Abstract
BACKGROUND
Because there is increasing concern that economic data are not used in the clinical guideline development process, our objective was to evaluate the extent to which economic analyses are incorporated in guideline development.
METHODS
We searched medline and HealthSTAR databases to identify English-language clinical practice guidelines (1996–1999) and economic analyses (1990–1998). Additional guidelines were obtained from The National Guidelines Clearinghouse Internet site available at http://www.guideline.gov. Eligible guidelines met the Institute of Medicine definition and addressed a topic included in an economic analysis. Eligible economic analyses assessed interventions addressed in a guideline and predated the guideline by 1 or more years. Economic analyses were defined as incorporated in guideline development if 1) the economic analysis or the results were mentioned in the text or 2) listed as a reference. The quality of economic analyses was assessed using a structured scoring system.
RESULTS
Using guidelines as the unit of analysis, 9 of 35 (26%) incorporated at least 1 economic analysis of above-average quality in the text and 11 of 35 (31%) incorporated at least 1 in the references. Using economic analyses as the unit of analysis, 63 economic analyses of above-average quality had opportunities for incorporation in 198 instances across the 35 guidelines. Economic analyses were incorporated in the text in 13 of 198 instances (7%) and in the references in 18 of 198 instances (9%).
CONCLUSIONS
Rigorous economic analyses may be infrequently incorporated in the development of clinical practice guidelines. A systematic approach to guideline development should be used to ensure the consideration of economic analyses so that recommendations from guidelines may impact both the quality of care and the efficient allocation of resources.
Keywords: guidelines, econimic analyses, cost-effectiveness
Evidence-based practice guidelines have been advocated as a mechanism for reducing practice variation and improving the quality of care. Systematic approaches to guideline development have been defined and advocated by leading medical journals and authorities.1–5 Recently, a review of the guideline development process has outlined several deficiencies, including the omission of economic data.6,7
In an era of increasingly constrained financial resources, allocation of scarce medical resources has become an important factor in health policy decision making. Such policy should rely on the best available evidence as promulgated in evidence-based practice guidelines.8 Evidence-based medicine and economic analyses address the values of effectiveness and efficiency critical to allocation decision making,9 and economic analyses are an important type of evidence that could inform the practice of evidence-based medicine.10 Several sources, including the Consensus Statement on the Role of Cost-Effectiveness Analysis in Health and Medicine, recommend that cost-effectiveness analyses be used as an aid to decision makers11 and that economic data should be incorporated into guidelines where possible.12
Because 80% or more of healthcare costs may be directly related to clinical decisions, safely controlling health care costs will require influencing clinical decisions. Guidelines are often used to inform and improve clinical decision making. If guidelines are to address economic issues relevant to clinical decision making, they should be informed by the highest quality economic evaluations available in the published literature. Our objective was to evaluate the extent to which economic analyses are incorporated in the guideline development process.
METHODS
Literature Review and Selection Process
A systematic search of the medline and HealthSTAR computerized bibliographic databases was performed to identify English-language clinical practice guidelines using the MESH heading “Practice Guidelines” for the years 1996–1999. Guidelines were also obtained from The National Guidelines Clearinghouse Internet site (http://www.guideline.gov).
English-language economic analyses—articles reporting data on direct and indirect costs—were identified through a systematic search of the databases using the MESH headings “Cost-Benefit Analysis” or “Costs and Cost Analysis” for the years 1990–1998. The search time frames for economic analyses and guidelines were chosen such that the economic analyses would likely predate the clinical guidelines. Duplicates, editorials, letters, and reviews were excluded. Then, on the basis of titles, the clinical practice guidelines and economic analyses were grouped by condition within 5 clinical categories: acute therapy, chronic therapy, risk factor reduction, screening, and surgical therapy. These categories were selected in an attempt to include guidelines and economic analyses across a broad spectrum of medical conditions. The medical condition with the greatest number of guidelines and at least 10 economic analyses was selected from each of the 5 categories.
After the 5 conditions were selected, a second literature search was conducted using the same search strategy as before but also including the medical conditions' MESH headings. Four researchers under the supervision of 2 health economists reviewed the identified guidelines and economic analyses and finalized the list for further testing on the basis of several criteria. These 4 researchers included a pharmacist trained in pharmacoeconomics (who was the lead investigator), a physician/health services researcher, a health economist, and an epidemiologist. All 4 researchers had training and experience in health services research methods. Guidelines were eligible for analysis if they: 1) met the Institute of Medicine definition of “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances,”6 2) were described clearly and made recommendations as to appropriate care,13–15 and 3) addressed a topic included in an economic analysis. Economic analyses were eligible for analysis if they met the following inclusion criteria: 1) assessed interventions specifically addressed in the guideline and 2) predated the guideline publication by 1 or more years. Hand searches of bibliographies of the guidelines that were accepted for analyses further identified additional economic analyses not identified in our search.
Scoring of Economic Analyses
Eligible economic analyses for the 5 conditions were then assessed for methodological quality using a structured scoring system (Table 1). Since no published checklist or appraisal system enables a quantitative ranking of the quality of economic analyses, we developed a compendium of questions from the most well-known published checklists for evaluating economic studies.16–20 Items were selected from the existing instruments if they represented a domain present in all existing checklists, and if the item could be operationalized into a yes/no question without changing the content of the item. To ensure content validity, each domain represented in the published checklists was also represented in the final composite instrument. The 4 researchers who finalized the list of guidelines and economic analyses also evaluated the quality of economic analyses with the scoring system. In a pilot test, pairs of reviewers (the lead investigator paired with each of the 3 other reviewers) assessed articles together and resolved the discrepancy among their ratings through several rounds of discussion overseen by the health economist. The agreement between 3 pairs of reviewers was assessed on 3 separate samples of 8 randomly selected economic analyses. The κ values for the samples were 0.87, 0.88, and 0.78. Subsequently, the economic analyses from the 5 conditions were divided among the 4 reviewers, with the lead investigator evaluating articles from 2 conditions. Mean and median scores were calculated for all economic analyses. Analyses scoring higher than the mean were defined as above-average quality.
Table 1.
Scoring System for Economic Analyses*
| 1. | Was the study objective clearly defined and measurable? | Y / N |
| 2. | Was the perspective for the analysis stated clearly? | Y / N |
| 3. | Was the pharmacoeconomic tool used appropriate for the study? | Y / N |
| 4. | Was this the tool that was actually used? | Y / N |
| 5. | Did the study provide a comparison of alternative treatments for patients with the same clinical condition? | Y / N |
| 6. | Was a complete description of the alternatives given? | Y / N |
| 7. | Was the evidence of efficacy established through randomized trials? | Y / N |
| 8. | Was this evidence of efficacy supplemented by evidence of effectiveness applicable to the patient population or subgroups considered in the study? | Y / N |
| 9. | Were the methods and analysis displayed in a clear and transparent manner? | Y / N |
| 10. | Were the components of the numerator and denominator displayed? | Y / N |
| 11. | Were costs and consequences measured in the appropriate physical units? | Y / N |
| 12. | Were costs and outcomes relevant to the analysis tool chosen? | Y / N |
| 13. | If the study time was greater than one year, were costs and consequences that occur in the future discounted to their present value? | Y / N |
| 14. | Equity assumptions made during analysis: if quality-adjusted life years gained by an individual were considered equal, is this acceptable? | Y / N |
| 15. | Were the results practical for medical decision makers? | Y / N |
| 16. | Were sensitivity analyses performed by incorporating ranges of values for variables with uncertainty? | Y / N |
| 17. | Were the assumptions and limitations of the study discussed? | Y / N |
| 18. | Was an incremental analysis performed? | Y / N |
| 19. | Were the conclusions of the study justified? | Y / N |
| 20. | Can the conclusions be generalized to other populations? | Y / N |
Analysis
Each guideline was then evaluated as to whether eligible economic analyses were incorporated into the eligible guidelines on the basis of the following criteria: 1) the economic analysis or the result of the economic analysis was mentioned in the body of the text, or 2) the economic analysis was listed as a reference. When neither of these criteria was met, the developers of each guideline were then contacted to determine if any economic analyses were incorporated in the guideline development process. Four telephone or e-mail attempts were made to contact the guideline committee/task force chairperson or other members knowledgeable about the guideline development process. A standardized questionnaire was administered to each contact to determine if there were records of additional references used in the development process that could be reviewed for the analysis. If no additional records were available, the contact was asked if there had been any consideration of economic issues by members of the task force. If the contact indicated that there had been such consideration, a list of eligible economic analyses was sent for review. Successful contact (yes/no), consideration of economic issues (yes/no/no recollection), and consideration of eligible economic analyses (yes/no) were documented.
