Abstract
OBJECTIVE
To assess the association of specialty training and experience in the care of HIV disease with HIV-specific knowledge, referral patterns, and HIV-related education activities.
DESIGN
Cross-sectional survey.
SETTING
The United States.
PARTICIPANTS
Physicians caring for patients in the HIV Costs and Service Utilization Study, a study of a probability sample of HIV-infected individuals in the United States.
MEASUREMENTS AND MAIN RESULTS
Measures included physicians' reports of specialty training and HIV caseload, scores on an HIV-specific knowledge test, referral patterns, and attendance rates at HIV-related educational activities. Approximately 72% (379) of the eligible physicians completed a survey. Of these, 152 (40%) had infectious disease (ID) training, and 213 (56%) were generalists; 4% of ID-trained physicians and 37% of generalist physicians did not consider themselves HIV experts. The median current caseloads were 150 and 200 patients for ID experts and generalist experts, respectively. In contrast, the median caseload for non-expert generalists was 5. Mean scores on the knowledge scale were similar for ID and generalist experts (9.0 items correct out of 11 vs 8.5; P = not significant), but lower for generalist non-experts (6.5 items correct; P < .01). Experts had attended more local and national HIV meetings than non-experts (9.3 vs 2.7; P < .01, and 2.3 vs .40; P < .01, respectively) in the past year. Fewer ID experts ever referred than generalist experts (13.0% vs 27.3%; P = .01). In multivariable models that included specialty training and caseload, physicians with caseloads of 20 to 49 and >50 were more likely to have a high knowledge score (defined as 80% or more correct, odds ratio [OR], 2.8; P = .04 and OR, 5.7; P < .001, respectively), and the effect of specialty was attenuated (OR, 2.7; P = .02 decreased from OR, 7.8; P < .001 in a model without caseload). In the models predicting referral practices, both experience (OR, .25; P < .01 and OR, .17; P < .01 for caseloads of 20 to 49 and >50, respectively) and specialty (OR, .19; P < .01 and OR, .09; P < .01 for generalist and ID experts, respectively) were significant.
CONCLUSIONS
In a national sample of physicians, HIV-specific knowledge was more strongly associated with HIV caseload than with specialty training. In addition, although referral practices were related to both experience and specialty, generalist experts and ID physicians reported similar behaviors. This suggests that generalist physicians, through clinical experience and self-education, can develop specialized knowledge in HIV care.
Keywords: HIV, Acquired Immunodeficiency Syndrome, specialism, physicians' practice patterns
There is not a consensus in the medical community about what types of physicians are best suited to taking care of HIV-infected patients.1–3 As HIV treatment becomes more complex, physicians with specialty training in infectious diseases (IDs) are often delivering care. While some advocate for care by specialists,2 many believe that AIDS care should continue to be based in primary care settings because AIDS care often requires teams of providers with different skills.1,3,4 Optimizing quality of care requires specialty care as well as core features of primary care such as accessibility, coordination, comprehensiveness, and continuity.1
Consequently, what constitutes expertise in the context of HIV care is a complex issue that may not conform to traditional distinctions between specialists and generalists. We theorized that expertise in the treatment of a particular condition would be related to both specialty training and specialization. By specialty training we mean residency, fellowship, or other formal training and/or board certification in a particular area. By specialization we mean experience and focus in a particular clinical area. A physician without formal training in a particular area might take a particular interest in a condition by following the current literature, attending conferences, seeking out patients with the condition for care, and/or participating in clinical research. For instance, a general internist might choose to focus on women's health or a cardiologist might choose to focus on congestive heart failure. Thus, we use the term “specialization” here to refer to the experience, skills, and knowledge necessary to competently care for patients with specific conditions, irrespective of formal training.
No study that we are aware of has examined the relationship between formal specialty training and informal specialization for providers of HIV care or for other chronic conditions. To better understand this issue, we surveyed physicians caring for a national probability sample of patients with HIV infection. We first examined the relationship between specialty training in infectious diseases and HIV specialization, using current HIV caseload and self-assessed expertise as indicators of specialization. Next, we examined the relationship of specialty training and HIV specialization to HIV-care knowledge levels, referral rates, and rates of attendance at HIV-related continuing medical education (CME) activities. We hypothesized that generalist physicians who identified themselves as HIV experts would have knowledge levels, referral rates, and attendance at HIV-related CME activities that were similar to infectious disease specialists.
METHODS
Sample
Study subjects were physicians identified by HIV-infected patients participating in the HIV Cost and Services Utilization Study (HCSUS).5,6 The HCSUS employed a multistage sample design to enroll a probability sample of persons in care for HIV in the contiguous United States.6–8 In the first stage of sampling, we randomly selected 28 metropolitan statistical areas and 24 clusters of rural counties that, in the aggregate, contained nearly 70 percent of all U.S. cases of the acquired immunodeficiency syndrome (AIDS).9,10 In the second stage, we randomly selected 58 institutional or individual providers known to care for patients with HIV infection in urban areas and 28 in rural areas, who had been identified by local physicians or public health officials, as well as 87 other providers in urban areas and 23 in rural areas, who had confirmed in a screening survey of approximately 4,000 physicians that they cared for eligible patients with HIV infection. In the third stage, we randomly selected patients by participating providers during January and February 1996. The study enrolled 2,864 patients and these patients completed a baseline interview and 2 subsequent interviews about aspects of their care, including costs and access to care.
