Abstract
OBJECTIVE
Primary spontaneous pneumothorax (PSP) is not uncommon in young men and is associated with frequent recurrence. The frequent recurrence after conservative treatment and resultant anxiety for recurrence are sources of disability. We explored which procedure is more appropriate as the initial therapy in terms of quality-adjusted life expectancy (QALE).
DESIGN
Decision analysis using a Markov model.
DATA SOURCES
Structured literature review for clinical probability. Utility derived from patients and medical staff using time trade-off method.
SETTING
Hypothetical cohort.
PATIENTS
Twenty-year-old men with a first episode of PSP for which simple aspiration was ineffective.
INTERVENTIONS
One of the following treatment options: 1) thoracoscopic surgery, 2) pleural drainage followed by thoracoscopic surgery for recurrence, 3) pleural drainage followed by thoracoscopic surgery for the second recurrence, 4) pleurodesis followed by thoracoscopic surgery for recurrence, 5) pleurodesis followed by thoracoscopic surgery for the second recurrence, 6) pleural drainage followed by pleurodesis for the first recurrence and thoracoscopic surgery for the second recurrence.
MEASUREMENTS AND MAIN RESULTS
During the 1-year period after one of the initial treatments, the QALE was 9.49 months for thoracoscopic surgery, 9.47 for pleurodesis, and 7.80–7.99 for pleural drainage. The QALE for thoracoscopic surgery was the longest among the 6 strategies during the period from 5 to 24 months. None of the variables in sensitivity analyses altered the main results except for thoracoscopic surgical death rate. When it exceeds 0.3%, pleurodesis becomes the preferred strategy.
CONCLUSION
On the basis of the current best available data and patients' preference, thoracoscopic surgery can be considered the treatment of choice for the first episode of PSP.
Keywords: decision theory, pleurodesis, pneumothorax, quality-adjusted life years, video-assisted thoracoscopic surgery
Primary spontaneous pneumothorax (PSP) usually occurs in young, otherwise healthy men in their 20s.1–4 The incidence is reported to be 74 cases per million per year among men.5 Treatment options include conservative treatments such as observation, simple aspiration, pleural drainage, and pleurodesis. Some guidelines recommend these conservative treatments for the first episode of PSP.6,7 However, recent studies have shown poor compliance with these guidelines on the part of clinicians.4,8 One of the reasons for marked variation in clinical management is that neither simple aspiration nor pleural drainage has resulted in a satisfactory cure rate: recurrence occurs in 34% to 65% of patients in 1.5 years.9,10 Such a high recurrence rate and anxiety associated with the anticipation of recurrence are sources of disability for young patients.11 An optimal strategy therefore should provide patients with satisfaction, in addition to safety and low recurrence rate. Most of the current guidelines, however, do not deal with patient satisfaction as an important outcome.6,7
Video-assisted thoracoscopic surgery has been clinically employed since the early 1990s. There are now many pulmonologists who regard thoracoscopic surgery as the treatment of choice for PSP because it allows the safe and successful resection of apical blebs, pleurodesis, or pleural ablation.12,13 It has been shown to be superior to open thoracotomy in terms of decreased postoperative pain and morbidity and to have a recurrence rate comparable to that of open thoracotomy.14–17 Since no prospective randomized trials have been done to compare thoracoscopic surgery, pleural drainage, and pleurodesis, we conducted a decision analysis to determine which procedure is most efficacious as an initial therapy in terms of quality-adjusted life expectancy (QALE).
METHODS
Decision Model (Appendix A)
The hypothetical cohort consists of patients who had complete unilateral lung collapse or severe dyspnea due to PSP without underlying diseases and failed to be treated by simple aspiration. Simple aspiration was set at starting point because current guidelines6,7 recommend it as the initial treatment for these patients, and successful aspiration presents no clinical difficulty. The fraction of patients in whom simple aspiration failed to re-expand the affected lung was reported to be 55% to 75%.18–20
A Markov model is an analytic structure that tracks the clinical events occurring in a hypothetical cohort of patients in various scenarios over time.21,22 Baumann and Strange recently reported that most pulmonologists considered pleural drainage as the treatment of choice after observation or simple drainage for young patients with the first episode of PSP. However, their choices were diversified into pleural drainage, pleurodesis, and thoracoscopic surgery for the second episode.4 It was also shown that open thoracotomy was not chosen for the first episode. We constructed a model based on these findings to compare 6 therapeutic options for 20-year-old men with a first episode of PSP for which simple aspiration was ineffective. The options consisted of, 1) thoracoscopic surgery for the first episode (thoracoscopic surgery strategy), 2) pleural drainage for the first episode followed by thoracoscopic surgery for the second episode (pleural drainage once strategy), 3) pleural drainage for the first and second episodes followed by thoracoscopic surgery for the third episode (pleural drainage twice strategy), 4) pleurodesis for the first episode followed by thoracoscopic surgery for the second episode (pleurodesis once strategy), 5) pleurodesis for the first and second episodes followed by thoracoscopic surgery for the third episode (pleurodesis twice strategy), and 6) pleural drainage for the first episode and pleurodesis for the second followed by thoracoscopic surgery for the third episode (pleural drainage followed by pleurodesis strategy).
Since most recurrence occurred in the first year,23 all patients in the cohort were followed up for 1 year and each patient was in 1 of the 2 clinical states at any given point in time: either hospitalized and treated with pleural drainage (with or without pleurodesis), thoracoscopic surgery, or open thoracotomy, or discharged after having undergone one of the treatments with some recurrence risk. The length of one cycle was set at 1 month and the patients' clinical status could change with specific probabilities during 1 cycle.
We used this model to calculate the QALE for each of the strategies, taking into account discomfort caused by the disease itself, anxiety about the recurrence, and the magnitude of the risk of recurrence. All analyses were performed with the aid of DATA (version 3.5, TreeAge Software, Inc., Williamstown, Mass), a decision analysis computer program.
