL between 130 and 160 mg/dL. By these criteria, 61% of patients diagnosed with diabetes had either hyperglycemia, HTN, or hyperlipidemia whose ideal management would be altered by the diagnosis of diabetes (Table 3). Of the 42 patients diagnosed with diabetes who identified a primary care physician, 23 had a treatment-altering diagnosis. This percentage was no different from that for patients reporting no primary care provider (P = .20).
DISCUSSION
In a population of patients enrolled at the Durham VA Medical Center we found a prevalence of unrecognized diabetes of approximately 5%. The prevalence is similar to that seen in men age 45 to 64 in the Third National Health and Nutrition Examination Survey.9 This prevalence suggests that there is opportunity for diabetes case-finding even in populations receiving medical treatment. The majority of these patients with diabetes diagnosed at screening have disease severe enough that they can potentially benefit from the diagnosis by altered treatment of diabetes or more intense treatment of hypertension or hyperlipidemia.
While the prevalence of unrecognized diabetes in our clinic population was similar to that found in the community, the frequency of comorbid illness was higher in our cohort than in the community. For example, the prevalence of pre-existing HTN was much higher in our sample than in the Atherosclerosis Risk in Communities Study (ARIC) population (53% vs 30% in ARIC).10,22 This relatively high prevalence and severity of disease suggest that for diabetes screening, opportunistic screening may be preferred to community screening because follow-up can be obtained more easily without a compromise in yield from the screening strategy. However, this assumes that the follow-up obtained is adequate and helpful to the patient. We are currently following this cohort of patients to determine the quality and success of follow-up of the newly diagnosed patients.
We also found that the prevalence of unrecognized diabetes is very low in the absence of 3 associated risk factors: hypertension, obesity, and family history. This suggests that these associated risks could be used to select patients to increase the yield for diabetes screening. Health care providers or public health organizations that wish to undertake systematic screening for diabetes may concentrate resources by exempting patients without any of these associated risks from any screening protocol, because the yield of cases will be low (<1%) in these patients.
The finding that coincident hypertension and obesity are the 2 strongest risk factors for diabetes reinforces the concept of the “metabolic syndrome.”23,24 The metabolic syndrome is the clustering of obesity, insulin resistance, hypertension, hypercoagulable state, and atherogenic lipid profile with exceedingly high risk for cardiovascular outcomes. The fact that the 2 strongest risks for unrecognized diabetes are part of this profile further increases the likelihood that patients with unrecognized diabetes have the potential for improved cardiovascular outcomes after recognition and treatment of diabetes and its comorbid conditions.
In classical clinical epidemiology, 5 conditions must be met if screening for a particular disease is to benefit society.25 With a heavy burden of disease, effective diagnostic testing, and a long asymptomatic phase of disease, diabetes meets the first 3 of these criteria. The other two criteria, ability of the health care system to manage additional cases of the disease and demonstrated effectiveness for early treatment, remain to be determined. Our study shows that the majority of outpatients with diabetes found at screening have reasonable expectation of benefiting from a change in their medical treatment. This frequent need for treatment is necessary but not sufficient evidence to support routine screening for diabetes. The evidence is not sufficient because patients identified by screening early in the course of their diabetes may or may not derive the same benefits from treatment of diabetes and comorbid HTN or hypercholesterolemia as patients with either symptomatic or advanced diabetes. However, in the U.K. Prospective Diabetes Study Group, which showed improved outcomes with treatment of blood pressure and blood sugar in a population of patients treated at the time of diagnosis, 29% of the patients were diagnosed at asymptomatic screening.26 This provides a hint that treatment of early diabetes may be as effective as treatment of diabetes at its usual time of diagnosis.
Other important findings of our study include the fact that the prevalence of undiagnosed diabetes is unrelated to whether patients are receiving primary care. This suggests that primary care providers do not systematically screen for diabetes, which is in keeping with the lack of firm evidence supporting screening at this time. Finally, because patients do not always present to the medical center in the fasting state, it is important for opportunistic screening protocols to begin with a sensitive nonfasting test. We show that a pragmatic screening protocol beginning with HbA1c testing and using a low threshold for further evaluation can detect a large number of cases in a screening protocol.
There are significant limitations inherent in our study. We were unable to preselect patients randomly for the study. Nonresponders were slightly less likely to be obese than were patients in our study sample, suggesting that patients with reason to be concerned that they had diabetes may have volunteered for the study. This nonresponse bias may also have occurred for a risk factor we were unable to ascertain from medical records, family history of diabetes. It is therefore possible that the actual prevalence of unrecognized diabetes at the DVAMC may be slightly lower than our measured prevalence. In addition, users of VA Medical Centers have a greater burden of disease than do patients in other health care systems,27 and conclusions may not generalize to patients of other health care providers. A second limitation is that our definition of diabetes was pragmatic, and so we may have missed a few patients with diabetes and subdiabetic fasting glucose levels (e.g., normal FPG, HbA1c between 6% and 7%, but elevated 2-hour post-load glucose). However, our screening algorithm reflects a clinical approach that can be easily generalized to most medical center situations. Finally, we had inadequate power to detect the influence of weaker risk factors. For example, there was a noticeable difference in the prevalence of heart disease between patients with and without unrecognized diabetes (45% to 36%) but this difference was not statistically significant. A multi-site study would be necessary to overcome this limitation.