The incorporation of economic analyses in guidelines was evaluated in 2 ways. The first approach used guidelines as the unit of analysis or denominator, and determined the proportion of guidelines that incorporated economic analyses in the text or references. The second approach used economic analyses as the unit of analysis, with a denominator equal to the number of opportunities for economic analyses to be incorporated into guidelines. Economic analyses with opportunities for incorporation were those that met the inclusion criteria described above, i.e., addressing a topic within, and predating, the guideline. Some economic analyses had opportunities for incorporation in more than 1 guideline within each clinical category.
We tested whether there was variation between the conditions studied in the degree to which economic analyses were incorporated into guidelines. Differences between groups were tested using χ2 and Fisher exact tests. Because there may be a time lag between publication of an economic analysis and the incorporation into a guideline, we performed a secondary analysis comparing the rates of incorporation of economic analyses into guidelines for those economic analyses published 1 or more years prior to guideline development and 2 or more years prior to guideline development.
RESULTS
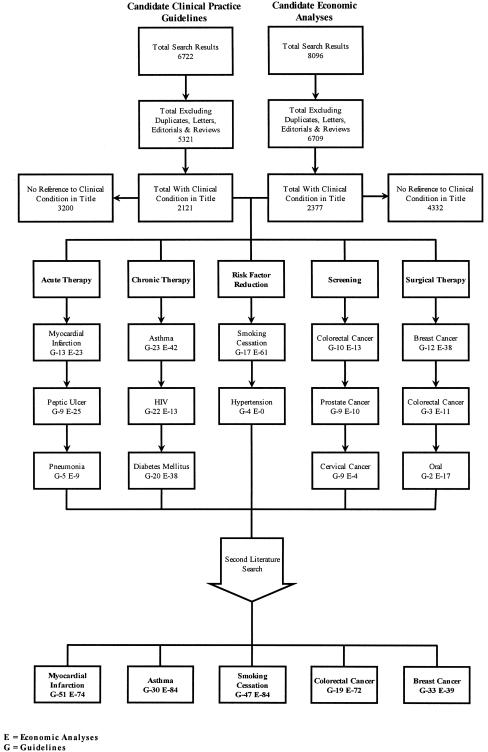
The systematic literature review resulted in 6,722 candidate practice guidelines (356 from the National Guideline Clearinghouse and 6,366 from medline) and 8,096 candidate economic analyses (Fig. 1). After excluding duplicates, letters, editorials, and reviews and classifying by medical condition on the basis of the title, there were a total of 2,121 candidate practice guidelines and 2,377 candidate economic analyses. The medical conditions with the most candidate practice guidelines and at least 10 candidate economic analyses for each category were acute myocardial infarction,21–56 asthma,57–90 smoking cessation,91–113 colorectal cancer,114–140 and breast cancer.141–166 The second literature search with the addition of each condition's MESH heading resulted in the identification of additional guidelines and economic analyses. A total of 180 guidelines and 353 economic analyses were subjected to further review (Fig. 1).
FIGURE 1.
Results of literature search and grouping process to identify the top medical conditions with the most guidelines and at least 10 economic analyses for each category.
A total of 35 guidelines across the 5 conditions met the inclusion criteria (Table 2). Hand searches of their bibliographies resulted in 14 additional economic analyses. Among the 367 economic analyses, 112 met the inclusion criteria (Table 2). Fourteen of these guidelines (40%) were developed by a governmental agency, 11 (31%) were developed by a medical society, 8 (23%) were developed by a nonprofit nonmedical society, and 2 (6%) were published in peer-reviewed medical journals but were not sponsored by an agency. The mean quality score for the 112 eligible economic analyses was 69% (median, 72%). Sixty-three of the 112 eligible economic analyses (56%) had scores greater than 69% and were defined as above-average quality. The distribution of quality scores was similar to that found in previous work.167
Table 2.
Number of Guidelines and Economic Analyses Identified and Included in the Analysis
| Category | ||||||
|---|---|---|---|---|---|---|
| Acute Therapy | Chronic Therapy | Risk Factor Reduction | Screening | Surgical Therapy | Total | |
| Condition | Myocardial infarction | Asthma | Smoking cessation | Colorectal cancer | Breast cancer | |
| Included | ||||||
| Guidelines, n | 621–23,28,49,50 | 557–60,63 | 691–93,106,112 | 6115–118,130,131 | 12141–147,149,153,161,162,164 | 35 |
| Economic analyses, n | 3024–27,29–48,51–56 | 2961,62,64–90 | 1894–105,107–111,113 | 21114,119–129,132–140 | 14148,150–152,154–160,163,165,166 | 112 |
| Economic analyses scoring > mean* | 17 | 13 | 10 | 16 | 7 | 63 |
The mean score for all economic analyses is 69% (median, 72%).
Incorporation of Economic Analyses by Guidelines
Using the guidelines as the unit of analysis, the incorporation of economic analyses by guideline is reported across the 5 conditions in Table 3. Eleven of 35 guidelines (31%) incorporated at least 1 eligible economic analysis in the text, and 15 (43%) incorporated at least 1 eligible economic analysis in the references. Nine (26%) of the guidelines incorporated at least 1 economic analysis of above-average quality in the text, and 11 (31%) incorporated at least 1 eligible economic analysis of above-average quality in the references. The 5 conditions were also analyzed individually, revealing statistically significant (P < .001) variation in the degree to which economic analyses were considered in guideline development (Table 3). We also evaluated variation when only including economic analyses of above-average quality. The percentage of economic analyses incorporated by guidelines across the 5 conditions ranged from 0 of 12 (0%) for breast cancer to 4 of 6 (67%) in smoking cessation and colorectal cancer and the variation was statistically significant (P < .005).
Table 3.
Incorporation of Economic Analyses by Guidelines
| Category | ||||||
|---|---|---|---|---|---|---|
| Acute Therapy | Chronic Therapy | Risk Factor Reduction | Screening | Surgical Therapy | Total | |
| Condition | Myocardial infarction | Asthma | Smoking cessation | Colorectal cancer | Breast cancer | |
| Any economic analysis | ||||||
| Guidelines, n | 6 | 5 | 6 | 6 | 12 | 35 |
| With economic analyses in text, n (%) | 3 (50) | 0 (0) | 4 (67) | 4 (67) | 0 (0) | 11 (31) |
| With economic analyses in references, n (%) | 4 (67) | 3 (60) | 4 (67) | 4 (67) | 0 (0) | 15 (43) |
| With economic analyses considered by task force, n (%) | 5 (83) | 3 (60) | 4 (67) | 4 (67) | 0 (0) | 16 (46) |
| Economic analyses scoring > mean* | ||||||
| Guidelines, n | 6 | 5 | 6 | 6 | 12 | 35 |
| With economic analyses in text, n (%) | 1 (17) | 0 (0) | 4 (67) | 4 (67) | 0 (0) | 9 (26) |
| With economic analyses in references, n (%) | 2 (33) | 1 (20) | 4 (67) | 4 (67) | 0 (0) | 11 (31) |
| With economic analyses considered by task force, n (%) | 3 (50) | 1 (20) | 4 (67) | 4 (67) | 0 (0) | 12 (34) |
The mean score for all economic analyses is 69% (median, 72%).
Of the 20 guidelines failing to incorporate an eligible economic analysis into the body of the text or in the references, 12 of the chairpersons (60%) were successfully interviewed. For 1 of 12 guidelines (8%), the chairperson reported consideration of economic analyses by the task force in guideline development
Incorporation of Economic Analyses by Opportunities for Incorporation
Using the economic analyses as the unit of analysis, the incorporation of economic analyses by opportunities for incorporation is reported across the 5 conditions in Table 4. One hundred twelve eligible economic analyses had an opportunity for incorporation in 300 instances across the 35 guidelines. Economic analyses were incorporated in the body of the text and in the references in 16 of 300 (5%) and 29 of 300 (10%) instances, respectively. Similarly, 63 eligible economic analyses of above-average quality had an opportunity for incorporation in 198 instances across the 35 guidelines. Economic analyses were incorporated in the body of the text and in the references in 13 of 198 (7%) and 18 of 198 (9%) instances, respectively. Twenty-two of the 112 economic analyses (20%) and 16 of the 63 economic analyses of above-average quality (24%) were incorporated into any of the guidelines meeting our inclusion criteria.
Table 4.