Assumptions of the Model
We adopted the following 8 assumptions for this model (Table 1): 1) there will be no second thoracoscopic surgery, which means that open thoracotomy is performed when PSP recurs after thoracoscopic surgery; 2) because of the fairly good performance results for open thoracotomy as reported in the current literature,24,25 no recurrence occurs after the second open thoracotomy, i.e., open thoracotomy is performed twice at most; 3) patients for whom pleural drainage or pleurodesis were ineffective to re-expand pneumothorax undergo thoracoscopic surgery in the next cycle; 4) if thoracoscopic surgery fails to re-expand pneumothorax, open thoracotomy is performed in the next cycle; 5) the agent of pleurodesis (talc or tetracycline) makes no difference in efficacy as reported in the literature26,27; 6) similarly, the surgical procedure (resection of apical blebs, pleurodesis, or pleural ablation) does not cause a difference in efficacy23,28,29; 7) chest computed tomography (CT) and other work-up for surgery are performed when thoracoscopic surgery or open thoracotomy is to be performed. The decision whether to perform conservative treatment (pleural drainage and pleurodesis) is not made on the basis of chest CT results; and 8) patients under consideration in this study are otherwise healthy and lead an active daily life that is adversely affected by hospitalization.
Table 1.
Assumptions for Decision Model
| 1. No second thoracoscopic surgery |
| 2. No recurrence after the second open thoracotomy |
| 3. If pleural drainage or pleurodesis fails, thoracoscopic surgery in the next cycle |
| 4. If thoracoscopic surgery fails, open thoracotomy in the next cycle |
| 5. Equal efficacies of all pleurodesis agents |
| 6. Equal efficacies of all surgical procedures |
| 7. Chest CT and other work-up just before decided surgery |
| 8. Otherwise healthy patients with active daily life |
CT indicates computed tomography.
Probabilities for Clinical Outcomes
A systematic literature search for studies on clinical outcomes of PSP published between January 1966 and April 2001 was performed with the aid of medline. The inclusion criteria for studies were: 1) the study population is clearly identified as patients with PSP; 2) the sample size is larger than 30; 3) the details of the involved lung and the treatment modality are clearly described; 4) the precise number of events (failure to re-expand, recurrence, affected lung, drop out) and length of observation are mentioned; and 5) the studies are published in English. If the relevant literature that met the criteria was not available, we consulted other articles (e.g., review articles, studies dealing with secondary pneumothorax). Relevant publications provided us with weighted averages for treatment-related mortality, success rates, and recurrence rates in the ipsilateral lung. General population mortality rates for Japanese people based on the data for 1997 published by the Ministry of Health and Welfare of Japan, adjusted for age and gender, were used as background mortality rates for the model. Since the length of the cycle in our study is shorter than that of observed periods in the literature, the transition probabilities from one state to another within a specified time frame were calculated by using the DEALE (declining exponential approximation of life expectancy) method.30,31 The probability ranges for the sensitivity analyses were determined on the basis of the variances reported in the literature or on expert opinions, when such variances were not available in the literature. The experts were 1 pulmonologist and 1 thoracic surgeon at a teaching hospital, and they reported no cases of death among young men with PSP undergoing invasive treatment in the hospital. Therefore, we set 1.0% as maximum surgical death rate and 0.1% as maximum pleural drainage or pleurodesis death rate to assess the robustness of our analysis. Also, we obtained the range of 10% to 50% per year for recurrence after second pleural drainage from these experts. Probabilities for clinical outcomes and their ranges are summarized in Table 2, and the characteristics of the studies used in this analysis are shown in Appendix B.32–52
Table 2.
Probability Estimates for Decision Model
| Probability | Base Case (Range) | Reference(s) |
|---|---|---|
| Failure to re-expand with thoracoscopic surgery, % | 3.13 (0.24 to 16.7) | 17, 24, 32–39 |
| Surgical death with thoracoscopic surgery, % | 0 (0 to 1.0) | 17, 26, 34, 35, 37–44 |
| Recurrence after thoracoscopic surgery, %/y | 2.31 (0.84 to 10.21) | 16, 17, 24, 34, 35, 37–46 |
| Mortality after thoracoscopic surgery, %/y | 0 (NA) | 16, 17, 24, 34, 35, 37–46 |
| Failure to re-expand with open thoracotomy, % | 0 (NA) | 24, 34, 37, 47, 48 |
| Surgical death with open thoracotomy, % | 0 (0 to 1.0) | 24, 34, 37, 47, 48 |
| Recurrence after the first open thoracotomy, %/y | 0.65 (0.06 to 2.40) | 14, 24, 34, 37, 47 |
| Recurrence after the second open thoracotomy, %/y | 0 (NA) | 14, 24, 34, 37, 47 |
| Mortality after open thoracotomy, %/y | 0 (NA) | 14, 24, 34, 37, 47 |
| Failure to re-expand with pleural drainage, % | 27.4 (18.0 to 34.4) | 3, 49 |
| Surgical death with pleural drainage, % | 0 (0 to 0.1) | 3, 49–51 |
| Recurrence after the first pleural drainage, %/y | 21.7 (11.5 to 29.1) | 3, 9, 10, 29 |
| Mortality after pleural drainage first, %/y | 0 (NA) | 3, 9, 10, 29 |
| Recurrence after the second pleural drainage, %/y | 36.3 (10.0 to 50.0) | 10 |
| Mortality after the second pleural drainage, %/y | 0 (NA) | 9, 10 |
| Failure to re-expand with pleurodesis, % | 9.0 (0 to 12.0) | 52 |
| Surgical death with pleurodesis, % | 0 (0 to 0.1) | 52 |
| Recurrence after pleurodesis, %/y | 5.49 (2.29 to 7.73) | 23, 28, 29 |
| Mortality after pleurodesis, %/y | 0 (NA) | 23, 28, 29 |
NA, not applicable from model assumptions.