Physician Sample Identification
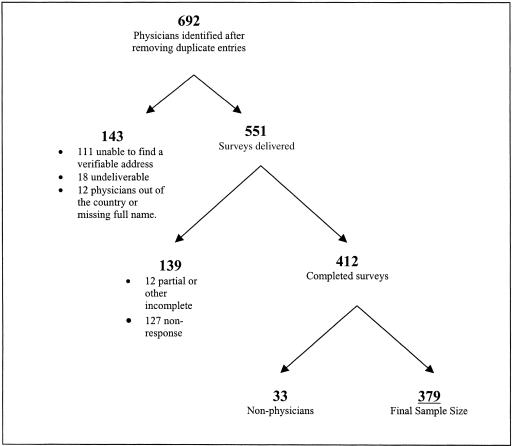
As part of the second interview (conducted primarily in 1997), patients were asked to identify and provide contact information for all major health care providers, including primary care physicians, specialist physicians, nurse practitioners, and/or physician's assistants. As part of this sequence of questions, each patient was also asked to name the single physician most important to their HIV care. This person was designated the “principal HIV physician” and 85% of patients were able to identify such a physician. In cases in which the patient was unable to identify a principal HIV physician, we created an algorithm to match the patient with the physician most likely to be responsible for his/her care. First, we selected the physician the patient said he or she saw most recently for HIV care and if there was no such physician, then we selected a non-HIV primary care physician that they identified. For the 13% of patients missing all of this information in the second interview, we used the primary HIV physician that the patient identified in the baseline survey, or, if they did not identify such a physician, the non-HIV primary care physician identified from the baseline survey. These physicians constituted the sample for the physician survey (Fig. 1).
FIGURE 1.
Physician sample definition.
For identified physicians, we confirmed mailing addresses by telephoning their practice site or local contacts, checking the AMA website, and conducting Internet searches to obtain addresses.11 We were successful in obtaining current addresses for 80% of the physicians.
Survey Methods
The surveys were administered between September 1998 and July 1999. We sent each physician a survey with a $25 check as a token of appreciation for study participation. Nonrespondents received 3 follow-up calls and were offered the chance to complete the survey by phone on the third round. Completed surveys were reviewed and calls were made to query missing items and to clarify ambiguous responses. Only 34 providers completed the interview by phone.
Survey Content
The survey first asked about the physician's outpatient practice sites, including the number of half days they spent in each site and whether or not they see HIV-infected patients. It also asked about year of birth, gender, year of graduation from medical school, race and ethnicity (African American, white, Hispanic, or other), and formal training, including whether the respondent had completed or was completing a residency or fellowship in 1 of 11 specific specialties, such as internal medicine, family practice, or obstetrics and gynecology, and whether they were board certified or eligible. Responders were also asked if they were exclusively heterosexual, mostly heterosexual, bisexual, mostly homosexual, or exclusively homosexual.
The survey then asked about the physician's current and past HIV practice experience, including the number of patients currently cared for (categorized for analyses as 0 to 19, 20 to 49, and >49), the cumulative number of cases cared for in the previous 5 years, the percentage of cases that were women, and the proportions with various risk factors for acquisition of HIV in their patient population. We created variables indicating whether the respondent said that 50% or more of his or her patients had intravenous drug use, homosexual contact, or heterosexual contact as a risk factor. The physicians were also asked whether they considered themselves “specialists” (hereafter referred to as “experts”) in the treatment of HIV and whether they would consider themselves extremely, very, somewhat, or not at all knowledgeable about HIV-related treatment. There also were questions about the proportion of their HIV-infected patients enrolled in clinical trials (categorized for analyses as 0% to 5%, 6% to 10%, 11% to 30%, and >30%) and the number of local (categorized for analyses as 0 to 4 and ≥5) and national (categorized for analyses as 0, 1 to 3, and ≥4) HIV-related CME activities they had attended in the last 12 months.
Physicians also were asked whether they ever needed to consult a colleague to discuss clinical management of HIV and, if so, whether they had access to a colleague in their practice, nearby or accessible by phone, or not at all. They were then asked how often (never, less than half the time, about half the time, more than half the time, or always) they “referred patients to another physician with specialized HIV knowledge” for 5 situations: initial evaluation of the HIV-infected patient; initiating antiretroviral therapy; evaluating possible changes in antiretroviral therapy; interpretation of a viral load or other test result; and choosing an alternative opportunistic infection prophylactic regimen.
The survey included a series of true/false/don't know questions that asked about the physicians' knowledge of current HIV treatment practices. These questions were developed with input from HIV experts. They covered 2 main domains: screening/prophylaxis and management of antiretroviral therapy. A larger set of questions was pretested with convenience samples of approximately 50 general internists with no specific interest in HIV and 50 infectious diseases or other HIV specialists attending a national HIV conference. We chose 12 questions for inclusion into the questionnaire that demonstrated both good variability and discriminate validity on the pretest (Appendix A). Six of the questions related to prophylaxis against opportunistic infections and screening and 6 were related to viral load testing and antiretroviral drug therapy.
Analytical Weights
To better understand the impact of physician characteristics on patient care, we also present physician characteristics weighted to reflect the HIV-infected patients under care nationality. To do this, we used the sum of patient weights for the patients treated by each physician, using the HCSUS weights.6,7,12 The patient weights are an estimate of the number of persons represented by each respondent. In addition, these weights are further adjusted to account for physician nonresponse.
Analytic Variables
For the knowledge questions, the number of correct responses out of 12 were summed to form a knowledge score. “Don't know” responses were classified as incorrect. Each item in the scale was weighted equally. Factor analysis of the scale revealed a single underlying construct to the scale. Each item was found to be highly correlated with the scale (range, 0.20 to 0.49) with 1 exception. That item, related to vaccination for Haemophilus influenzae, was negatively correlated with the scale and was therefore dropped from analyses, leaving 11 items in the scale. Further investigation revealed that this topic had become controversial and that many HIV experts no longer agreed with the recommendation. The Cronbach's α for the resulting scale was 0.64. Because of the skewed distribution of the score, we also created a dichotomous variable indicating whether the physician was correct on 80% or more of these questions. Items assessing referral patterns were also skewed, so we dichotomized those questions into “ever referred” versus “never referred.” We created a summary variable coded as “yes” if the physician indicated that they ever referred for any of the 5 situations. Physicians with training in internal medicine, family practice, general practice, pediatrics, and medicine/pediatrics were classified as generalist physicians. The small numbers of physicians (14 in total) with non-ID subspecialty training or training in other specialties (for instance, 4 in hematology/oncology and 2 in obstetrics/gynecology) were classified with the generalist physicians. Only 6 of 152 (4%) ID physicians in the sample did not consider themselves HIV experts. To examine the joint effects of training and expertise in multivariable models, we therefore created a composite training/expertise variable with 3 levels: ID physicians, generalist experts, and generalist nonexperts. When analyzing experience, we examined both current and cumulative caseload. Because these were highly correlated (r = .58), we used only current caseload in analyses presented here.