Economic Analyses Guidelines by Opportunities for Incorporation
| Category | ||||||
|---|---|---|---|---|---|---|
| Acute Therapy | Chronic Therapy | Risk Factor Reduction | Screening | Surgical Therapy | Total | |
| Condition | Myocardial infarction | Asthma | Smoking cessation | Colorectal cancer | Breast cancer | |
| Economic analyses | ||||||
| Opportunities for economic analyses to be incorporated in guidelines, n | 94 | 35 | 36 | 92 | 43 | 300 |
| Economic analyses incorporated in text, n (%) | 4 (4) | 0 (0) | 4 (11) | 8 (9) | 0 (0) | 16 (5) |
| Economic analyses incorporated in references, n (%) | 9 (10) | 3 (9) | 7 (19) | 10 (11) | 0 (0) | 29 (10) |
| Economic analyses scoring >mean* | ||||||
| Opportunities for economic analyses to be incorporated in guidelines, n | 61 | 18 | 25 | 68 | 26 | 198 |
| Economic analyses incorporated in text, n (%) | 2 (3) | 0 (0) | 4 (16) | 7 (10) | 0 (0) | 13 (7) |
| Economic analyses incorporated in references, n (%) | 3 (5) | 1 (6) | 6 (24) | 8 (12) | 0 (0) | 18 (9) |
The mean score for economic analyses is 69% (median, 72%).
As shown in Table 4, risk reduction guidelines (as represented by smoking cessation) had the highest percentage of economic analyses incorporated (i.e., 19%). Eighteen economic analyses had opportunities for incorporation in 36 instances across the 6 risk reduction guidelines. Economic analyses were incorporated in the text and in the references in 4 of 36 (11%) and 7 of 36 (19%) instances, respectively. Smoking cessation also had the highest percentage of analyses of above-average quality incorporated. Ten analyses of above-average quality had opportunities for incorporation in 25 instances across the 6 smoking cessation guidelines. Economic analyses were incorporated in the text and in the references in 4 of 25 (16%) and 6 of 25 (24%) instances, respectively.
Analysis by Number of Years Between Publication of Economic Analysis and Guideline
We also compared the rates of incorporation of economic analyses into guidelines for economic analyses published 1 or more years prior to guideline development with those published 2 or more years prior to guideline development. There were no significant differences between economic analyses published 1 or more years prior to guideline development and those published 2 or more years prior to guideline development for incorporation in the body of guideline (5.3% vs 4.6%; P = .69) or in the references (9.6% vs 10.5%; P = .76).
COMMENT
Economic analyses appear to be infrequently incorporated in the development of clinical practice guidelines. Twenty of the 35 guidelines (57%) failed to incorporate any eligible economic analysis into the text or in the references. Ninety of the 112 economic analyses (80%) failed to be incorporated into any of the guidelines meeting our inclusion criteria. These findings are inconsistent with recommendations that economic analyses should be taken into account when developing guidelines.
The paucity of high-quality economic data within the clinical guideline development process makes it increasingly difficult for decision makers to allocate limited health care resources efficiently. It is unlikely that this is due to the lack of higher quality, published economic analyses, based on the number identified in this study. Certain clinical conditions were more likely to incorporate economic analyses than others. Conditions amenable to risk factor reduction or preventative care, such as smoking cessation and colorectal cancer screening, were more likely to incorporate economic analyses than, for example, surgical therapy for breast cancer. This suggests there may be greater rationale to justify program benefits economically when the benefits will occur in the future compared to treatments with more immediate impact.
There are some limitations to this analysis. We attempted, a priori, to stratify our analysis by the quality of the economic analysis because we do not believe that guidelines should incorporate the results of economic analyses that do not meet standards for reporting and methodological rigor. Because we were unable to identify a valid scoring system for quality,167 we developed one from existing instruments.1–20 This composite system assumes that each item carries equal weight, and it has not been formally validated. While construct validity was not tested in this study, we believe that the composite instrument has face, or content, validity since each domain from existing checklists is represented and the distribution of quality scores obtained with this instrument appears similar to the distributions found in other studies using different methods. Moreover, there was high inter-rater agreement, suggesting adequate reliability. A limitation of our composite instrument is that items that are relevant to only specific types of economic analyses may be lacking, such as a specific item about utility values. Further study of the construct and discriminant validity of the composite scoring system appears warranted. One reason for the lack of incorporation of economic analyses in guidelines may be that there is no standardized method for developers of guidelines to determine which economic analyses merit incorporation on the basis of their quality.
Another limitation of our analysis is that we did not grade the quality of the practice guidelines. While appraisal systems exist to identify certain elements that guidelines should include, there is no acceptable system for ranking or comparing the relative quality of clinical practice guidelines. In light of this, a priori we assumed that if economic analyses were considered in the guideline development process, consideration would most likely occur in high-profile guidelines developed by national societies and published in the peer-reviewed literature.
A third limitation is that we used a very specific (MESH headings) rather than sensitive (keywords) search strategy, recognizing that we wanted a “sample” of the universe of published economic analyses and guidelines. Thus, our sample may not be completely representative of all relevant articles. Moreover, because this analysis included only guidelines published in peer-reviewed journals or on the National Guidelines Clearinghouse website, it may be susceptible to publication bias.
Furthermore, although we attempted to analyze a representative sample of published guidelines, we nonetheless submitted a sample of 5 conditions to evaluation. Since we only examined 1 condition in each category, all of the analyses performed in the current study were based on relatively small samples. This may influence the generalizability of the findings. However, the conditions chosen were those with the greatest number of clinical guidelines and at least 10 published economic analyses predating the guidelines. We also recognized that the recollection of the guideline committee regarding the consideration of economic analyses by the group might not be completely reliable. Nevertheless, we felt that this was the only available method for obtaining information regarding whether the group developing the guideline did not consider any economic analyses or simply could not identify any economic analyses.
Finally, it may be considered unlikely that economic analyses published only 1 year prior to guideline development would be incorporated into a practice guideline. We explored this by comparing the rates of incorporation of economic analyses into guidelines for economic analyses published 1 or more years prior to guideline development and 2 or more years prior to guideline development. When we limited our analysis to only economic analyses published 2 or more years prior to guideline development, the results were unchanged.
In an era of increasing scrutiny of the costs and benefits of medical interventions, clinical practice guidelines are needed to inform practitioners about the most effective and efficient management strategies. Because there is a proliferation of economic evaluations and methodological standards for these evaluations in the literature, economic findings should be incorporated in the guideline development process. However, incorporating economic analyses of poor quality in the guideline could potentially decrease the value of the guideline and mislead the users. Therefore, practical standardized methods for assessing the quality of economic analyses appear to be needed. We are concerned that guidelines and ensuing policy recommendations may not be informed by economic evaluations that elucidate and promote the most efficient utilization of health care resources. Further research is necessary to identify barriers to the incorporation of economic analyses in the guideline development process.
Acknowledgments
This investigator-initiated work was supported by a research grant from TAP Pharmaceutical Products, Inc.