Adjustments for Quality of Life
We adjusted life expectancy for quality of life by using health state utilities, which represent an individual patient's preference for a given health state and are graded from 0 to 1. Zero means death and 1 represents perfect health without any physical or mental discomfort.53 QALEs are calculated by multiplying the time spent in a given health state by the utility value for that health state. We used the time trade-off method54,55 to obtain the utilities for this model, based on a hypothetical scenario, from 8 young male patients with PSP and 7 male medical staff members in a teaching hospital. The 8 interviewed patients suffered the first episode of primary spontaneous pneumothorax, which was confirmed by chest x-ray. All of them had been admitted to the teaching hospital because they had unilateral pneumothorax completely collapsed and dyspnea. Simple aspiration failed to re-expand the affected lung and they underwent pleural drainage. All of them received the information about thoracic surgery when the pneumothorax recurred. A trained research nurse interviewed each of them using the hypothetical scenario. All the medical staff were internists who had experience in treating patients with pneumothorax using pleural drainage. They were interviewed in the same way as the patients, by the same research nurse. The scenario consisted of thoracoscopic surgery, open thoracotomy, pleural drainage, and follow-up state after these treatment options. Detailed information was also provided in terms of pain or confinement for intervention and risk of recurrence during the follow-up period based on the literature review. Pleurodesis and pleural drainage did not differ in hospital stay, duration of drainage, and symptoms.28 Therefore, we substituted the utility value of pleural drainage for that of pleurodesis. In addition, our review of the literature indicated that the recurrence rates after pleurodesis and thoracoscopic surgery were equivalent, and we assumed that patients' preference during the follow-up period would depend primarily on the risk of recurrence. Therefore, we substituted the utility value of the follow-up state after thoracoscopic surgery for that after pleurodesis.
The mean values thus obtained were used for a base case analysis. The range for sensitivity analyses was set at a 95% confidence interval of obtained values. The utility of a given cycle, with the length of hospitalization for any intervention set at 7 days for the base case analysis, was calculated as follows:
Base Case Analysis and Sensitivity Analyses
For each of the treatment strategies, we calculated the QALE by multiplying the probability of each health status by the corresponding utility for 12 cycles with discounting at the rate of 0.05 per year (range, 0 to 0.10).56 The fact that health preferences expressed or costs incurred at an earlier stage are more keenly felt than those that are expressed or incurred later is accounted for by discounting as they accrue over time.56 Although we set 1 year for the base case analysis, we varied the number of cycles from 4 (4 months) to 24 (2 years) to gain a better understanding of the impact of recurrence for an extended period of time on overall QALEs. Because of the wide variation of probabilities for clinical outcomes published in reports as well as of utilities for individual patients, extensive sensitivity analyses were performed with the simulation model. First, all variables of interest were subjected to 1-way sensitivity analyses with the range shown in Table 2. Second, 2-way and 3-way sensitivity analyses were also performed. Third, the length of hospitalization, ranging from 4 to 10 days, was subjected to sensitivity analyses according to the expert opinions. Finally, we checked the effect of discounting rate ranging from 0 to 0.1.
RESULTS
Utility Values
The utility values obtained are shown in Table 3. The mean age of the male subjects was 29.1 years with a range of 16 to 42. The mean utilities of intervention were 0.45 for pleural drainage, 0.37 for thoracoscopic surgery, and 0.32 for open thoracotomy. The utility values for health status were similar for patients and staff, which meant that the patients understood the treatment modalities and sequelae correctly.
Table 3.
Quality of Life Estimates (Utilities)
| Patients (n = 8) | Medical Staff (n = 7) | Combined (N = 15) | |
|---|---|---|---|
| Variable | Mean (Range) | Mean (Range) | Mean (95% Confidence Interval) |
| Age, y | 25 (16 to 35) | 34 (26 to 42) | 29 (25 to 34) |
| Thoracoscopic surgery | 0.38 (0.07 to 0.71) | 0.36 (0.07 to 0.71) | 0.37 (0.25 to 0.49) |
| Follow-up after thoracoscopic surgery/follow-up state after pleurodesis | 0.84 (0.23 to 1.0) | 0.79 (0.67 to 0.97) | 0.82 (0.70 to 0.93) |
| Open thoracotomy | 0.35 (0.07 to 0.71) | 0.29 (0.07 to 0.71) | 0.32 (0.19 to 0.45) |
| Follow-up state after the first open thoracotomy | 0.97 (0.83 to 1.0) | 0.86 (0.67 to 1.0) | 0.92 (0.86 to 0.97) |
| Follow-up after second open thoracotomy | 0.99 (0.97 to 1.0) | 0.91 (0.83 to 1.0) | 0.96 (0.92 to 0.99) |
| Pleural drainage/pleurodesis | 0.38 (0.07 to 0.71) | 0.53 (0.36 to 1.0) | 0.45 (0.33 to 0.58) |
| Follow-up state after the first pleural drainage | 0.63 (0.17 to 1.0) | 0.64 (0.5 to 0.83) | 0.63 (0.51 to 0.76) |
| Follow-up state after the second pleural drainage | 0.46 (0.17 to 0.67) | 0.55 (0.33 to 0.83) | 0.50 (0.40 to 0.60) |
Base Case Analysis
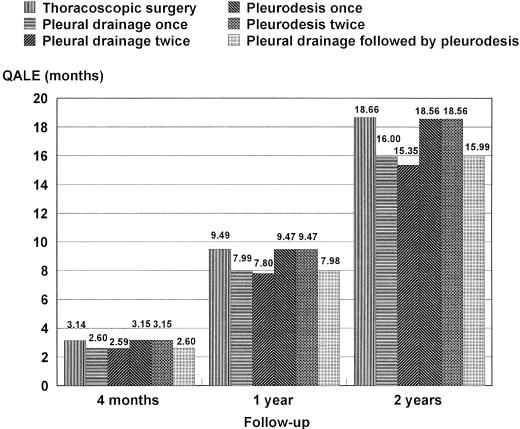
During the 1-year period, the longest QALE for 20-year-old male patients with PSP was 9.49 months for thoracoscopic surgery strategy and the shortest, 7.80 months for pleural drainage twice strategy (Fig. 1). Thoracoscopic surgery strategy was superior to drainage once strategy by 1.5 months and to drainage twice strategy by 1.69 months but similar to pleurodesis once or twice strategy. We then changed the time horizon in the simulation model from 4 months (4 cycles) to 2 years (24 cycles). For any follow-up periods except for 4 months, the result was consistent with the longest QALE for thoracoscopic surgery strategy among the 6 strategies. For drainage once strategy, only 70% of the patients were managed without undergoing thoracoscopic surgery or open thoracotomy during the follow-up period. For drainage twice strategy, 74% of the patients were managed without one of these surgical interventions (Table 4). As the follow-up period becomes longer, the percentage of patients who have undergone thoracoscopic surgery or open thoracotomy increases approaching 50% at 2 years for drainage once strategy and drainage twice strategy (Table 4). Overall mortality rates for 2 years were the same at 0% for all 6 strategies, with an actual life expectancy (i.e., not quality-adjusted) of 3.97 months for 4-month observation, 11.71 months for 1-year observation, and 22.86 months for 2-year observation for any strategy.