This study does not prove that screening for diabetes is an effective or cost-effective intervention. However, our results demonstrate that there is room for diabetes case-finding within medical centers. Our data also point to the potential usefulness of screening patients for diabetes in order to identify patients at high risk for cardiovascular disease so that they can have more aggressive cardiovascular risk reduction. Longitudinal studies are needed to confirm the effectiveness of such a screening strategy.
Acknowledgments
Support for this research was received from the Department of Veterans' Affairs Cooperative Studies. Dr. Edelman was supported by a VA Health Services Research Career Development Award.
REFERENCES
- 1.Harris MI. Noninsulin-dependent diabetes mellitus in black and white Americans. Diabetes Metab Rev. 1990;6:71–90. doi: 10.1002/dmr.5610060202. [DOI] [PubMed] [Google Scholar]
- 2.Harris MI. Impaired glucose tolerance in the U.S. population. Diabetes Care. 1989;12:464–74. doi: 10.2337/diacare.12.7.464. [DOI] [PubMed] [Google Scholar]
- 3.Huse DM, Oster G, Killen AR, Lacey MJ, Colditz GA. The economic costs of non-insulin-dependent diabetes mellitus. JAMA. 1989;262:2708–13. doi: 10.1001/jama.262.19.2708. [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Modan M. Screening for NIDDM. Diabetes Care. 1994;17:440–4. doi: 10.2337/diacare.17.5.440. [DOI] [PubMed] [Google Scholar]
- 5.CDC Cost-Effectiveness Study Group. The cost-effectiveness of screening for type 2 diabetes. JAMA. 1998;280:1757–63. [PubMed] [Google Scholar]
- 6.Engelgau MM, Narayan KMV, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000;23:1563–80. doi: 10.2337/diacare.23.10.1563. [DOI] [PubMed] [Google Scholar]
- 7.Home PD. Diagnosing the undiagnosed with diabetes. BMJ. 1994;308:611–2. doi: 10.1136/bmj.308.6929.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luckmann R, Melville SK. Periodic health evaluation of adults: a survey of family physicians. J Fam Pract. 1995;40:547–54. [PubMed] [Google Scholar]
- 9.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 10.Brancati FL, Kao WHL, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetesm mellitus in African American and white adults: The Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–60. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 11.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187–91. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 12.Perry RC, Shankar RR, Fineberg N, McGill J, Baron AD. HbA1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levels of fasting plasma glucose: the Early Diabetes Intervention Program (EDIP) Diabetes Care. 2001;24(3):465–71. doi: 10.2337/diacare.24.3.465. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan MH, Feinstein AR. The importance of classifying initial comorbidity in evaluating the outcome of diabetes mellitus. J Chron Dis. 1974;27:387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 14.Marks GC, Habicht J-P, Mueller WH. Reliability, dependability, and precision of anthropometric measurements. Am J Epidemiol. 1989;130:578–87. doi: 10.1093/oxfordjournals.aje.a115372. [DOI] [PubMed] [Google Scholar]
- 15.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 16.UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 19.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;154:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 20.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G The Scandinavian Simvastatin Survival Study (4S) Group. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. Diabetes Care. 1997;20:614–20. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (Adult Treatment Panel II). Summary of the second report of the National Cholesterol Education Program (NCEP) JAMA. 1993;269:3015–31. [PubMed] [Google Scholar]
- 22.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. New Engl J Med. 2000;342:905–12. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 23.Liese AD, Mayer-Davis EJ, Haffner SM. Development of the multiple metabolic syndrome: an epidemiologic perspective. Epidemiol Rev. 1998;20:157–72. doi: 10.1093/oxfordjournals.epirev.a017978. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MI, Duncan BB, Watson RL, Sharrett AR, Brancati FL, Heiss G. A metabolic syndrome in whites and African Americans: the Atherosclerosis Risk in Communities Baseline Study. Diabetes Care. 1996;19:414–8. doi: 10.2337/diacare.19.5.414. [DOI] [PubMed] [Google Scholar]
- 25.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. second edition. Boston: Little, Brown and Company; 1991. [Google Scholar]
- 26.Prospective Diabetes Study UK. (UKPDS). IV. Characteristics of newly presenting type 2 diabetic patients: male preponderance and obesity at different ages. Diabetic Med. 1988;5:154–9. [PubMed] [Google Scholar]
- 27.Kazis LE, Ren XS, Lee A, et al. Health status in VA patients: results from the Veterans Health Study. Am J Med Qual. 1999;14:28–38. doi: 10.1177/106286069901400105. [DOI] [PubMed] [Google Scholar]