REFERENCES
- 1.Brook RH. Practice guidelines: to be or not to be. Lancet. 1996;348:1005–6. [PubMed] [Google Scholar]
- 2.Cluzeau F, Littlejohns P, Grimshaw JM. Appraising clinical guidelines: towards a “which” guide for purchasers. Qual Health Care. 1994;3(3):121–2. doi: 10.1136/qshc.3.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook DJ, Greengold NL, Ellrodt AG, Weingarten SR. The relation between systematic reviews and practice guidelines. Ann Intern Med. 1997;127(3):210–6. doi: 10.7326/0003-4819-127-3-199708010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Eccles M, Clapp Z, Grimshaw J, et al. Developing valid guidelines: methodological and procedural issues from the North of England Evidence Based Guideline Development Project. Qual Health Care. 1996;5(1):44–50. doi: 10.1136/qshc.5.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellrodt G, Cook DJ, Lee J, Cho M, Hunt D, Weingarten S. Evidence-based disease management. JAMA. 1997;278(20):1687–92. [PubMed] [Google Scholar]
- 6.Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA. 1999;281(20):1900–5. doi: 10.1001/jama.281.20.1900. [DOI] [PubMed] [Google Scholar]
- 7.Grilli R, Magrini N, Penna A, Mura G, Liberati A. Practice guidelines developed by specialty societies: the need for a critical appraisal. Lancet. 2000;355(9198):103–6. doi: 10.1016/S0140-6736(99)02171-6. [DOI] [PubMed] [Google Scholar]
- 8.Lohr KN, Eleazer K, Mauskopf J. Health policy issues and applications for evidence-based medicine and clinical practice guidelines. Health Policy. 1998;46(1):1–19. doi: 10.1016/s0168-8510(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 9.Eddy DM. Clinical decision making: from theory to practice. Benefit language: criteria that will improve quality while reducing costs. JAMA. 1996;275(8):650–7. doi: 10.1001/jama.275.8.650. [DOI] [PubMed] [Google Scholar]
- 10.Singer PA. Resource allocation: beyond evidence-based medicine and cost-effectiveness analysis. ACP J Club. 1997;127(3):A16–8. [PubMed] [Google Scholar]
- 11.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(14):1172–7. [PubMed] [Google Scholar]
- 12.Cook D, Giacomini M. The trials and tribulations of clinical practice guidelines. JAMA. 1999;281(20):1950–1. doi: 10.1001/jama.281.20.1950. [DOI] [PubMed] [Google Scholar]
- 13.Hayward RS, Wilson MC, Tunis SR, Bass EB, Guyatt G. Users' guides to the medical literature. VIII. How to use clinical practice guidelines. A. Are the recommendations valid? The Evidence-Based Medicine Working Group. JAMA. 1995;274(7):570–4. doi: 10.1001/jama.274.7.570. [DOI] [PubMed] [Google Scholar]
- 14.Wilson MC, Hayward RS, Tunis SR, Bass EB, Guyatt G. Users' guides to the Medical Literature. VIII. How to use clinical practice guidelines. B. What are the recommendations and will they help you in caring for your patients? The Evidence-Based Medicine Working Group. JAMA. 1995;274(20):1630–2. doi: 10.1001/jama.274.20.1630. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Clinical Practice Guidelines: Directions of a New Program. Washington DC: National Academy Press; 1990. [Google Scholar]
- 16.Drummond MF, Stoddart GL, Torrance GW. Methods for Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1987. [Google Scholar]
- 17.Torrance GW, Blaker D, Detsky A, et al. Canadian guidelines for economic evaluation of pharmaceuticals. Canadian Collaborative Workshop for Pharmacoeconomics. Pharmacoeconomics. 1996;9(6):535–59. doi: 10.2165/00019053-199609060-00008. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez LA. Evaluating the quality of published pharmacoeconomic evaluations. Hosp Pharm. 1995;30(2):146–8. 152–2. [PubMed] [Google Scholar]
- 19.Clemens K, Townsend R, Luscombe F, Mauskopf J, Osterhaus J, Bobula J. Methodological and conduct principles for pharmacoeconomic research. Pharmaceutical Research and Manufacturers of America. Pharmacoeconomics. 1995;8(3):169–74. doi: 10.2165/00019053-199508020-00008. [DOI] [PubMed] [Google Scholar]
- 20.Sacristan JA, Soto J, Galende I. Evaluation of pharmacoeconomic studies: utilization of a checklist. Ann Pharmacother. 1993;27(3):1126–33. doi: 10.1177/106002809302700919. [DOI] [PubMed] [Google Scholar]
- 21.The Heart and Stroke Foundation of Canada, the Canadian Cardiovascular Society and the Canadian Association of Emergency Physicians for the Emergency Cardiac Care Coalition. Recommendations for ensuring early thrombolytic therapy for acute myocardial infarction. CMAJ. 1996;154(3):483–7. [PMC free article] [PubMed] [Google Scholar]
- 22.The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Acute myocardial infarction pre-hospital and in-hospital management. Eur Heart J. 1996;17(1):43–63. doi: 10.1093/oxfordjournals.eurheartj.a014691. [DOI] [PubMed] [Google Scholar]
- 23.American College of Physicians. Guidelines for risk stratification after myocardial infarction. Ann Intern Med. 1997;126(7):556–60. [PubMed] [Google Scholar]
- 24.Ades PA, Pashkow FJ, Nestor JR. Cost-effectiveness of cardiac rehabilitation after myocardial infarction. J Cardiopulm Rehabil. 1997;17(4):222–31. doi: 10.1097/00008483-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Castillo PA, Palmer CS, Halpern MT, Hatziandreu EJ, Gersh BJ. Cost-effectiveness of thrombolytic therapy for acute myocardial infarction. Ann Pharmacother. 1997;31(5):596–603. doi: 10.1177/106002809703100515. [DOI] [PubMed] [Google Scholar]
- 26.Davey PJ, Schulz M, Gliksman M, Dobson M, Aristides M, Stephens NG. Cost-effectiveness of vitamin E therapy in the treatment of patients with angiographically proven coronary narrowing (CHAOS trial). Cambridge Heart Antioxidant Study. Am J Cardiol. 1998;82(4):414–7. doi: 10.1016/s0002-9149(98)00354-3. [DOI] [PubMed] [Google Scholar]
- 27.de Boer MJ, van Hout BA, Liem AL, Suryapranata H, Hoorntje JC, Zijlstra F. A cost-effective analysis of primary coronary angioplasty versus thrombolysis for acute myocardial infarction. Am J Cardiol. 1995;76(11):830–3. doi: 10.1016/s0002-9149(99)80238-0. [DOI] [PubMed] [Google Scholar]
- 28.Deedwania PC, Amsterdam EA, Vagelos RH. Evidence-based, cost-effective risk stratification and management after myocardial infarction. California Cardiology Working Group on Post-MI Management. Arch Intern Med. 1997;157(3):273–80. [PubMed] [Google Scholar]
- 29.Gaspoz JM, Lee TH, Weinstein MC, et al. Cost-effectiveness of a new short-stay unit to “rule out” acute myocardial infarction in low risk patients. J Am Coll Cardiol. 1994;24(5):1249–59. doi: 10.1016/0735-1097(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons RJ, Holmes DR, Reeder GS, Bailey KR, Hopfenspirger MR, Gersh BJ. Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. N Engl J Med. 1993;328:685–91. doi: 10.1056/NEJM199303113281003. [DOI] [PubMed] [Google Scholar]
- 31.Goel V, Naylor CD. Potential cost effectiveness of intravenous tissue plasminogen activator versus streptokinase for acute myocardial infarction. Can J Cardiol. 1992;8(1):31–8. [PubMed] [Google Scholar]
- 32.Grines CL, Marsalese DL, Brodie B, et al. Safety and cost-effectiveness of early discharge after primary angioplasty in low risk patients with acute myocardial infarction. PAMI-II Investigators. Primary Angioplasty in Myocardial Infarction. J Am Coll Cardiol. 1998;31(5):967–72. doi: 10.1016/s0735-1097(98)00031-x. [DOI] [PubMed] [Google Scholar]
- 33.Herve C, Castiel D, Gaillard M, Boisvert R, Leroux V. Cost-benefit analysis of thrombolytic therapy. Eur Heart J. 1990;11(11):1006–10. doi: 10.1093/oxfordjournals.eurheartj.a059627. [DOI] [PubMed] [Google Scholar]