FIGURE 1.
QALE after follow-up. Thoracoscopic surgery strategy offers longest QALE at 1-year (base case) and 2-year follow-up. The QALE of pleurodesis once and twice strategy is longest at 4 months follow-up, but thoracoscopic surgery strategy becomes longest after 4.7 months by sensitivity analysis.
Table 4.
Health Status After Follow-up
| Follow-up, % (Months) | ||||
|---|---|---|---|---|
| Therapeutic Strategy | Health Status | 4 | 12 | 24 |
| Thoracoscopic surgery strategy | Follow-up state after thoracoscopic surgery | 96 | 95 | 92 |
| Follow-up state after open thoracotomy | 4 | 5 | 8 | |
| Dead | 0 | 0 | 0 | |
| Pleural drainage once strategy | Follow-up state after pleural drainage | 70 | 59 | 46 |
| Follow-up state after thoracoscopic surgery | 29 | 39 | 50 | |
| Follow-up state after open thoracotomy | 1 | 2 | 4 | |
| Dead | 0 | 0 | 0 | |
| Pleural drainage twice strategy | Follow-up state after pleural drainage | 74 | 70 | 61 |
| Follow-up state after thoracoscopic surgery | 25 | 29 | 36 | |
| Follow-up state after open thoracotomy | 1 | 1 | 3 | |
| Dead | 0 | 0 | 0 | |
| Pleurodesis once strategy | Follow-up state after pleurodesis | 89 | 86 | 81 |
| Follow-up state after thoracoscopic surgery | 10 | 13 | 18 | |
| Follow-up state after open thoracotomy | 1 | 1 | 1 | |
| Dead | 0 | 0 | 0 | |
| Pleurodesis twice strategy | Follow-up state after pleurodesis | 91 | 90 | 89 |
| Follow-up state after thoracoscopic surgery | 9 | 9 | 10 | |
| Follow-up state after open thoracotomy | 0 | 1 | 1 | |
| Dead | 0 | 0 | 0 | |
| Pleural drainage followed by pleurodesis strategy | Follow-up state after pleural drainage | 70 | 59 | 46 |
| Follow-up state after pleurodesis | 5 | 13 | 22 | |
| Follow-up state after thoracoscopic surgery | 25 | 27 | 30 | |
| Follow-up state after open thoracotomy | 0 | 1 | 2 | |
Sensitivity Analyses
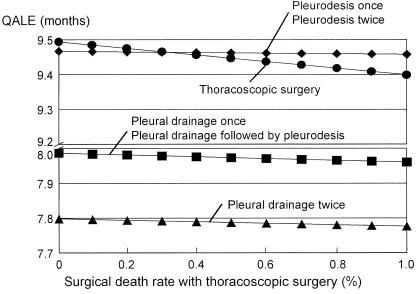
None of the probability estimates for treatment options in 1-way sensitivity analyses altered the main results except for the thoracoscopic surgical death rate. When it exceeds 0.3%, pleurodesis once or twice strategy becomes most preferable (Fig. 2). The 2-way and 3-way sensitivity analyses did not affect these results by changing probabilities for clinical outcomes, utilities, hospital days, the number of cycles, and discounting rate.
FIGURE 2.
One-way sensitivity analysis on surgical rate with thoracoscopic surgery. Pleurodesis once or twice strategy becomes preferable when surgical death rate exceeds 0.3%.
DISCUSSION
QALE is an expected life length that takes into account quality of life measured as utility.56 Benign diseases like PSP affect patients' daily life but not survival. Patients may wish to achieve good, active daily life without disability to the greatest possible extent. The goal of physicians is therefore not only to extend the length of life of patients but also to improve the quality of their health status. QALE, the product of quality and length of life, is preferable for evaluating the efficacy of interventions for such a non-fatal disease.
Our analysis showed young male patients with PSP could benefit most by selecting thoracoscopic surgery as the initial treatment of choice from this viewpoint. This preference is mainly due to the far superior efficacy of thoracoscopic surgery to pleural drainage in terms of recurrence rate. Moreover, as the observation period becomes longer than 1 year, thoracoscopic surgery strategy may become even more advantageous than other strategies and eventually a substantial number of patients will undergo thoracoscopic surgery or open thoracotomy. Although chemical pleurodesis is not widely considered the treatment of choice for young patients with a first episode of PSP, and the efficacy of pleurodesis remained to be proved in a clinical trial, our analysis suggests its potential efficacy for the first episode. When pleurodesis is used for the second episode (the first recurrence), its efficacy looks similar to that of thoracoscopic surgery used for the second episode and inferior to that used for the first episode.
Simple observation or simple aspiration is recommended as the first choice of treatment in some guidelines.6,7 However, we do see patients with pneumothorax who have severe symptoms or fail to improve after simple aspiration. In such cases, we have to select one of more invasive procedures.4 We started the present analysis from this point. These patients may have larger bullae or tear of pleura, for which simple aspiration is ineffective in re-expanding the lungs. Our analysis shows that thoracoscopic surgery may be preferable to pleural drainage for the first episode of PSP in young men in such a situation.