Analyses
We compared physicians with formal training in infectious diseases to those without formal training in infectious diseases, primarily generalist physicians. Within each of these classifications, we stratified by whether the physician rated himself or herself as an expert in HIV. Proportions were compared using the χ2 test, and continuous variables were compared using the t test.
We used multivariable logistic regression to examine the influence of specialization on 2 dependent variables: whether the physician answered more than 80% of the knowledge items correctly and whether the physician ever referred patients. All multivariable models included years in practice, gender, race, and risk factors for HIV acquisition in the physician's practice as covariates. In the knowledge analyses, we first examined the relationship of ID training, self-assessed expertise, the composite training/expertise variable, and current HIV caseload to knowledge levels. We then examined the joint effects of the composite training/expertise variable and current HIV caseload. We used the same approach to develop models of referral practices, except that in the final referral model we also included the knowledge variable.
RESULTS
Of the 551 clinicians with correct addresses, 412 (75%) responded to the survey (60% of all physicians identified). Thirty-three of these were nurse practitioners or physician's assistants whom HCSUS participants had identified as physicians; we eliminated these. This left a total of 379 responses (Fig. 1). In general, there was no difference in response rates by region of the country, but respondents tended to have more patients enrolled in HCSUS (mean number identified, 4.8 vs 2.3; P < .01). We had no other information with which to compare responders to nonresponders. Item completion rates ranged from 88% (income) to 100%, with most items completed by 99% or more of the respondents.
The average age of the respondents was 44 years, and 73% were male, although male physicians treated 83% of patients (Table 1). Fifty-six percent of the physicians identified themselves as general physicians. Physicians with ID training, however, cared for 46% of the patients. Seventy-eight percent classified themselves as white while 7% were African American. Approximately a third of the sample had an annual income of less than $100,000/year. The physicians spent approximately 33 hours per week in patient care and derived approximately 72% of their income from patient related activities. The average physician saw approximately 60 patients/week. Twenty percent of the physicians identified their sexual orientation as mostly or exclusively homosexual.
Table 1.
Physician Demographics and Training*
| Physician Characteristics (N = 379) | Physician Characteristics Weighted to Reflect Patients Under Care Nationally† | |
|---|---|---|
| Personal characteristics | ||
| Age, y (±SD) | 43.9 (7.7) | 45.8 (6.5) |
| Male | 73 | 83 |
| Number of years since graduation from medical school, y (±SD) | 16.5(7.8) | 19.0(6.7) |
| Sexual preference (mostly or exclusively homosexual) | 20.0 | 20.0 |
| Primary fields of medicine | ||
| General medicine | 56 | 51 |
| Infectious diseases | 40 | 47 |
| Other (largely medical subspecialties) | 4 | 3 |
| Race/ethnicity | ||
| African American | 7 | 5 |
| White | 78 | 71 |
| Other | 15 | 24 |
| Annual income (n = 370) | ||
| Less than $100,000 | 33 | 21 |
| $100,000–$150,000 | 44 | 42 |
| More than $150,000 | 23 | 37 |
| Physicians receiving more than half their pay in salary (n = 306) | 85 | 85 |
| Patient care activities (±SD) | ||
| Half days per week in patient care, n | 6.3 (3.1) | 6.4 (2.8) |
| Outpatients per week, n | 60.6 (45.0) | 78.9 (42.3) |
| Hours in direct patient care per week, n | 32.8 (18.1) | 37.2 (18.4) |
| Mean percent of income from patient care | 71.9 (34.1) | 79.2 (29) |
All numbers represent percents unless otherwise noted.
Weighted to reflect the proportion of HIV-infected patients under care in the United States who receive care from physicians with each characteristic. N = 379 unless otherwise noted.
HIV-Specific Experience and Training
Table 2 presents data on HIV-specific experience and practice characteristics by specialty training (classified as general medicine/other or ID) and then, within specialty, by whether the physician was a self-rated expert in HIV care. Physicians trained in infectious diseases were more likely to consider themselves expert in HIV care than physicians in the general medicine or other category (96% vs 63%; P < .01). For most of the characteristics in Table 2, infectious disease physicians and self-rated experts within general medicine were similar. Median current caseloads were greater for general medicine experts than for ID experts (200 vs 150 patients; P < .05). In contrast, the average caseload for other general medicine physicians was 5. Three percent of ID and 4% of general medicine experts had fewer than 20 active cases compared with 74% of general medicine physicians who did not consider themselves HIV experts (P < .01). These low-volume physicians cared for an estimated 13,115 HIV-infected patients across the country, or about 8.5% of patients in care for HIV. General medicine physicians spent more time in outpatient practice and saw more patients per week than did ID physicians, although the amount of time spent in clinical practice was similar for both specialties. For 21% of nonexpert general physicians, the majority of their patients had injection drug use as a risk factor for acquiring HIV, as compared to just 14% of general medicine specialists and 16% of ID specialists (P = not significant [NS]).
Table 2.