- 34.Kalish SC, Gurwitz JH, Krumholz HM, Avorn J. A cost-effectiveness model of thrombolytic therapy for acute myocardial infarction. J Gen Intern Med. 1995;10(6):321–30. doi: 10.1007/BF02599951. [DOI] [PubMed] [Google Scholar]
- 35.Krumholz HM, Pasternak RC, Weinstein MC, et al. Cost effectiveness of thrombolytic therapy with streptokinase in elderly patients with suspected acute myocardial infarction. N Engl J Med. 1992;327(1):7–13. doi: 10.1056/NEJM199207023270102. [DOI] [PubMed] [Google Scholar]
- 36.Krumholz HM, Cohen BJ, Tsevat J, Pasternak RC, Weinstein MC. Cost-effectiveness of a smoking cessation program after myocardial infarction. J Am Coll Cardiol. 1993;22(6):1697–702. doi: 10.1016/0735-1097(93)90598-u. [DOI] [PubMed] [Google Scholar]
- 37.Kuntz KM, Tsevat J, Goldman L, Weinstein MC. Cost-effectiveness of routine coronary angiography after acute myocardial infarction. Circulation. 1996;94(5):957–65. doi: 10.1161/01.cir.94.5.957. [DOI] [PubMed] [Google Scholar]
- 38.Levin LA, Jonsson B. Cost-effectiveness of thrombolysis—a randomized study of intravenous rt-PA in suspected myocardial infarction. Eur Heart J. 1992;13(1):2–8. doi: 10.1093/oxfordjournals.eurheartj.a060041. [DOI] [PubMed] [Google Scholar]
- 39.Lieu TA, Gurley RJ, Lundstrom RJ, et al. Projected cost-effectiveness of primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1997;30(7):1741–50. doi: 10.1016/s0735-1097(97)00391-4. [DOI] [PubMed] [Google Scholar]
- 40.Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96(4):1089–96. doi: 10.1161/01.cir.96.4.1089. [DOI] [PubMed] [Google Scholar]
- 41.Lorenzoni R, Pagano D, Mazzotta G, et al. Pitfalls in the economic evaluation of thrombolysis in myocardial infarction. The impact of national differences in the cost of thrombolytics and of differences in the efficacy across patient subgroups. Eur Heart J. 1998;19(10):1518–24. doi: 10.1053/euhj.1998.1092. [DOI] [PubMed] [Google Scholar]
- 42.Machecourt J, Dumoulin J, Calop J, et al. Cost effectiveness of thrombolytic treatment for myocardial infarction: comparison of anistreplase, alteplase and streptokinase in 270 patients treated within 4 hours. Eur Heart J. 1993;14(1):75–83. doi: 10.1093/eurheartj/14.1.75. [DOI] [PubMed] [Google Scholar]
- 43.Mark DB, Hlatky MA, Califf RM, et al. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med. 1995;332(21):1418–24. doi: 10.1056/NEJM199505253322106. [see comments]. [published erratum appears in N Engl J Med 1995 Jul 27;333(4) 267] [DOI] [PubMed] [Google Scholar]
- 44.Midgette AS, Wong JB, Beshansky JR, Porath A, Fleming C, Pauker SG. Cost-effectiveness of streptokinase for acute myocardial infarction: a combined meta-analysis and decision analysis of the effects of infarct location and of likelihood of infarction. Med Decis Making. 1994;14(2):108–17. doi: 10.1177/0272989X9401400203. [DOI] [PubMed] [Google Scholar]
- 45.Oldridge N, Furlong W, Feeny D, et al. Economic evaluation of cardiac rehabilitation soon after acute myocardial infarction. Am J Cardiol. 1993;72(2):154–61. doi: 10.1016/0002-9149(93)90152-3. [DOI] [PubMed] [Google Scholar]
- 46.Pedretti RF, Migliori GB, Mapelli V, Daniele G, Podrid PJ, Tramarin R. Cost-effectiveness analysis of invasive and noninvasive tests in high risk patients treated with amiodarone after acute myocardial infarction. J Am Coll Cardiol. 1998;31(7):1481–9. doi: 10.1016/s0735-1097(98)00171-5. [DOI] [PubMed] [Google Scholar]
- 47.Radensky PW, Hilton TC, Fulmer H, McLaughlin BA, Stowers SA. Potential cost effectiveness of initial myocardial perfusion imaging for assessment of emergency department patients with chest pain. Am J Cardiol. 1997;79(5):595–9. doi: 10.1016/s0002-9149(96)00822-3. [DOI] [PubMed] [Google Scholar]
- 48.Reeder GS, Bailey KR, Gersh BJ, Holmes DR, Jr, Christianson J, Gibbons RJ. Cost comparison of immediate angioplasty versus thrombolysis followed by conservative therapy for acute myocardial infarction: a randomized prospective trial. Mayo Coronary Care Unit and Catheterization Laboratory Groups. Mayo Clin Proc. 1994;69(1):5–12. doi: 10.1016/s0025-6196(12)61604-8. [DOI] [PubMed] [Google Scholar]
- 49.Ryan TJ, Anderson JL, Antman EM, et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) J Am Coll Cardiol. 1996;28(5):1328–428. doi: 10.1016/s0735-1097(96)00392-0. [DOI] [PubMed] [Google Scholar]
- 50.Ryan TJ, Antman EM, Brooks NH, et al. 1999 Update: ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) J Am Coll Cardiol. 1999;34(3):890–911. doi: 10.1016/s0735-1097(99)00351-4. [DOI] [PubMed] [Google Scholar]
- 51.Simoons ML, Vos J, Martens LL. Cost-utility analysis of thrombolytic therapy. Eur J Heart. 1991;12(6):694–9. doi: 10.1093/eurheartj/12.6.694. [DOI] [PubMed] [Google Scholar]
- 52.Talat A, Hay J, Pitt B, et al. Cost-effectiveness of pravastatin in secondary prevention of coronary artery disease. Am J Cardiol. 1996;78(4):409–14. doi: 10.1016/s0002-9149(96)00328-1. [DOI] [PubMed] [Google Scholar]
- 53.Tsevat J, Duke D, Goldman L, et al. Cost-effectiveness of captopril therapy after myocardial infarction. J Am Coll Cardiol. 1995;26(4):914–9. doi: 10.1016/0735-1097(95)00284-1. [DOI] [PubMed] [Google Scholar]
- 54.Vale L, Silcock J, Rawles J. An economic evaluation of thrombolysis in a remote rural community. BMJ. 1997;314(7080):570–2. doi: 10.1136/bmj.314.7080.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Bergen PF, Jonker JJ, van Hout BA, et al. Costs and effects of long-term oral anticoagulant treatment after myocardial infarction. JAMA. 1995;273(12):925–8. doi: 10.1001/jama.273.12.925. [DOI] [PubMed] [Google Scholar]
- 56.Zijlstra F, de Boer MJ, Beukema WP, et al. Mortality, reinfarction, left ventricular ejection fraction and costs following reperfusion therapies for acute myocardial infarction. Eur Heart J. 1996;17:382–7. doi: 10.1093/oxfordjournals.eurheartj.a014869. [DOI] [PubMed] [Google Scholar]
- 57.Scottish Intercollegiate Guidelines Network. Hospital In-Patient Management of Acute Asthma Attacks. A National Clinical Guideline Recommended for Use in Scotland. Edinburgh, Scotland: SIGN; 1996. p. 28. Pilot edition. [Google Scholar]
- 58.National Heart, Lung and Blood Institute. New NHLBI guidelines for the diagnosis and management of asthma. Lippincott Health Promot Lett. 1997;2(7):1, 8–9. [PubMed] [Google Scholar]
- 59.The Institute for Clinical Systems Integration. Diagnosis and management of asthma. Postgrad Med. 1999;105(4):191–202. 207. [PubMed] [Google Scholar]
- 60.Scottish Intercollegiate Guidelines Network. Primary Care Management of Asthma. A National Clinical Guideline. Edinburgh, Scotland: SIGN; 1998. p. 39. [Google Scholar]
- 61.Grampian Asthma Study of Integrated Care (GRASSIC) Integrated care for asthma: a clinical, social, and economic evaluation. BMJ. 1994;308(6928):559–64. [PMC free article] [PubMed] [Google Scholar]
- 62.Balkrishnan R, Norwood GJ, Anderson A. Outcomes and cost benefits associated with the introduction of inhaled corticosteroid therapy in a medicaid population of asthmatic patients. Clin Ther. 1998;20(3):567–80. doi: 10.1016/s0149-2918(98)80066-0. [DOI] [PubMed] [Google Scholar]
- 63.Beveridge RC, Grunfeld AF, Hodder RV, Verbeek PR. Guidelines for the emergency management of asthma in adults. CAEP/CTS Asthma Advisory Committee. Canadian Association of Emergency Physicians and the Canadian Thoracic Society. CMAJ. 1996;155(1):25–37. [PMC free article] [PubMed] [Google Scholar]
- 64.Bolton MB, Tilley BC, Kuder J, Reeves T, Schultz LR. The cost and effectiveness of an education program for adults who have asthma. J Gen Intern Med. 1991;6(5):401–7. doi: 10.1007/BF02598160. [DOI] [PubMed] [Google Scholar]