Our findings here differ from those of published guidelines or reviews.6,7 The difference is mainly due to the fact that our analysis placed the emphasis on recurrence incidence and quality of life. PSP is a fairly common disease among young, otherwise healthy men and the disability associated with the recurrence cannot be disregarded from an individual as well as a societal point of view. We did an extensive literature search for the recurrence rates for each treatment strategy and included the best data available into our analyses.
To adequately interpret our analysis, a comment is necessary about utility values. These values elicited from patients and medical staff seem to be extremely low for the follow-up state after treatment. They reflect, however, not only the suffering from recurrence per se, but also patients' anxiety about the recurrence. Some patients may have strong anxiety and avoid risky activities such as air trips or scuba diving.11 Although the range of utility values varies widely, our main results do not change, because these values are equally weighted in all the strategies. Consistent results shown by sensitivity analyses suggest the robustness of our analysis.
While our analyses were focused on young male patients with the highest incidence rate of PSP, other populations, such as women, the elderly, and patients with comorbidity, may have different utilities for a variety of reasons. Some patients may have much lower utility values for follow-up periods of thoracoscopic surgery and open thoracotomy due to the detrimental cosmetic effects. In contrast, some patients may have higher utility values for follow-up periods of pleural drainage without feeling concern about future recurrence. Patients with comorbidities or short life expectancies might not attach much importance to long-term efficacy. Moreover, treatment-related mortality would increase significantly in the case of elderly patients and patients with comorbidities. Our analysis showed that the pleurodesis once or twice strategy may turn out to be the strategy most preferred when the surgical death rate exceeds 0.3% (Fig. 2). Thus, pleurodesis might be the treatment of choice for elderly patients and those with underlying lung disease.
Also, the cost required for the management of PSP cannot be disregarded in the current cost-conscious world. Schramel et al. demonstrated that thoracoscopic surgery is more cost-effective than conservative treatment as an initial treatment for PSP.57 However, their study compared the cost of thoracoscopic surgery with that of pleural drainage and the management for recurrence for the first PSP episode without randomization or reference to utility.57 Therefore, a standard cost-effectiveness analysis56,58 should be performed to validate our findings, in addition to a well-designed controlled study.
Limitations
This analysis was done for a hypothetical cohort of patients and involved some important limitations regarding probability estimates and utilities. Our analysis was done with a 1-year time horizon. Since no data are available concerning the long-term prognosis of PSP, it is not clear whether recurrence occurs at a constant rate beyond 2 years. The current results can therefore not necessarily be extrapolated beyond 2 years. However, our sensitivity analyses implied that the difference of QALE between thoracoscopic surgery and other treatment extended when the observation period became longer.
We did not incorporate chest CT to evaluate bullae before or after pleural drainage. Since chest CT is usually performed only for preoperative evaluation in daily practice, our main objective, i.e., to determine whether surgical treatment should be considered for the patients with a first episode of PSP, was not compatible with incorporating bullae evaluation by chest CT.
We did not take into account technical differences among surgeries as well as different chemical agents used for pleurodesis in our model. Although these differences could affect treatment results, sensitivity analyses that included all reported probabilities in this respect did not change the results.
The size of the sample from which we obtained utilities was relatively small. However, its effect on the main results seems unlikely because of the consistency in sensitivity analyses covering a wide range of utility values. The substantial overlap of the utility values for each health status in different treatment strategies might also serve to make the influence of the small sample size miniscule.
In terms of pleurodesis once strategy, a long-term sequela, i.e., difficulty of performing the next chest surgery20 was not taken into consideration in the model. In that case, the strategy of thoracoscopic surgery after pleurodesis might not be as effective as shown by our results, and the superiority of thoracoscopic surgery strategy over pleurodesis once strategy would become more prominent.
Finally, although we retrieved extensively the relevant literatures, no article mentioned the failure of simple aspiration to re-expand PSP as one of the inclusion criteria. Therefore, the severity of PSP among the subject patients in the literature may be less than that among our hypothetical cohort of patients in whom simple aspiration fails to re-expand the affected lung at the onset. This difference of severity of PSP may bias our analyses in favor of pleural drainage because all the probability estimates used in this study were derived from patients with possibly less severe PSP. Therefore, it is fair to say that our main finding, i.e., that thoracoscopic surgery is the treatment of choice, is robust.
Conclusion
Our decision analysis showed that thoracoscopic surgery might be the treatment of choice in terms of QALE for the first episode of PSP in young, otherwise healthy men. The results were robust for a wide range of probabilities and utilities subjected to sensitivity analyses. Since PSP is a fairly common disease and patients' preference cannot be disregarded, our results may represent a reference point for both patient and physician in deciding the best therapeutic strategy.
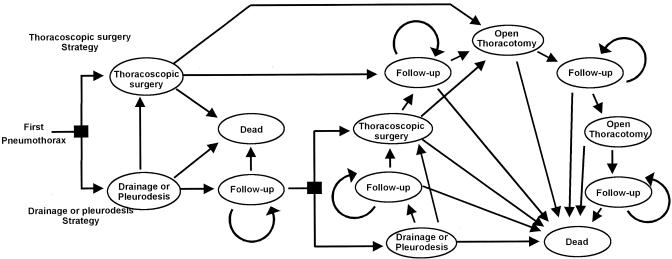
APPENDIX A: Decision Model for Primary Spontaneous Pneumothorax
Square nodes represent the decision to treat primary spontaneous pneumothorax with thoracoscopic surgery, pleural drainage, or pleurodesis. When pleurodesis are selected at the first square decision node, pleural drainage without pleurodesis cannot be selected at the second node. With each 1-month cycle, patients move to a different state or remain in the same state, as indicated by arrows.