Practice Characteristics and Behaviors of Physicians by Training and Self-assessed HIV Expertise
| Self-rated “Expert in HIV” | ||||
|---|---|---|---|---|
| Infectious Diseases–trained | General Medicine and Others | |||
| No (n = 6) | Yes (n = 146)* | No (n = 83) | Yes (n = 144)†,‡ | |
| HIV-related experience | ||||
| Current case load, median (±SD) | 22.5 (98) | 150 (209)‖ | 5 (73) | 200 (259)¶,+ |
| With fewer than 20 current cases, n (%) | 2 (33.3) | 5 (3.4)¶ | 61 (73.5) | 6 (4.2)¶ |
| Cumulative case load, median (±SD) | 100 (237) | 400 (1,300)¶ | 20 (256) | 500 (1,423)¶ |
| HIV patient population risk factors, % | ||||
| Majority of cases with IV drug use as a risk factor | 0 | 16.4 | 20.5 | 13.9 |
| Majority of cases with homosexual contact as a risk factor | 83.3 | 65.8 | 55.4 | 72.2‖ |
| Majority of cases with heterosexual contact as a risk factor | 16.7 | 11.6 | 21.7 | 11.8‖ |
| Practice characteristics, mean (±SD)§ | ||||
| Half days per week in patient care | 2.0 (2.0) | 4.9 (3.0)‖ | 7.0 (3.0) | 7.5 (2.7)++ |
| Outpatients per week | 15.5 (18.0) | 40.0 (33.5)‖ | 79.9 (54.3) | 72.2 (40.4)++ |
| Hours in direct patient care per week | 12.5 (15.1) | 30.7 (20.8)‖ | 35.5 (18.2) | 34.2 (14.4) |
‖ or ¶ in this column indicates significant test (t or χ2) for column 3 versus column 2.
‖ or ¶ in this column indicates significant test for column 5 versus column 4.
+ or ++ indicates significant test for column 5 versus column 3‖, or + for P < .05, and ¶ or ++ for P < .01.
Practice characteristics refers to the physician's entire practice.
HIV-Specific Knowledge
On average, physicians were correct on 8.2 (75%) of the 11 questions in the HIV knowledge scale and about one half were correct on more than 80% (Table 3). Infectious disease physicians had an average of 1 more correct question than generalist physicians and 69% had more than 80% correct, as compared to only 45% of general physicians (P < .01). Self-rated experts had higher average scores than did those who did not consider themselves experts (8.7 vs 6.6; P < .01), and self-rated experts within general medicine had scores that were similar to scores for ID specialists (8.5 vs 9.0; P = NS). Physicians with higher current patient volumes (>50 patients) scored higher than did those with fewer than 20 patients (8.8 vs 6.4; P < .01). For physicians with fewer than 20 patients, there was a positive association between caseload and score (e.g., an average score of 6.0 for physicians with fewer than 10 patients versus a score of 7.2 for those with between 10 and 18 patients; P < .05).
Table 3.
Physician Formal Training and Experience and HIV-specific Knowledge
| Number of Respondents | Average Number Correct (%) | Proportion >80% Correct | |
|---|---|---|---|
| All providers | 379 | 8.2 (75) | 0.54 |
| Formal training* | |||
| Infectious diseases | 152 | 8.9 (81) | 0.69 |
| General medicine | 213 | 7.9 (71) | 0.45 |
| Other | 14 | 6.7 (61) | 0.36 |
| Self-rated “experts”* | |||
| No | 89 | 6.6 (60) | 0.27 |
| Yes | 290 | 8.7 (79) | 0.63 |
| Formal training and self-rated expertise* | |||
| Infectious diseases | |||
| Non “experts” | 6 | 7.8 (71) | 0.50 |
| “Experts” | 146 | 9.0 (81) | 0.70 |
| General medicine or other | |||
| Non “experts” | 83 | 6.5 (59) | 0.25 |
| “Experts” | 144 | 8.5 (77) | 0.56 |
| Self-rated HIV knowledge* | |||
| Extremely knowledgeable | 205 | 8.8 (80) | 0.65 |
| Very knowledgeable | 121 | 8.3 (76) | 0.55 |
| Somewhat or not knowledgeable | 53 | 5.9 (53) | 0.11 |
| Current HIV case load* | |||
| 0–19 | 74 | 6.4 (58) | 0.20 |
| 20–49 | 44 | 8.4 (76) | 0.50 |
| ≥50 | 261 | 8.8 (80) | 0.65 |
Indicates P < .01 by ANOVA for differences within the category for average number correct.
Referral Patterns and Rates of CME Activity
Similar associations were observed for the relationships of experience and training with HIV-related referral patterns, involvement of patients in clinical trials, and attending local and national HIV-related CME activities. Fourteen percent of infectious disease physicians reported that they would ever refer versus 46% of general and other physicians (P < .01; Table 4). General medicine physicians who rated themselves as experts reported that they would refer less often than nonexperts (27% vs 82%; P < .01) and had rates closer to those reported by ID physicians. Compared to ID physicians, general medicine experts were more likely to refer for evaluating possible changes in antiretroviral therapy (22% vs 11%; P = .01) and choosing alternative prophylactic regimens for opportunistic infections (16% vs 8%; P = .02). The referral rates for the individual items were lowest for the initial evaluation of an HIV-infected patient (4% for general medicine experts, 2% for ID physicians, and 44% for general medicine physicians without expertise) and highest for evaluating possible changes in antiretroviral therapy (22% for general medicine experts, 11% for ID physicians, and 78% for general medicine physicians without expertise).
Table 4.