- 65.Booth PC, Wells NE, Morrison AK. A comparison of the cost effectiveness of alternative prophylactic therapies in childhood asthma. Pharmacoeconomics. 1996;10(3):262–8. doi: 10.2165/00019053-199610030-00007. [DOI] [PubMed] [Google Scholar]
- 66.Doan T, Grammer LC, Yarnold PR, Greenberger PA, Patterson R. An intervention program to reduce the hospitalization cost of asthmatic patients requiring intubation. Ann Allergy Asthma Immunol. 1996;76(6):513–8. doi: 10.1016/S1081-1206(10)63270-X. [DOI] [PubMed] [Google Scholar]
- 67.Greineder DK, Loane KC, Parks P. Outcomes for control patients referred to a pediatric asthma outreach program: an example of the Hawthorne effect. Am J Manag Care. 1998;4(2):196–202. [PubMed] [Google Scholar]
- 68.Greineder DK, Loane KC, Parks P. A randomized controlled trial of a pediatric asthma outreach program. J Allergy Clin Immunol. 1999;103:436–40. doi: 10.1016/s0091-6749(99)70468-9. [DOI] [PubMed] [Google Scholar]
- 69.Holzer SS, Engelhart L, Crown WH, L'Herrou TA, Kennedy ST. Asthma treatment costs using inhaled corticosteroids. Am J Manag Care. 1997;3(6):891–7. [PubMed] [Google Scholar]
- 70.Jasper AC, Mohsenifar Z, Kahan S, Goldberg HS, Koerner SK. Cost-benefit comparison of aerosol bronchodilator delivery methods in hospitalized patients. Chest. 1987;91(4):614–8. doi: 10.1378/chest.91.4.614. [DOI] [PubMed] [Google Scholar]
- 71.Kauppinen R, Sintonen H, Tukiainen H. One-year economic evaluation of intensive vs conventional patient education and supervision for self-management of new asthmatic patients. Respir Med. 1998;92(2):300–7. doi: 10.1016/s0954-6111(98)90113-5. [DOI] [PubMed] [Google Scholar]
- 72.Kelloway JS, Wyatt R. A cost-effectiveness analysis of breath-actuated metered-dose inhalers. Manag Care Interface. 1997;10(9):99–107. [PubMed] [Google Scholar]
- 73.Kerridge RK, Glasziou PP, Hillman KM. The use of “quality-adjusted life years” (QALYs) to evaluate treatment in intensive care. Anaesth Intensive Care. 1995;23(3):322–31. doi: 10.1177/0310057X9502300309. [DOI] [PubMed] [Google Scholar]
- 74.Lahdensuo A, Haahtela T, Herrala J, et al. Randomised comparison of cost effectiveness of guided self management and traditional treatment of asthma in Finland. BMJ. 1998;316(7138):1138–9. doi: 10.1136/bmj.316.7138.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levenson T, Grammer LC, Yarnold PR, Patterson R. Cost-effective management of malignant potentially fatal asthma. Allergy Asthma Proc. 1997;18(2):73–8. doi: 10.2500/108854197778605455. [DOI] [PubMed] [Google Scholar]
- 76.Liljas B, Stadhl E, Pauwels RA. Cost-effectiveness analysis of a dry powder inhaler (Turbuhaler) versus a pressurised metered dose inhaler in patients with asthma. Pharmacoeconomics. 1997;12:267–77. doi: 10.2165/00019053-199712020-00017. [DOI] [PubMed] [Google Scholar]
- 77.McDermott MF, Murphy DG, Zalenski RJ, et al. A comparison between emergency diagnostic and treatment unit and inpatient care in the management of acute asthma. Arch Intern Med. 1997;157(18):2055–62. [PubMed] [Google Scholar]
- 78.Neri M, Migliori GB, Spanevello A, et al. Economic analysis of two structured treatment and teaching programs on asthma. Allergy. 1996;51(5):313–9. [PubMed] [Google Scholar]
- 79.O'Connor JF, Singer ME, Richter JE. The cost-effectiveness of strategies to assess gastroesophageal reflux as an exacerbating factor in asthma. Am J Gastroenterol. 1999;94(6):1472–80. doi: 10.1111/j.1572-0241.1999.1129_p.x. [DOI] [PubMed] [Google Scholar]
- 80.Perera BJ. Efficacy and cost effectiveness of inhaled steroids in asthma in a developing country. Arch Dis Child. 1995;72(4):312–5. doi: 10.1136/adc.72.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rutten-van Molken MP, van Doorslaer EK, Till MD. Cost-effectiveness analysis of formoterol versus salmeterol in patients with asthma. Pharmacoeconomics. 1998;14(6):671–84. doi: 10.2165/00019053-199814060-00007. [DOI] [PubMed] [Google Scholar]
- 82.Rutten-van Molken MP, van Doorslaer EK, Jansen MC, Kerstjens HA, Rutten FF. Costs and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151(4):975–82. doi: 10.1164/ajrccm.151.4.7697275. [DOI] [PubMed] [Google Scholar]
- 83.Rutten-van Molken MP, van Doorslaer EK, Jansen MC, Van Essen-Zandvliet EE, Rutten FF. Cost effectiveness of inhaled corticosteroid plus bronchodilator therapy versus bronchodilator monotherapy in children with asthma. Pharmacoeconomics. 1993;4(4):257–70. doi: 10.2165/00019053-199304040-00004. [DOI] [PubMed] [Google Scholar]
- 84.Sculpher MJ, Buxton MJ. The episode-free day as a composite measure of effectiveness: an illustrative economic evaluation of formoterol versus salbutamol in asthma therapy: see comments. Pharmacoeconomics. 1993;4(5):345–52. doi: 10.2165/00019053-199304050-00005. [DOI] [PubMed] [Google Scholar]
- 85.Soondergaard B, Davidsen F, Kirkeby B, Rasmussen M, Hey H. The economics of an intensive education programme for asthmatic patients: a prospective controlled trial. Pharmacoeconomics. 1992;1(3):207–12. doi: 10.2165/00019053-199201030-00008. [DOI] [PubMed] [Google Scholar]
- 86.Taitel MS, Kotses H, Bernstein IL, Bernstein DI, Creer TL. A self-management program for adult asthma. Part II: Cost-benefit analysis. J Allergy Clin Immunol. 1995;95(3):672–6. doi: 10.1016/s0091-6749(95)70171-0. [DOI] [PubMed] [Google Scholar]
- 87.Thomas K, Peter JV, Cherian AM, Guyatt G. Cost-effectiveness of inhaled beta-agonists v. oral salbutamol in asthma: a randomized double-blind cross-over study. Natl Med J India. 1996;9(4):159–62. [PubMed] [Google Scholar]
- 88.Trautner C, Richter B, Berger M. Cost-effectiveness of a structured treatment and teaching programme on asthma. Eur Respir J. 1993;6(10):1485–91. [PubMed] [Google Scholar]
- 89.Westley CR, Spiecher B, Starr L, et al. Cost effectiveness of an allergy consultation in the management of asthma. Allergy Asthma Proc. 1997;18(1):15–8. doi: 10.2500/108854197778612835. [DOI] [PubMed] [Google Scholar]
- 90.Williams J, Richards KA. Ease of handling and clinical efficacy of fluticasone propionate Accuhaler/Diskus inhaler compared with the Turbohaler inhaler in paediatric patients. UK Study Group. Br J Clin Pract. 1997;51(3):147–53. [PubMed] [Google Scholar]
- 91.The Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline. JAMA. 1996;275(16):1270–80. [PubMed] [Google Scholar]
- 92.Institute for Clinical Systems Integration. Tobacco-use prevention and cessation. For infants, children, adolescents, and adults. Postgrad Med. 1997;101(3):292–300. doi: 10.3810/pgm.1997.03.190. 302. [DOI] [PubMed] [Google Scholar]
- 93.Trepka MJ, DiGiuseppi G. Guide to Clinical Preventive Services. 2nd ed. Baltimore, Md: Williams and Wilkins; 1996. Counseling to prevent tobacco use. [Google Scholar]
- 94.Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA. 1997;278(21):1759–66. [PubMed] [Google Scholar]
- 95.Cummings SR, Rubin SM, Oster G. The cost-effectiveness of counseling smokers to quit. JAMA. 1989;261(1):75–9. [PubMed] [Google Scholar]
- 96.Elder JP, Campbell NR, Mielchen SD, Hovell MF, Litrownik AJ. Implementation and evaluation of a community-sponsored smoking cessation contest. Am J Health Promot. 1991;5(3):200–7. doi: 10.4278/0890-1171-5.3.200. [DOI] [PubMed] [Google Scholar]
- 97.Ershoff DH, Quinn VP, Mullen PD, Lairson DR. Pregnancy and medical cost outcomes of a self-help prenatal smoking cessation program in a HMO. Public Health Rep. 1990;105(4):340–7. [PMC free article] [PubMed] [Google Scholar]
- 98.Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians' smoking cessation counseling. JAMA. 1996;275(16):1247–51. [PubMed] [Google Scholar]