APPENDIX B
Characteristics of Reference Articles
| Reference | Author | Year | Design | Patients with PSP | Mean Age | Intervention | Death With Treatment | Treatment Failure | Death During Follow-up | Recurrence | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | Elfeldt RJ | 1994 | Cohort | 96 | — | First pleural drainage | 0 | 33 | 0 | 23 | 36 |
| 9 | Abolnik IZ | 1993 | Cohort | 285 | 25.3 | First pleural drainage | 0 | — | 0 | 118 | 24.1 |
| 118 | — | Second pleural drainage | 0 | — | 0 | 50 | 17.6 | ||||
| 10 | Ferraro P | 1994 | Cohort | 239 | 30.5 | First pleural drainage | 0 | — | 0 | 82 | 18.8 |
| 82 | — | Second pleural drainage | 0 | — | 0 | 53 | 17.2 | ||||
| 14 | Waller DA | 1994 | Cohort | 30 | 28 | Open thoracotomy | 0 | 0 | 0 | 1 | 16.3 |
| 16 | Rieger R | 1998 | Cohort | 57 | 31.7 | Thoracoscopic surgery | 0 | 0 | 0 | 4 | 34 |
| 17 | Passlick B | 1998 | Cohort | 65 | 31 | Thoracoscopic surgery | 0 | 6 | 0 | 4 | 29 |
| 23 | Light RW | 1990 | Cohort* | 104 | 53.8 | Pleurodesis | 0 | — | 0 | 26 | 34.4 |
| 24 | Dumont P | 1997 | Cohort | 82 | 35 | Thoracoscopic surgery | 0 | 3 | 0 | 3 | 16 |
| 170 | 31 | Open thoracotomy | 0 | 0 | 0 | 1 | 80 | ||||
| 26 | Ayed AK | 2000 | Cohort | 72 | 25 | Thoracoscopic surgery | 0 | 0 | 0 | 4 | 42 |
| 28 | Almind M | 1989 | Cohort* | 72 | — | Pleurodesis | 0 | — | 0 | 14 | 72 |
| 29 | Altageme I | 1994 | Cohort | 34 | 34 | First pleural drainage | 0 | — | 0 | 11 | 58 |
| 39 | 39 | Pleurodesis | 0 | — | 0 | 5 | 45 | ||||
| 32 | Kaiser D | 1993 | Case series | 66 | — | Thoracoscopic surgery | 0 | 3 | — | — | — |
| 33 | Cole FH | 1995 | Case series | 30 | 33 | Thoracoscopic surgery | 0 | 5 | — | — | — |
| 34 | Bertrand PC | 1996 | Cohort | 163 | 29.7 | Thoracoscopic surgery | 0 | 5 | 0 | 3 | 24.5 |
| 87 | 30.7 | Open thoracotomy | 0 | 1 | 0 | 1 | 33 | ||||
| 35 | Kim KH | 1996 | Cohort | 33 | 28 | Thoracoscopic surgery | 0 | 0 | 0 | 4 | 24 |
| 36 | Freixinet J | 1997 | Case series | 132 | 26† | Thoracoscopic surgery | 0 | 2 | — | — | — |
| 37 | Horio H | 1998 | Cohort | 51 | 34.8† | Thoracoscopic surgery | 0 | 3 | 0 | 7 | 15.3 |
| 44 | 33.3† | Open thoracotomy | 0 | 0 | 0 | 3 | 39.4 | ||||
| 38 | Maruyama R | 2000 | Cohort | 115 | 28† | Thoracoscopic surgery | 0 | 8 | 0 | 8 | 27 |
| 39 | Ohno K | 2000 | Cohort | 424 | 28 | Thoracoscopic surgery | 0 | 1 | 0 | 40 | 424 |
| 40 | Inderbitzi RG | 1994 | Cohort | 57 | 37 | Thoracoscopic surgery | 0 | 1 | 0 | 6 | 19.6 |
| 41 | Naunheim KS | 1995 | Cohort | 121 | 35.1 | Thoracoscopic surgery | 0 | 0 | 0 | 5 | 13.1 |
| 42 | Yim AP | 1997 | Cohort | 518 | — | Thoracoscopic surgery | 0 | 0 | 0 | 9 | 20 |
| 43 | Andres B | 1998 | Cohort | 44 | 23.2 | Thoracoscopic surgery | 0 | 1 | 0 | 1 | 24 |
| 44 | Waller DA | 1999 | Cohort | 118 | 32.1 | Thoracoscopic surgery | 0 | 0 | 0 | 8 | 24 |
| 45 | McCarthy JF | 1997 | Cohort | 38 | 36.7 | Thoracoscopic surgery | 0 | 1 | 0 | 0 | 18 |
| 46 | Hatz RA | 2000 | Cohort | 95 | 32.5 | Thoracoscopic surgery | 0 | 0 | 0 | 4 | 53.2 |
| 47 | Nkere UU | 1994 | Cohort | 60 | 22† | Open thoracotomy | 0 | 0 | 0 | 2 | 36 |
| 48 | Athanassiadi K | 1998 | Case series | 347 | — | Open thoracotomy | 0 | 0 | — | — | — |
| 49 | Shoenenberger RA | 1991 | Cohort | 72 | 33.8 | First pleural drainage | 0 | 13 | 0 | 20 | 7 |
| 50 | Chan TB | 1985 | Cohort | 36 | 28.4 | Pleural drainage | 0 | — | — | — | — |
| 52 | Kennedy L | 1994 | Review* | 681 | — | Pleurodesis | 0 | 64 | — | — | — |
Data for patients with secondary pneumothorax because data for patients with primary spontaneous pneumothorax are not available.
Median.
(—), data are not available.