Relationships of Specialty Training, Experience, and Self-assessed Specialization to Referral Patterns and Participation in CME Activities/Meetings
| Number of Respondents | Ever Refer,*% | Local Meetings Attended*† Mean (±SD) | National Meetings Attended*† Mean (±SD) | Enrollment in Clinical Trials,‡% (±SD) | |
|---|---|---|---|---|---|
| All providers | 379 | 33.5 | 7.7 (10.7) | 1.9 (2.8) | 14.2 (16.9) |
| Formal training | § | ‖ | § | ||
| Infectious diseases | 152 | 13.8 | 9.4 (11.7) | 2.4 (3.1) | 18.2 (18.9) |
| General medicine | 213 | 46.2 | 6.7 (10.1) | 1.6 (2.6) | 11.8 (15.2) |
| Other | 14 | 57.1 | 6.1 (7.6) | 0.8 (1.1) | 9.2 (9.9) |
| Self-rated experts | § | § | § | ||
| Yes | 290 | 20.1 | 9.3 (11.5) | 2.3 (3.1) | 15.0 (15.9) |
| No | 89 | 78.1 | 2.7 (5.0) | 0.4 (0.8) | 11.6 (19.7) |
| Formal training and self-rated expertise | § | § | § | § | |
| Infectious diseases | |||||
| Non “expert” | 6 | 33.3 | 4.5 (4.3) | 1.8 (1.2) | 45.3 (32.6)¶ |
| “Expert” | 146 | 13.0 | 9.6 (11.8) | 2.4 (3.2) | 17.0 (17.4) |
| General medicine or other | |||||
| Non “expert” | 83 | 81.5 | 2.6 (5.0) | 0.3 (0.6) | 9.2 (16.2) |
| “Expert” | 144 | 27.3 | 9.0 (11.2) | 2.2 (3.0) | 13.0 (14.1) |
| Self-rated HIV knowledge | § | § | § | ||
| Extremely knowledgeable | 205 | 16.6 | 10.2 (12.4) | 2.6 (3.5) | 16.1 (15.9) |
| Very knowledgeable | 121 | 40.8 | 6.2 (8.2) | 1.3 (1.3) | 11.8 (14.8) |
| Somewhat or not knowledgeable | 53 | 84.3 | 1.6 (2.3) | 0.3 (0.6) | 12.6 (23.5) |
| Current HIV caseload | § | § | § | ||
| 0–19 | 74 | 83.1 | 2.7 (5.4) | 0.46 (0.8) | 12.0 (20.4) |
| 20–49 | 44 | 34.1 | 7.2 (11.0) | 1.39 (1.4) | 14.8 (17.8) |
| ≥50 | 261 | 19.9 | 9.3 (11.4) | 2.3 (3.2) | 14.8 (15.7) |
Notes physicians who answered that they would ever refer for one of 5 different clinical scenarios in HIV care (see METHODS section). N = 376 respondents for this series of questions.
Local and national meetings refer to meetings attended in the past 12 months.
Percent of patient population enrolled in clinical trials.
Indicates P < .01 by ANOVA or χ2 for differences within the category.
Indicated P < .05.
Only 6 physicians were in this category.
Self-rated experts in both infectious diseases and general medicine reported that they attended more local (9.6 and 9.0, respectively) and national (2.4 and 2.2, respectively) CME meetings than did nonexpert generalists (2.6 local meetings and 0.3 national meetings; P < .01). In contrast, ID physicians had more patients enrolled in clinical trials (17%) when compared with general medicine experts (13%) and general medicine nonexperts (9%; P < .01).
For physicians who rated themselves as extremely, very, and somewhat or not knowledgeable, rates of ever referring were 17%, 41%, and 84%, respectively (P < .01 for trend). Physicians who rated themselves as extremely knowledgeable attended 10.2 local CME meetings per year, compared with 6.2 and 1.6 meetings per year for those in the next 2 knowledge categories (P < .01).
Twenty percent of physicians with high caseloads (>50 patients) reported that they would ever refer versus 34% of those with 20 to 50 patients and 83% of those with fewer than 20 patients (<.01). Similarly, physicians with high caseloads also attended more local and national CME meetings, although enrollment in clinical trials was similar for both high- and low-volume physicians.
Multivariable Correlates of Knowledge and Referrals
Table 5 shows the relationships of the composite training/experience variable and current caseload to knowledge levels. When current caseload is considered individually (column 2), physicians following 20 to 49 patients (odds ratio [OR], 4.3; 95% confidence interval [CI], 1.8 to 10.1) or 50 or more patients (OR, 8.9; 95% CI, 4.6 to 17.1) were more likely than were those following fewer than 20 patients to score 80% or higher on the knowledge scale. When the composite training/experience variable is considered individually (column 2), generalist HIV experts (OR, 4.3; 95% CI, 2.3 to 7.9) and ID trained physicians (OR, 7.8; 95% CI, 4.1 to 14.7) were both more likely than were nonexpert generalists to score 80% or higher on the knowledge scale. When these 2 variables are examined simultaneously (column 3), a caseload of 50 or more patients is still significantly associated with knowledge (OR, 5.7; 95% CI, 2.4 to 13.6). The association of ID training with knowledge, however, is now markedly attenuated (OR, 2.7; 95% CI, 1.2 to 6.2), suggesting that the effects of specialty training on knowledge may largely be explained by HIV caseload.
Table 5.
Multivariable Effects of Specialty Training and Caseload on Knowledge Levels and Referrals, Individually and in Combination*
| Knowledge Level | Referrals | |||
|---|---|---|---|---|
| Effects of Specialty Training and Caseload Looked at Individuality | Effects of Specialty Training and Caseload Looked at in Combination | Effects of Specialty Training, Caseload, and Knowledge Looked at Individually | Effects of Specialty Training, Caseload, and Knowledge Looked at in Combination | |
| Caseload | ||||
| 0–19 (omitted category) | — | — | — | — |
| 20–49 | 4.3 (1.8 to 10.1) | 2.8 (1.1 to 7.3) | .08 (.03 to 0.21) | .25 (.09 to 0.75) |
| 50+ | 8.9 (4.6 to 17.1) | 5.7 (2.4 to 13.6) | 0.04 (0.02 to 0.08) | 0.17 (0.07 to 0.45) |
| Specialty training/expertise | ||||
| General medicine nonexpert | — | — | — | — |
| General medicine expert | 4.3 (2.3 to 7.9) | 1.4 (0.6 to 3.2) | 0.06 (0.03 to 0.13) | 0.19 (0.08 to 0.49) |
| ID trained | 7.8 (4.1 to 14.7) | 2.7 (1.2 to 6.2) | 0.03 (0.01 to 0.06) | 0.09 (0.03 to 0.22) |
| Knowledge level† | — | — | 0.20 (0.13 to 0.33) | 0.39 (0.22 to 0.69) |
All results are adjusted for race, gender, and patient HIV risk factor profile. Results represent odds ratios and 95% confidence interval.
Knowledge level refers to the proportion with >80% correct.
ID, infectious diseases.