- 99.Hueston WJ, Mainous AG, III, Farrell JB. A cost-benefit analysis of smoking cessation programs during the first trimester of pregnancy for the prevention of low birthweight. J Fam Pract. 1994;39(4):353–7. [PubMed] [Google Scholar]
- 100.Marks JS, Koplan JP, Hogue CJ, Dalmat ME. A cost-benefit/cost-effectiveness analysis of smoking cessation for pregnant women. Am J Prev Med. 1990;6(5):282–9. [PubMed] [Google Scholar]
- 101.McGhan WF, Smith MD. Pharmacoeconomic analysis of smoking-cessation interventions. Am J Health Syst Pharm. 1996;53(1):45–52. doi: 10.1093/ajhp/53.1.45. [DOI] [PubMed] [Google Scholar]
- 102.Mudde AN, de Vries H, Strecher VJ. Cost-effectiveness of smoking cessation modalities: comparing apples with oranges? Prev Med. 1996;25(6):708–16. doi: 10.1006/pmed.1996.0110. [DOI] [PubMed] [Google Scholar]
- 103.Oster G, Huse DM, Delea TE, Colditz GA. Cost-effectiveness of nicotine gum as an adjunct to physician's advice against cigarette smoking. JAMA. 1986;256(10):1315–8. [PubMed] [Google Scholar]
- 104.Parrott S, Godfrey C, Raw M, West R, McNeill A. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Health Educational Authority. Thorax. 1998;53:1S–38S. [PMC free article] [PubMed] [Google Scholar]
- 105.Prathiba BV, Tjeder S, Phillips C, Campbell IA. A smoking cessation counsellor: should every hospital have one? J R Soc Health. 1998;118(3):356–9. doi: 10.1177/146642409811800614. [DOI] [PubMed] [Google Scholar]
- 106.Raw M, McNeill A, West R. Smoking cessation guidelines for health professionals. A guide to effective smoking cessation interventions for the health care system. Health Education Authority. Thorax. 1998;53:1S–19S. doi: 10.1136/thx.53.2008.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serxner S, Adams VG, Hundahl LS, Lau S, Adessa CJ, Jr, Hopkins D. A smoking cessation pilot program. Hawaii Med J. 1993;52(10):266–72. [PubMed] [Google Scholar]
- 108.Shipp M, Croughan-Minihane MS, Petitti DB, Washington AE. Estimation of the break-even point for smoking cessation programs in pregnancy. Am J Public Health. 1992;82(3):383–90. doi: 10.2105/ajph.82.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sofian NS, McAfee T, Wilson J, Levan S. Telephone smoking cessation intervention: the free and clear program. HMO Pract. 1995;9(3):144–6. [PubMed] [Google Scholar]
- 110.Warner KE, Smith RJ, Smith DG, Fries BE. Health and economic implications of a work-site smoking-cessation program: a simulation analysis. J Occup Environ Med. 1996;38(10):981–92. doi: 10.1097/00043764-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 111.Wasley MA, McNagny SE, Phillips VL, Ahluwalia JS. The cost-effectiveness of the nicotine transdermal patch for smoking cessation. Prev Med. 1997;26(2):264–70. doi: 10.1006/pmed.1996.0127. [DOI] [PubMed] [Google Scholar]
- 112.Wetter DW, Fiore MC, Gritz ER, et al. The Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline. Findings and implications for psychologists. Am Psychol. 1998;53(6):657–69. doi: 10.1037//0003-066x.53.6.657. [DOI] [PubMed] [Google Scholar]
- 113.Windsor RA, Lowe JB, Perkins LL, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Public Health. 1993;83(2):201–6. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.U.S. Congress, Office of Technology Assessment. Cost-Effectiveness of Colorectal Cancer Screening in Average-Risk Adults. OTA-BP-H-146. Washington DC: U. S. Government Printing Office; April, 1995. [Google Scholar]
- 115.American College of Physicians. Suggested technique for fecal occult blood testing and interpretation in colorectal cancer screening. Ann Intern Med. 1997;126(10):808–10. [PubMed] [Google Scholar]
- 116.Institute for Clinical Systems Improvement (ICSI) Health Care Guideline: Colorectal cancer screening. Available at: http://www icsi org/guide/Colon pdf 1999.
- 117.American Gastroenterology Association issues guidelines for colorectal cancer screening. Am Fam Physician. 1997;55(8):2860–2. [PubMed] [Google Scholar]
- 118.Screening for colorectal cancer–United States, 1992–1993, and new guidelines. MMWR Morb Mortal Wkly Rep. 1996;45(5):107–10. [PubMed] [Google Scholar]
- 119.Barry MJ, Mulley AG, Richter JM. Effect of workup strategy on the cost-effectiveness of fecal occult blood screening for colorectal cancer. Gastroenterology. 1987;93(2):301–10. doi: 10.1016/0016-5085(87)91019-5. [DOI] [PubMed] [Google Scholar]
- 120.Brown K, Burrows C. A prospective cost-effectiveness study of alternative work-up strategies in colorectal cancer screening. Aust Health Rev. 1992;15(2):176–89. [PubMed] [Google Scholar]
- 121.Brown ML, Kessler LG. The use of gene tests to detect hereditary predisposition to cancer: economic considerations. J Natl Cancer Inst. 1995;87(15):1131–6. doi: 10.1093/jnci/87.15.1131. [DOI] [PubMed] [Google Scholar]
- 122.Daniels K, McKee M. Options for screening for colorectal cancer in the Royal Air Force: a cost-effectiveness evaluation. J R Army Med Corps. 1995;141(3):142–50. doi: 10.1136/jramc-141-03-04. [published erratum appears in J R Army Med Corps 1996 Feb;142(1) 49] [DOI] [PubMed] [Google Scholar]
- 123.Eddy DM. Screening for colorectal cancer. Ann Intern Med. 1990;113(5):373–84. doi: 10.7326/0003-4819-113-5-373. [DOI] [PubMed] [Google Scholar]
- 124.Eddy DM, Nugent FW, Eddy JF, et al. Screening for colorectal cancer in a high-risk population. Results of a mathematical model. Gastroenterology. 1987;92(3):682–92. doi: 10.1016/0016-5085(87)90018-7. [DOI] [PubMed] [Google Scholar]
- 125.Fric P, Zavoral M, Dvorakova H, Zoubek V, Roth Z. An adapted program of colorectal cancer screening—7 years experience and cost-benefit analysis. Hepatogastroenterology. 1994;41(5):413–6. [PubMed] [Google Scholar]
- 126.Gyrd-Hansen D. Is it cost effective to introduce screening programmes for colorectal cancer? Illustrating the principles of optimal resource allocation. Health Policy. 1997;41(3):189–99. doi: 10.1016/s0168-8510(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 127.Joseph AM, Crowson TW, Rich EC. Cost effectiveness of HemoQuant versus Hemoccult for colorectal cancer screening. J Gen Intern Med. 1988;3(2):132–8. doi: 10.1007/BF02596117. [DOI] [PubMed] [Google Scholar]
- 128.Lieberman DA. Cost-effectiveness model for colon cancer screening. Gastroenterology. 1995;109(6):1781–90. doi: 10.1016/0016-5085(95)90744-0. [DOI] [PubMed] [Google Scholar]
- 129.Manus B, Bragelmann R, Armbrecht U, Stolte M, Stockbrugger RW. Screening for gastrointestinal neoplasia: efficacy and cost of two different approaches in a clinical rehabilitation centre. Eur J Cancer Prev. 1996;5(1):49–55. [PubMed] [Google Scholar]
- 130.Markowitz AJ, Winawer SJ. Screening and surveillance for colorectal carcinoma. Hematol Oncol Clin North Am. 1997;11(4):579–608. doi: 10.1016/s0889-8588(05)70452-4. [DOI] [PubMed] [Google Scholar]
- 131.Morey SS. ACS updates guidelines on screening for colorectal cancer. Am Fam Physician. 1997;56(7):1887–8. [PubMed] [Google Scholar]
- 132.Neilson AR, Whynes DK. Cost-effectiveness of screening for colorectal cancer: a simulation model. IMA J Math Appl Med Biol. 1995;12:355–67. doi: 10.1093/imammb/12.3-4.355. [DOI] [PubMed] [Google Scholar]
- 133.Petrelli NJ, Palmer M, Michalek A, et al. Massive screening for colorectal cancer. A single institution's public commitment. Arch Surg. 1990;125(8):1049–51. doi: 10.1001/archsurg.1990.01410200113018. [DOI] [PubMed] [Google Scholar]
- 134.Salkeld G, Young G, Irwig L, Haas M, Glasziou P. Cost-effectiveness analysis of screening by faecal occult blood testing for colorectal cancer in Australia. Aust N Z J Public Health. 1996;20(2):138–43. doi: 10.1111/j.1753-6405.1996.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 135.Shimbo T, Glick HA, Eisenberg JM. Cost-effectiveness analysis of strategies for colorectal cancer screening in Japan. Int J Technol Assess Health Care. 1994;10(3):359–75. doi: 10.1017/s0266462300006607. [DOI] [PubMed] [Google Scholar]