REFERENCES
- 1.Mattila S, Kostiainen S. Spontaneous pneumothorax. Scand J Thorac Cardiovasc Surg. 1977;11:259–63. [PubMed] [Google Scholar]
- 2.Hagen RH, Reed W, Solheim K. Spontaneous pneumothorax. Scand J Thorac Cardiovasc Surg. 1987;21:183–5. doi: 10.3109/14017438709106521. [DOI] [PubMed] [Google Scholar]
- 3.Elfeldt RJ, Schroder DW, Thies J. Long-term follow-up of different therapy procedures in spontaneous pneumothorax. J Cardiovasc Surg (Torino) 1994;35:229–33. [PubMed] [Google Scholar]
- 4.Baumann MH, Strange C. The clinician's perspective on pneumothorax management. Chest. 1997;112:822–8. doi: 10.1378/chest.112.3.822. [DOI] [PubMed] [Google Scholar]
- 5.Melton LJ, III, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379–82. doi: 10.1164/arrd.1979.120.6.1379. [DOI] [PubMed] [Google Scholar]
- 6.Miller AC, Harvey JE. Guidelines for the management of spontaneous pneumothorax. Standards of Care Committee, British Thoracic Society. BMJ. 1993;307:114–6. doi: 10.1136/bmj.307.6896.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann MH, Strange C. Treatment of spontaneous pneumothorax: a more aggressive approach? Chest. 1997;112:789–804. doi: 10.1378/chest.112.3.789. [DOI] [PubMed] [Google Scholar]
- 8.Soulsby T. British Thoracic Society guidelines for the management of spontaneous pneumothorax: do we comply with them and do they work? J Accid Emerg Med. 1998;15:317–21. doi: 10.1136/emj.15.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abolnik IZ, Lossos IS, Gillis D, Breuer R. Primary spontaneous pneumothorax in men. Am J Med Sci. 1993;305:297–303. doi: 10.1097/00000441-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Ferraro P, Beauchamp G, Lord F, Emond C, Bastien E. Spontaneous primary and secondary pneumothorax: a 10-year study of management alternatives. Can J Surg. 1994;37:197–202. [PubMed] [Google Scholar]
- 11.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med. 2000;342:868–74. doi: 10.1056/NEJM200003233421207. [DOI] [PubMed] [Google Scholar]
- 12.Schramel FM, Postmus PE, Vanderschueren RG. Current aspects of spontaneous pneumothorax. Eur Respir J. 1997;10:1372–9. doi: 10.1183/09031936.97.10061372. [DOI] [PubMed] [Google Scholar]
- 13.Massard G, Thomas P, Wihlm JM. Minimally invasive management for first and recurrent pneumothorax. Ann Thorac Surg. 1998;66:592–9. [PubMed] [Google Scholar]
- 14.Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg. 1994;58:372–7. doi: 10.1016/0003-4975(94)92210-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu HP, Lin PJ, Hsieh MJ, Chang JP, Chang CH. Thoracoscopic surgery as a routine procedure for spontaneous pneumothorax. Results from 82 patients. Chest. 1995;107:559–62. doi: 10.1378/chest.107.2.559. [DOI] [PubMed] [Google Scholar]
- 16.Rieger R, Woisetschlager R, Schrenk P, Wayand W. Thoracoscopic bleb resection selectively combined with pleurectomy for complicated spontaneous pneumothorax. Eur J Surg. 1998;164:333–8. doi: 10.1080/110241598750004355. [DOI] [PubMed] [Google Scholar]
- 17.Passlick B, Born C, Haussinger K, Thetter O. Efficiency of video-assisted thoracic surgery for primary and secondary spontaneous pneumothorax. Ann Thorac Surg. 1998;65:324–7. doi: 10.1016/s0003-4975(97)01128-4. [DOI] [PubMed] [Google Scholar]
- 18.Sassoon CS. The etiology and treatment of spontaneous pneumothorax. Curr Opin Pulm Med. 1995;1:331–8. [PubMed] [Google Scholar]
- 19.Light RW. Management of spontaneous pneumothorax. Am Rev Respir Dis. 1993;148:245–8. doi: 10.1164/ajrccm/148.1.245. [DOI] [PubMed] [Google Scholar]
- 20.Baumann MH. Treatment of spontaneous pneumothorax. Curr Opin Pulm Med. 2000;6:275–80. doi: 10.1097/00063198-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–58. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 23.Light RW, O'Hara VS, Moritz TE, et al. Intrapleural tetracycline for the prevention of recurrent spontaneous pneumothorax. Results of a Department of Veterans Affairs cooperative study. JAMA. 1990;264:2224–30. [PubMed] [Google Scholar]
- 24.Dumont P, Diemont F, Massard G, Toumieux B, Wihlm JM, Morand G. Does a thoracoscopic approach for surgical treatment of spontaneous pneumothorax represent progress? Eur J Cardiothorac Surg. 1997;11:27–31. doi: 10.1016/s1010-7940(96)01021-4. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez Merchan R, Garcia Diaz F, Arenas Linares C, Giron Arjona JC, Congregado Loscertales M, Loscertales J. Comparative retrospective study of surgical treatment of spontaneous pneumothorax. Thoracotomy vs thoracoscopy. Surg Endosc. 1997;11:919–22. doi: 10.1007/s004649900487. [DOI] [PubMed] [Google Scholar]
- 26.Ayed AK, Al-Din HJ. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest. 2000;118:235–8. doi: 10.1378/chest.118.1.235. [DOI] [PubMed] [Google Scholar]
- 27.Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg. 2000;69:357–62. doi: 10.1016/s0003-4975(99)01299-0. [DOI] [PubMed] [Google Scholar]
- 28.Almind M, Lange P, Viskum K. Spontaneous pneumothorax: comparison of simple drainage, talc pleurodesis, and tetracycline pleurodesis. Thorax. 1989;44:627–30. doi: 10.1136/thx.44.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfageme I, Moreno L, Huertas C, Vargas A, Hernandez J, Beiztegui A. Spontaneous pneumothorax. Long-term results with tetracycline pleurodesis. Chest. 1994;106:347–50. doi: 10.1378/chest.106.2.347. [DOI] [PubMed] [Google Scholar]