The relationships between current caseload, the composite knowledge/experience, and knowledge levels to referral rates are also shown in Table 5 (columns 4 and 5). In column 4, each of these 3 variables is considered individually. After controlling for race, gender, and patient HIV risk factor profile, physicians with caseloads of 20 to 49 patients (OR, 0.08; 95% CI, 0.03 to 0.21) or 50 or more patients (OR, 0.04; 95% CI, 0.02 to 0.08) were less likely than were physicians with fewer than 20 patients to ever refer. Both generalist HIV experts (OR, 0.06; 95% CI, 0.03 to 0.13) and ID-trained physicians (OR, 0.03; 95% CI, 0.01 to 0.06) were less likely to ever refer than were generalist nonexperts. A higher knowledge score was also associated with referring less often (OR, 0.20; 95% CI, 0.13 to 0.33). When these 3 variables were considered jointly (column 5), we observed similar findings. Caseloads of 20 to 49 patients (OR, 0.25; 95% CI, 0.09 to 0.75) and caseloads of 50 or greater patients (OR, 0.17; 95% CI, 0.07 to 0.45) were associated with lower rates of ever referring. Both generalist experts (OR, 0.19; 95% CI, 0.08 to 0.49) and ID-trained physicians (OR, 0.09; 95% CI, 0.03 to 0.22) referred less often than did generalist nonexperts, and physicians with higher knowledge scores referred less often (OR, 0.39; 95% CI, 0.22 to 0.69).
DISCUSSION
The debate about who should care for patients with HIV infection or other chronic diseases often focuses on specialty training. This focus fails to account for disease-specific experience and the knowledge and skills that physicians might acquire through such experience and other clinical or educational activities. Our study is the first that we are aware of to provide national data on the relationships between specialty training and specialization, and how they relate to physician knowledge and selected physician behaviors. We hypothesized that expertise in the treatment of a particular condition would be most related to both specialty training and specialization, but that specialization, as primarily measured by experience, would be the most important factor.
These results confirm our hypotheses in a nationwide representative sample of physicians caring for adults with HIV infection. Among generalist physicians, expertise was strongly associated with caseload, knowledge, referral rates, and rates of participation in CME activities. We found no important difference between physicians trained in infectious diseases and general physicians who consider themselves experts in HIV care on caseload and attendance at local and national meetings. Infectiousdiseases–trained physicians, however, had slightly higher HIV-related knowledge scores and referred less often. In multivariable models that controlled for caseload and self-assessed expertise, caseload was the most important predictor of knowledge scores. This suggests that interest and involvement in HIV care by a physician, as manifested by caseload, keeping up with the literature, and attending meetings, are related to disease-specific expertise, regardless of specialty training.
Other studies have shown that specialists are usually more knowledgeable than generalists about diagnostic techniques13,14 and efficacious therapies.15–20 In addition, when processes of care are examined (using chart reviews or patients' reports), for acute myocardial infarction (MI),15,21 unstable angina,22 asthma,23 acute arthritis,24,25 multiple sclerosis,26 and depression,27 specialists tended to provide care deemed appropriate at higher rates than did generalists. While ID physicians in our study had slightly higher knowledge levels and different behaviors than generalist HIV experts, it appears that physicians not trained in ID can, by virtue of interest, clinical experience, CME activities, and other methods of self-education such as reading medical journals and newsletters, develop similar levels of HIV expertise.
We found that generalists who do not claim to have expertise in HIV refer more than generalists who do have expertise. As expected, physicians with training in infectious diseases had the lowest rate of referral for the 5 clinical scenarios presented. While we cannot examine the appropriateness of these referrals, one might assume that higher rates of referral are an appropriate response for nonexpert physicians and that this does not indicate lower quality or inappropriate care. Several studies of acute MI have shown the value of specialists and generalists working together in consultative relationships,28 and collaborative management has clearly been shown effective in the treatment of depression.29 In addition, constraints imposed by the health care delivery system might also contribute to lower rates of referrals for ID specialists, particularly for patients who require approval of referrals from primary care physicians.
Kitahata et al. found that AIDS patients in the Group Health Cooperative treated by more experienced clinicians (defined as >5 cases) had lower mortality, as compared with patients treated by other clinicians.30 That study, however, was conducted before the advent of newer therapies and in a single organization where most of the physicians were family practitioners with very low patient volumes. Turner et al. demonstrated that patients with HIV infection cared for by generalists were more frequently hospitalized, and Markson et al. showed that generalists were slower to adopt the use of zidovudine.31,32 In addition, Curtis et al. and Paauw et al. suggest that generalist physicians have fewer skills than do specialists in diagnosing HIV-related complications, including recognizing HIV-related skin conditions and diagnosing Pneumocystis carinii pneumonia.33,34 These studies, however, only examined formal training and were not able to account for HIV-specific experience and interests. Similarly, studies in cardiology21,35 and other specialties36 have also focused only on formal training. More recently, in a national survey, Stone et al. demonstrated that both training in infectious diseases and HIV experience were associated with prescribing appropriate antiretroviral regimens. That study, however, included physicians who were not necessarily the principal physicians for patients with HIV infection. Their findings were not significant when the analysis was restricted to physicians who would not refer patients for the management of HIV.37
Our study has several limitations. First, we do not know if the knowledge scale we used predicts care quality, and we do not have external criteria to assess the extent to which absence of knowledge will lead to poor care. However, nationally recognized experts selected questions that they thought reflected knowledge necessary to provide state-of-the-art care. Extensive pretesting that included groups of both HIV specialist physicians and generalist physicians with no particular expertise in the treatment of HIV infection demonstrated that the scale was able to discriminate between these 2 types of physicians. Second, as with all surveys, we rely on self-reports for our measures of specialty training, experience, and expertise. We see no reason, however, why error in reporting would tend to bias the associations we examined. Third, HCSUS did not include patients in prisons or the military and patients not receiving care. This is, however, the only nationally representative population of HIV-infected patients that we are aware of, and these patients identified our physician sample. We believe that our results can be generalized to physicians caring for patients with HIV infection in the United States. One additional caveat, however, is that HIV/AIDS may be atypical when compared to other chronic diseases in that when HIV first emerged, care was not uniformly embraced by ID specialists and was often delivered in community settings by generalists physicians. In the current environment, it might be more difficult for a non–ID-trained physician to develop similar expertise in HIV-related care. This would suggest, however, that in order to establish competency among current internal medicine residents, training programs should involve residents in the outpatient care of HIV-infected patients in settings where they can be supervised by experts in HIV-related care.