- 136.Tsuji I, Fukao A, Shoji T, Kuwajima I, Sugawara N, Hisamichi S. Cost-effectiveness analysis of screening for colorectal cancer in Japan. Tohoku J Exp Med. 1991;164(4):269–78. doi: 10.1620/tjem.164.269. [DOI] [PubMed] [Google Scholar]
- 137.Wagner JL. Cost-effectiveness of colorectal cancer screening in the elderly. Ann Intern Med. 1991;115(10):807–17. doi: 10.7326/0003-4819-115-10-807. [DOI] [PubMed] [Google Scholar]
- 138.Walker A, Whynes DK. Filtering strategies in mass population screening for colorectal cancer: an economic evaluation. Med Decis Making. 1992;12(1):2–7. doi: 10.1177/0272989X9201200102. [DOI] [PubMed] [Google Scholar]
- 139.Weller D, Moss J, Hiller J, Thomas D, Edwards J. Screening for colorectal cancer: what are the costs? Int J Technol Assess Health Care. 1995;11(1):26–39. doi: 10.1017/s0266462300005237. [DOI] [PubMed] [Google Scholar]
- 140.Whynes DK, Neilson AR, Walker AR, Hardcastle JD. Faecal occult blood screening for colorectal cancer: is it cost-effective? Health Econ. 1998;7(1):21–9. doi: 10.1002/(sici)1099-1050(199802)7:1<21::aid-hec306>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 141.The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer, Canadian Association of Radiation Oncologists. The palpable breast lump: information and recommendations to assist decision-making when a breast lump is detected. CMAJ. 1998;158:3S–8S. [PubMed] [Google Scholar]
- 142.Wright JR, Whelan TJ, McCready DR, O'Malley FP. Management of ductal carcinoma in situ of the breast. Provincial Breast Cancer Disease Site Group. Cancer Prev Control. 1998;2(6):312–9. [PubMed] [Google Scholar]
- 143.The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer, Canadian Association of Radiation Oncologists. The management of ductal carcinoma in situ (DCIS) CMAJ. 1998;158:27S–34S. [PubMed] [Google Scholar]
- 144.The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Adjuvant systemic therapy for women with node-positive breast cancer. CMAJ. 1998;158:52S–64S. [PubMed] [Google Scholar]
- 145.The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer, Canadian Association of Radiation Oncologists. Axillary dissection. CMAJ. 1998;158:22S–6S. [PubMed] [Google Scholar]
- 146.Blichert-Toft M, Smola MG, Cataliotti L, O'Higgins N. Principles and guidelines for surgeons—management of symptomatic breast cancer. On behalf of the European Society of Surgical Oncology. Ann Chir Gynaecol. 1998;87(1):101–9. [PubMed] [Google Scholar]
- 147.Cady B, Steele GDJ, Morrow M, et al. Evaluation of common breast problems: guidance for primary care providers. CA Cancer J Clin. 1998;48(1):49–63. doi: 10.3322/canjclin.48.1.49. [DOI] [PubMed] [Google Scholar]
- 148.Flett MM, Going JJ, Stanton PD, Cooke TG. Sentinel node localization in patients with breast cancer. Br J Surg. 1998;85(7):991–3. doi: 10.1046/j.1365-2168.1998.00746.x. [DOI] [PubMed] [Google Scholar]
- 149.Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Natl Cancer Inst. 1998;90(21):1601–8. doi: 10.1093/jnci/90.21.1601. [DOI] [PubMed] [Google Scholar]
- 150.Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16(3):979–85. doi: 10.1200/JCO.1998.16.3.979. [DOI] [PubMed] [Google Scholar]
- 151.Hayman JA, Hillner BE, Harris JR, Weeks JC. Cost-effectiveness of routine radiation therapy following conservative surgery for early-stage breast cancer. J Clin Oncol. 1998;16(3):1022–9. doi: 10.1200/JCO.1998.16.3.1022. [DOI] [PubMed] [Google Scholar]
- 152.Layfield LJ, Chrischilles EA, Cohen MB, Bottles K. The palpable breast nodule. A cost-effectiveness analysis of alternate diagnostic approaches. Cancer. 1993;72(5):1642–51. doi: 10.1002/1097-0142(19930901)72:5<1642::aid-cncr2820720525>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 153.Lazovich D, Solomon CC, Thomas DB, Moe RE, White E. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86(4):628–37. [PubMed] [Google Scholar]
- 154.Lee CH, Egglin TK, Philpotts L, Mainiero MB, Tocino I. Cost-effectiveness of stereotactic core needle biopsy: analysis by means of mammographic findings. Radiology. 1997;202(3):849–54. doi: 10.1148/radiology.202.3.9051045. [DOI] [PubMed] [Google Scholar]
- 155.Liberman L, Fahs MC, Dershaw DD, et al. Impact of stereotaxic core breast biopsy on cost of diagnosis. Radiology. 1995;195(3):633–7. doi: 10.1148/radiology.195.3.7753986. [DOI] [PubMed] [Google Scholar]
- 156.Liberman L, Feng TL, Dershaw DD, Morris EA, Abramson AF. US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208(3):717–23. doi: 10.1148/radiology.208.3.9722851. [DOI] [PubMed] [Google Scholar]
- 157.Liljegren G, Karlsson G, Bergh J, Holmberg L. The cost-effectiveness of routine postoperative radiotherapy after sector resection and axillary dissection for breast cancer stage I. Results from a randomized trial. Ann Oncol. 1997;8(8):757–63. doi: 10.1023/a:1008230000822. [DOI] [PubMed] [Google Scholar]
- 158.Lindfors KK, Rosenquist CJ. Needle core biopsy guided with mammography: a study of cost-effectiveness. Radiology. 1994;190(1):217–22. doi: 10.1148/radiology.190.1.8259408. [DOI] [PubMed] [Google Scholar]
- 159.Lockett MA, Metcalf JS, Baron PL, et al. Efficacy of reverse transcriptase-polymerase chain reaction screening for micrometastic disease in axillary lymph nodes of breast cancer patients. Am Surg. 1998;64(6):539–43. [PubMed] [Google Scholar]
- 160.Logan-Young W, Dawson AE, Wilbur DC, et al. The cost-effectiveness of fine-needle aspiration cytology and 14-gauge core needle biopsy compared with open surgical biopsy in the diagnosis of breast carcinoma. Cancer. 1998;82(10):1867–73. doi: 10.1002/(sici)1097-0142(19980515)82:10<1867::aid-cncr8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 161.Mirsky D, O'Brien SE, McCready DR, Newman TE, Whelan TJ, Levine MN. Surgical management of early stage invasive breast cancer (stage I and II). Provincial Breast Disease Site Group. Cancer Prev Control. 1997;1(1):10–7. [published erratum appears in Cancer Prev Control 1997 Jun;1(2) 132] [PubMed] [Google Scholar]
- 162.Morrow M, Bland KI, Foster R. Breast cancer surgical practice guidelines. Society of Surgical Oncology practice guidelines. Oncology (Huntingt) 1997;11(6):877–81. 885–6. [PubMed] [Google Scholar]
- 163.Norum J, Olsen JA, Wist EA. Lumpectomy or mastectomy? Is breast conserving surgery too expensive? Breast Cancer Res Treat. 1997;45(1):7–14. doi: 10.1023/a:1005804101106. [DOI] [PubMed] [Google Scholar]
- 164.O'Higgins N, Linos DA, Blichert-Toft M, et al. European guidelines for quality assurance in the surgical management of mammographically detected lesions. Eur J Surg Oncol. 1998;24(2):96–8. doi: 10.1016/s0748-7983(98)91329-4. [DOI] [PubMed] [Google Scholar]
- 165.Vetto J, Schmidt W, Pommier R, et al. Accurate and cost-effective evaluation of breast masses in males. Am J Surg. 1998;175(5):383–7. doi: 10.1016/S0002-9610(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 166.Warmerdam PG, de Koning HJ, Boer R, et al. Quantitative estimates of the impact of sensitivity and specificity in mammographic screening in Germany. J Epidemiol Community Health. 1997;51(2):180–6. doi: 10.1136/jech.51.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gerard K, Seymour J, Smoker I. A tool to improve quality of reporting published economic analyses. Int J Technol Assess Health Care. 2000;16(1):100–10. doi: 10.1017/s0266462300016196. [DOI] [PubMed] [Google Scholar]