- 30.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med. 1982;73:883–8. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 31.Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”). II. Use in medical decision-making. Am J Med. 1982;73:889–97. doi: 10.1016/0002-9343(82)90787-2. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser D, Ennker IC, Hartz C. Video-assisted thoracoscopic surgery—indications, results, complications, and contraindications. Thorac Cardiovasc Surg. 1993;41:330–4. doi: 10.1055/s-2007-1013884. [DOI] [PubMed] [Google Scholar]
- 33.Cole FH, Jr, Cole FH, Khandekar A, Maxwell JM, Pate JW, Walker WA. Video-assisted thoracic surgery: primary therapy for spontaneous pneumothorax? Ann Thorac Surg. 1995;60:931–5. doi: 10.1016/0003-4975(95)00598-f. [DOI] [PubMed] [Google Scholar]
- 34.Bertrand PC, Regnard JF, Spaggiari L, et al. Immediate and long-term results after surgical treatment of primary spontaneous pneumothorax by VATS. Ann Thorac Surg. 1996;61:1641–5. doi: 10.1016/0003-4975(96)00190-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim KH, Kim HK, Han JY, Kim JT, Won YS, Choi SS. Transaxillary minithoracotomy versus video-assisted thoracic surgery for spontaneous pneumothorax. Ann Thorac Surg. 1996;61:1510–2. doi: 10.1016/0003-4975(96)00113-0. [DOI] [PubMed] [Google Scholar]
- 36.Freixinet J, Canalis E, Rivas JJ, et al. Surgical treatment of primary spontaneous pneumothorax with video-assisted thoracic surgery. Eur J Respir. 1997;10:409–11. doi: 10.1183/09031936.97.10020409. [DOI] [PubMed] [Google Scholar]
- 37.Horio H, Nomori H, Fuyuno G, Kobayashi R, Suemasu K. Limited axillary thoracotomy vs video-assisted thoracoscopic surgery for spontaneous pneumothorax. Surg Endosc. 1998;12:1155–8. doi: 10.1007/s004649900805. [DOI] [PubMed] [Google Scholar]
- 38.Maruyama R, Oka T, Anai H. Video-assisted thoracoscopic treatment for spontaneous pneumothorax as two-day surgery. Am J Surg. 2000;180:171–3. doi: 10.1016/s0002-9610(00)00448-7. [DOI] [PubMed] [Google Scholar]
- 39.Ohno K, Miyoshi S, Minami M, et al. Ipsilateral recurrence frequency after video-assisted thoracoscopic surgery for primary spontaneous pneumothorax. Jpn J Thorac Cardiovasc Surg. 2000;48:757–60. doi: 10.1007/BF03218248. [DOI] [PubMed] [Google Scholar]
- 40.Inderbitzi RG, Leiser A, Furrer M, Althaus U. Three years' experience in video-assisted thoracic surgery (VATS) for spontaneous pneumothorax. J Thorac Cardiovasc Surg. 1994;107:1410–5. [PubMed] [Google Scholar]
- 41.Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg. 1995;109:1198–204. doi: 10.1016/S0022-5223(95)70203-2. [DOI] [PubMed] [Google Scholar]
- 42.Yim AP, Liu HP. Video assisted thoracoscopic management of primary spontaneous pneumothorax. Surg Laparosc Endosc. 1997;7:236–40. [PubMed] [Google Scholar]
- 43.Andres B, Lujan J, Robles R, Aguilar J, Flores B, Parrilla P. Treatment of primary and secondary spontaneous pneumothorax using videothoracoscopy. Surg Laparosc Endosc. 1998;8:108–12. [PubMed] [Google Scholar]
- 44.Waller DA. Video-assisted thoracoscopic surgery for spontaneous pneumothorax—a 7-year learning experience. Ann R Coll Surg Engl. 1999;81:387–92. [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy JF, Lannon D, McKenna S, Wood AE. Video-assisted thoracic surgery (VATS) for spontaneous pneumothorax. Ir J Med Sci. 1997;166:217–9. doi: 10.1007/BF02944237. [DOI] [PubMed] [Google Scholar]
- 46.Hatz RA, Kaps MF, Meimarakis G, Loehe F, Muller C, Furst H. Long-term results after video-assisted thoracoscopic surgery for first-time and recurrent spontaneous pneumothorax. Ann Thorac Surg. 2000;70:253–7. doi: 10.1016/s0003-4975(00)01411-9. [DOI] [PubMed] [Google Scholar]
- 47.Nkere UU, Kumar RR, Fountain SW, Townsend ER. Surgical management of spontaneous pneumothorax. Thorac Cardiovasc Surg. 1994;42:45–50. doi: 10.1055/s-2007-1016454. [DOI] [PubMed] [Google Scholar]
- 48.Athanassiadi K, Kalavrouziotis G, Loutsidis A, Hatzimichalis A, Bellenis I, Exarchos N. Surgical treatment of spontaneous pneumothorax: ten-year experience. World J Surg. 1998;22:803–6. doi: 10.1007/s002689900473. [DOI] [PubMed] [Google Scholar]
- 49.Schoenenberger RA, Haefeli WE, Weiss P, Ritz RF. Timing of invasive procedures in therapy for primary and secondary spontaneous pneumothorax. Arch Surg. 1991;126:764–6. doi: 10.1001/archsurg.1991.01410300110017. [DOI] [PubMed] [Google Scholar]
- 50.Chan TB, Tan WC, Teoh PC. Spontaneous pneumothorax in medical practice in a general hospital. Ann Acad Med Singapore. 1985;14:457–61. [PubMed] [Google Scholar]
- 51.Minami H, Saka H, Senda K, et al. Small caliber catheter drainage for spontaneous pneumothorax. Am J Med Sci. 1992;304:345–7. doi: 10.1097/00000441-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Kennedy L, Sahn SA. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215–22. doi: 10.1378/chest.106.4.1215. [DOI] [PubMed] [Google Scholar]
- 53.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31:697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 54.Torrance GW, Thomas WH, Sackett DL. A utility maximization model for evaluation of health care programs. Health Serv Res. 1972;7:118–33. [PMC free article] [PubMed] [Google Scholar]
- 55.Torrance GW. Preferences for health states: a review of measurement methods. Mead Johnson Symp Perinat Dev Med. 1982;20:37–45. [PubMed] [Google Scholar]
- 56.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 57.Schramel FM, Sutedja TG, Braber JC, van Mourik JC, Postmus PE. Cost-effectiveness of video-assisted thoracoscopic surgery versus conservative treatment for first time or recurrent spontaneous pneumothorax. Eur J Respir. 1996;9:1821–5. doi: 10.1183/09031936.96.09091821. [DOI] [PubMed] [Google Scholar]
- 58.Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 2nd ed. Oxford: Oxford University Press; 1997. [Google Scholar]