In summary, our results suggest that if we are to understand better the factors underlying reported associations between either specialty or volume and quality of care and/or outcomes, we should study both formal training and experience with a particular condition. Our data strongly suggest that general physicians are able to develop condition-specific knowledge similar to that of physicians with specialty training if they have a substantial case load and make an effort to stay current in a particular area. Specifically, we recommend that in future studies of HIV care, physicians be classified according to HIV caseload and whether they consider themselves experts in HIV care, in addition to classification by formal training. If there is concern that the latter type of measure is too subjective, other indicators, such as attendance at HIV clinical meetings, could be used. It remains to be determined whether these findings can be generalized to the care of patients with other chronic conditions. Further studies also are required to assess whether such knowledge translates into appropriate clinical practices and subsequent good outcomes. It will also be important to know if contextual factors, such as practicing with other specialists, modify the relationships described here.
Acknowledgments
Supported by a grant from the Robert Wood Johnson Foundation and by a cooperative agreement (U-01HS08578) between RAND and the Agency for Health Care Policy and Research. Dr. Bozzette is a Health Services Research and Development Senior Research Associate of the Department of Veterans Affairs.
We are indebted to Lin Ding, PhD for assistance with expert statistical programming; to Deborah Collins for assistance with manuscript preparation; to Shirley Nederend and Dana Perry for assistance with survey administration; and to Barbara J. McNeil, MD, PhD for comments on an earlier version of this manuscript.
Appendix A
True/False Knowledge Questions
| 1. In areas where the incidence of INH-resistant TB is >5%, HIV-infected patients should be given INH and rifampin for chemoprophylaxis of a positive PPD.(F) |
| 2. MAC prophylaxis should be given to all HIV-infected persons whose CD4 count is <100.(F) |
| 3. HIV-infected women require Pap smears more frequently than the general population. (T) |
| 4. An HIV viral load that doubles from 2,000 to 4,000 copies is indicative of a significant rise and antiretroviral therapy should be changed. (F) |
| 5. If a person's HIV viral load drops from 500,000 to 40,000 copies over 4 weeks of therapy and remains at that level for the next 4 months, their antiretrovial regimen should be changed.(T) |
| 6. AZT is contraindicated in patients who have thrombocytopenia.(F) |
| 7. If the HIV viral load rises from 2,000 to 40,000 copies on a 3-drug antiretrovial regimen, a fourth drug should be added.(F) |
| 8. Three determinations are necessary to establish an adequate baseline for viral loads.(F) |
| 9. Pneumococcal vaccine is recommended for HIV-infected adults.(T) |
| 10. Haemophilus influenza vaccine is recommended for HIV-infected adults.(F)(dropped) |
| 11. A patient whose HIV viral load became undetectable on AZT, 3TC, and Ritonavir has been forgetting to take their evening doses over the past 3 months since returning to work. Their HIV viral load is now 30,000 copies. This patient is likely to have an “undetectable” viral load after resuming these medications as prescribed. (F) |
| 12. Oral Ganciclovir is as effective as intravenous Ganciclovir in preventing relapses of CMV retinitis. (F) |
REFERENCES
- 1.Hecht FM, Wilson IB, Wu AW, Cook RL, Turner BJ. Optimizing care for persons with HIV infection. Ann Intern Med. 1999;131:136–43. doi: 10.7326/0003-4819-131-2-199907200-00011. [DOI] [PubMed] [Google Scholar]
- 2.Zuger A, Sharp VL. ‘HIV specialists’: the time has come. JAMA. 1997;278:1131–2. [PubMed] [Google Scholar]
- 3.Northfelt DW, Hayward RA, Shapiro MF. The acquired immunodeficiency syndrome is a primary care disease. Ann Intern Med. 1988;109:773–5. doi: 10.7326/0003-4819-109-10-773. [DOI] [PubMed] [Google Scholar]
- 4.Lewis CE. Management of patients with HIV/AIDS. Who should care? JAMA. 1997;278:1133–5. [PubMed] [Google Scholar]
- 5.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States. Results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 6.Bozzette SA, Berry SH, Duan N, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 7.Frankel MR, Shapiro JF, Duan N, et al. National probability samples in studies of low-prevalence diseases. Part II: designing and implementing the HIV Cost and Services Utilization Study sample. Health Serv Res. 1999;34:969–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro MF, Berk ML, Berry SH, et al. National probability samples in studies of low-prevalence diseases. Part I: perspectives and lessons from the HIV Cost and Services Utilization Study. Health Serv Res. 1999;34:951–68. [PMC free article] [PubMed] [Google Scholar]
- 9.Kish L. Survey Sampling. New York: John Wiley; 1965. [Google Scholar]
- 10.Lam NSN, Liu KB. Use of space-filling curves in generating a national rural sampling frame for HIV/AIDS research. Professional Geographer. 1996;48:321–32. [Google Scholar]
- 11.American Medical Association. Website located at http://www.ama-assn.org.
- 12.Duan N, McCaffrey D, Frankel M, et al. HCSUS Baseline Methods Technical Report. Santa Monica, Calif: RAND; 1998. Publication no. MR-1060-AHCPR. [Google Scholar]
- 13.Hnatiuk O, Moores L, Loughney T, Torrington K. Evaluation of internists' spirometric interpretations. J Gen Intern Med. 1996;11:204–8. doi: 10.1007/BF02642476. [DOI] [PubMed] [Google Scholar]
- 14.Dolan NC, Martin GJ, Robinson JK, Rademaker AW. Skin cancer control practices among physicians in a university general medicine practice. J Gen Intern Med. 1995;10:515–9. doi: 10.1007/BF02602405. [DOI] [PubMed] [Google Scholar]
- 15.Ayanian JZ, Guadagnoli E, McNeil BJ, Cleary PD. Treatment and outcomes of acute myocardial infarction among patients of cardiologists and generalist physicians. Arch Intern Med. 1997;157:2570–6. [PubMed] [Google Scholar]
- 16.Chin MH, Friedmann PD, Cassel CK, Lang RM. Differences in generalist and specialist physicians' knowledge and use of angiotensin-converting enzyme inhibitors for congestive heart failure. J Gen Intern Med. 1997;12:523–30. doi: 10.1046/j.1525-1497.1997.07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fendrick AM, Hirth RA, Chernew ME. Differences between generalist and specialist physicians regarding Helicobacter pylori and peptic ulcer disease. Am J Gastroenterol. 1996;91:1544–8. [PubMed] [Google Scholar]
- 18.Goldstein LB, Bonito AJ, Matchar DB, et al. US National Survey of Physician Practices for the Secondary and Tertiary Prevention of Ischemic Stroke. Design, service availability, and common practices. Stroke. 1995;26:1607–15. doi: 10.1161/01.str.26.9.1607. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein LB, Bonito AJ, Matchar DB, Duncan PW, Samsa GP. US National Survey of Physicians Practices for the Secondary and Tertiary Prevention of Ischemic Stroke. Carotid endarterectomy. Stroke. 1996;27:801–6. doi: 10.1161/01.str.27.5.801. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein LB, Bonito AJ, Matchar DB, Duncan PW, Samsa GP. US National Survey of Physician Practices for the Secondary and Tertiary Prevention of Ischemic Stroke. Medical therapy in patients with carotid artery stenosis. Stroke. 1996;27:1473–8. doi: 10.1161/01.str.27.9.1473. [DOI] [PubMed] [Google Scholar]
- 21.Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N Engl J Med. 1996;335:1880–7. doi: 10.1056/NEJM199612193352505. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber TL, Elkhatib A, Grines CL, O'Neill WW. Cardiologist versus internist management of patients with unstable angina: treatment patterns and outcomes. J Am Coll Cardiol. 1995;26:577–82. doi: 10.1016/0735-1097(95)00214-O. [DOI] [PubMed] [Google Scholar]
- 23.Legorreta AP, Christian-Herman J, O'Connor RD, Hasan MM, Evans R, Leung KM. Compliance with national asthma management guidelines and specialty care: a health maintenance organization experience. Arch Intern Med. 1998;158:457–64. doi: 10.1001/archinte.158.5.457. [DOI] [PubMed] [Google Scholar]
- 24.Panush RS, Carias K, Kramer N, Rosenstein ED. Acute arthritis in the hospital: comparison of rheumatologic with nonrheumatologic care. J Clin Rheumatol. 1995;1:74–80. [PubMed] [Google Scholar]
- 25.Evans TI, Wheeler MT, Small RE, Breitbach SA, Sanders KM, Roberts WN. A comprehensive investigation of inpatient intravenous colchicine use shows more education is needed. J Rheumatol. 1996;23:143–8. [PubMed] [Google Scholar]
- 26.Vickrey BG, Edmonds ZV, Shatin D, et al. General neurologist and subspecialist care for multiple sclerosis: patients' perceptions. Neurology. 1999;53:1190–7. doi: 10.1212/wnl.53.6.1190. [DOI] [PubMed] [Google Scholar]
- 27.Norquist G, Wells KB, Rogers WH, Davis LM, Kahn K, Brook R. Quality of care for depressed elderly patients hospitalized in the specialty psychiatric units or general medical wards. Arch Gen Psych. 1995;52:695–701. doi: 10.1001/archpsyc.1995.03950200085018. [DOI] [PubMed] [Google Scholar]
- 28.Willison DJ, Soumerai SB, McLaughlin TJ, et al. Consultation between cardiologists and generalists in the management of acute myocardial infarction: implications for quality of care. Arch Intern Med. 1998;158:1778–83. doi: 10.1001/archinte.158.16.1778. [DOI] [PubMed] [Google Scholar]
- 29.Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273:1026–31. [PubMed] [Google Scholar]
- 30.Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med. 1996;334:701–6. doi: 10.1056/NEJM199603143341106. [DOI] [PubMed] [Google Scholar]
- 31.Turner BJ, McKee L, Fanning T, Markson LE. AIDS specialist versus generalist ambulatory care for advanced HIV infection and impact on hospital use. Med Care. 1994;32:902–16. doi: 10.1097/00005650-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Markson LE, Cosler LE, Turner BJ. Implications of generalists' slow adoption of zidovudine in clinical practice. Arch Intern Med. 1994;154:1497–504. [PubMed] [Google Scholar]
- 33.Curtis JR, Paauw DS, Wenrich MD, Carline JD, Ramsey PG. Ability of primary care physicians to diagnose and manage Pneumocystis carinii pnuemonia. J Gen Intern Med. 1995;10:395–9. doi: 10.1007/BF02599841. [DOI] [PubMed] [Google Scholar]
- 34.Paauw DS, Wenrich MD, Curtis JR, Carline JD, Ramsey PG. Ability of primary care physicians to recognize physical findings associated with HIV infection. JAMA. 1995;274:1380–2. [PubMed] [Google Scholar]
- 35.Ayanian JZ, Hauptman PJ, Guadagnoli E, Antman EM, Pashos CL, McNeil BJ. Knowledge and practices of generalist and specialist physicians regarding drug therapy for acute myocardial infarction. N Engl J Med. 1994;331:1136–42. doi: 10.1056/NEJM199410273311707. [DOI] [PubMed] [Google Scholar]
- 36.Solomon DH, Bates DW, Panush RS, Katz JN. Costs, outcomes, and patient satisfaction by provider type for patients with rheumatic and musculoskeletal conditions: a critical review of the literature and proposed methodologic standards. Ann Intern Med. 1997;127:52–60. doi: 10.7326/0003-4819-127-1-199707010-00009. [DOI] [PubMed] [Google Scholar]
- 37.Stone VE, Mansourati FF, Poses RM, Mayer KH. Relation of physician specialty and HIV/AIDS experience to choice of guideline-recommended antiretroviral therapy. J Gen Intern Med. 2001;16:360–8. doi: 10.1046/j.1525-1497.2001.016006360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]